Abstract
Objectives
To assess feasibility and to conduct a preliminary evaluation of outcomes following Peaceful Mind, a CBT-based intervention for anxiety in dementia, relative to usual care (UC).
Design
Pilot randomized controlled trial including assessments at baseline, 3 and 6 months
Setting
Houston, TX
Participants
32 outpatients diagnosed with mild (47%) or moderate (53%) dementia receiving care through outpatient clinics at the Veterans Affairs medical center, Baylor College of Medicine, Harris County Hospital District and community day centers for dementia, and their collaterals, who spent at least 8 hours a week with them.
Intervention
Peaceful Mind included up to 12 weekly in-home sessions (mean = 8.7, SD = 2.27) during the initial 3 months and up to eight brief telephone sessions (mean = 5.4, SD = 3.17) during months 3 to 6, involving self-monitoring for anxiety, deep breathing, and optional skills (coping self-statements, behavioral activation and sleep management). Patients learned skills, and collaterals served as coaches. In UC, patients received diagnostic feedback; and providers were informed of inclusion status.
Measurements
Neuropsychiatric Inventory-Anxiety subscale, Rating Anxiety in Dementia scale, Penn State Worry Questionnaire-Abbreviated, Geriatric Anxiety Inventory, Geriatric Depression Scale, Quality of Life in Alzheimer’s disease, Patient Health Questionnaire, Client Satisfaction Questionnaire
Results
Feasibility was demonstrated with regard to recruitment, attrition, and treatment characteristics. At 3 months, clinicians rated patients receiving Peaceful Mind as less anxious, and patients rated themselves as having higher quality of life; collaterals reported less distress related to loved ones’ anxiety. Although significant positive effects were not noted in other outcomes or at 6-month follow-up, the pilot nature of the trial prohibits conclusions about efficacy.
Conclusions
Results support that Peaceful Mind is ready for future comparative clinical trials.
Keywords: anxiety, dementia, cognitive behavioral therapy, self-ratings, proxy ratings
Objective
Anxiety is common among persons with dementia. Symptom prevalence rates vary widely (8% to 71%), (1) but anxiety is a common coexistent complaint. Prevalence of anxiety disorders also ranges broadly (5% to 21%); (1) but generalized anxiety disorder (GAD) appears to be most common, occurring in 5% to 15% of patients with dementia. (2, 3) Anxiety may be more common in the mild-to-moderate stages of dementia; (1, 4) and anxiety themes relate to coming to terms with the dementia diagnosis, loss of skills, and health-related issues.(4, 5)
Anxiety coexistent with dementia is associated with increased behavioral problems and limitations in activities of daily living, (6,7) increased disability in social functioning and decreased independence, (7, 8) and increased risk of nursing-home placement. (9) Anxiety co-occurs commonly with depression, as is the case among older adults without cognitive impairment, (10) but even after controlling for depression and/or cognitive performance, anxiety contributes unique variance to functional impairment, (7, 9, 11) behavioral problems, (11) and quality of life. (12)
Despite the serious impact of coexistent anxiety in dementia, few treatment outcome data are available to guide clinical care. (13) Among older adults with anxiety and no cognitive impairment, randomized clinical trials (RCTs) demonstrate positive effects of both antidepressant treatment and cognitive behavior therapy (CBT), (14, 15) although effects following CBT are smaller than for younger adults and small relative to active control conditions. (16, 17) Data addressing the treatment of anxiety in dementia, however, are largely limited to post-hoc analyses of outcomes following medication for Alzheimer’s disease (18) and case series of patients with anxiety and dementia treated with pharmacological and psychosocial treatments. (19, 20) RCTs of anxiety treatment in people with dementia have been limited to tests of music therapy, with mixed results. (21, 22) No RCTs have examined pharmacological or CBT-based interventions for anxiety in patients with dementia, although other trials have demonstrated positive effects of cognitive and behavioral interventions for depression and other behavioral problems in people with dementia, (23-26) and those with cognitive impairment but not dementia. (27, 28) CBT-based interventions have the potential for reducing anxiety among persons with dementia, given that a variety of skills are available, many of which can be easily simplified for patients and their caregivers. In fact, guidelines for dementia care include recommendations to consider CBT for anxiety; (29) and certain learning strategies, particularly spaced retrieval, (30) may be beneficial for teaching anxiety-management skills to patients with dementia. Adaptations from traditional CBT are needed, however, to meet the needs of patients with dementia who require modifications in content (simpler and fewer skills), learning strategies (e.g., increased repetition, spaced retrieval), and delivery (in-home care, involvement of a collateral, different learning strategies).
We developed Peaceful Mind, a CBT-based intervention for anxiety in dementia. Treatment procedures were derived from evidence-based CBT for anxiety and depression in cognitively intact older adults, (31-33) but with significant modifications for participants with dementia. (34) Modifications included in-home sessions; simplified skills, materials, and practice exercises; a collateral to serve as “coach”; more repetition and practice; and cueing procedures and spaced retrieval to facilitate learning and memory. A recent open trial of eight patients supported the feasibility, utility, and positive outcomes of Peaceful Mind. (35) Based on experiences in the open trial and additional consultation with experts, further modifications were made to the intervention manual (e.g., wording changes; creation of handouts to address communication, dementia education, and stress reduction for collaterals; additional instructions for clinicians about in-home delivery). The next step in development and testing of the Peaceful Mind intervention was to conduct a randomized, controlled pilot study that would inform a subsequent Phase III trial. (36) Goals of this pilot study were to test feasibility of the modified Peaceful Mind intervention and to conduct a preliminary evaluation of outcomes relative to a control condition (usual care [UC]). Feasibility was assessed by relative success of different recruitment procedures, attrition rates, and treatment characteristics (average number of sessions completed; nature of skills used; home practice adherence). Although tests of intervention efficacy are not a primary goal of pilot studies (36) and should not be used to determine viability of a subsequent Phase III trial, (37) outcomes were evaluated. Peaceful Mind was expected to produce greater improvements in patient anxiety and worry, depression, and quality of life relative to UC. Positive effects also were expected for collateral distress and depression.
Methods
Participants
Participants were recruited from outpatient clinics at 1) the Veterans Affairs (VA) medical center (geriatrics, primary care, neurology, and psychiatry); 2) Baylor College of Medicine’s affiliated geriatric, primary care and Alzheimer’s disease center; 3) the Harris County Hospital District’s geriatric service; and 4) community day centers specializing in dementia care. Potential participants were identified, using three different recruitment strategies: (1) direct provider referral; (2) facilitated provider referral, using the electronic medical record (EMR) to identify patients with dementia diagnosis; and (3) self-referral. Patients identified via EMR screens either received a letter from or had an in-person conversation with the provider. Self-referrals were generated with educational brochures in clinic waiting areas and newsletters to patients and/or collaterals through community day centers.
Interested patients were contacted by study staff, who conducted a phone screen to assess whether patients had a dementia diagnosis and possible anxiety, could communicate in English, were willing to participate, and had a collateral (adult who spent at least 8 hours weekly with them) who was willing to participate. Anxiety was indicated by a positive response to at least one of three screening questions from the Neuropsychiatric Inventory – anxiety subscale (NPI-A). (38) Informed consents and initial assessment meetings were scheduled in the patient’s home. Demographic information was collected; and the NPI-A, Clinical Dementia Rating (CDR), (39) Dementia Rating Scale-2 (DRS) (40) and Mini International Neuropsychiatric Inventory (MINI) (41) were administered. The MINI was administered to the dyad, and ratings took into account information from the participant and collateral. Diagnoses of generalized anxiety disorder (GAD) were not made if symptoms occurred only in the presence of a depressive disorder. Clinical diagnosticians were Master’s-level graduate students and a predoctoral intern in clinical psychology who received extensive training. CDRs were based on results of the DRS and separate clinical interviews with the collateral and patient. All CDR and MINI interviews were audio taped, and a random 20% (n = 11) were rated by a second clinician. Agreement on the CDR was excellent (kappa = 1.0). Diagnostic agreement was excellent for depression (kappa = 1.0), good for GAD (kappa = 0.79), and adequate for other anxiety disorders (kappa = 0 .61). Included patients had a diagnosis of dementia (confirmed by the patient’s medical provider), an NPI-A score ≥4, and a CDR score between 0.5 and 2.0 (mild-to-moderate dementia severity). Patients with a primary psychiatric diagnosis of major depression, active psychosis, bipolar disorder, active suicidal intent, or recent verbal or physical aggression were excluded.
Measures
Primary outcomes
Primary outcomes, used in our prior open trial of Peaceful Mind, (35) assessed patient anxiety using clinician-rated and collateral report measures. The NPI-A, (38) a seven-item scale, assessed anxiety in patients with dementia based on collateral report of frequency and severity of the patient’s anxiety symptoms over the previous week. It has good interrater reliability, test-retest reliability, and convergent validity in patients with Alzheimer’s disease. (38) A score of 2.5 is average for outpatients with Alzheimer’s disease. (42) The Rating Anxiety in Dementia scale (RAID) (43) assessed anxiety symptoms in four categories: worry, apprehension and vigilance, motor tension, and autonomic hyperactivity. The 18 RAID items rate occurrence of anxiety symptoms from 0 (absent) to 3 (severe) over the previous week and take into account input from the patient and collateral. The RAID has good inter-rater reliability, internal consistency, and convergent/divergent validity. (43-46) A score of 11 indicates clinically significant anxiety, (45) and a score of 18 is characteristic of patients with dementia and GAD. (4) The RAID was administered as a clinical interview to the patient and caregiver. Regular calibration meetings were held between the Masters-level independent evaluators (IEs) who conducted all assessments and assessment supervisor (ALS; also blinded to study condition) to assure ongoing consistency in assessment. IEs were independent from clinical raters who conducted diagnostic interviews. All IE assessment sessions were audio taped, and a random 20% of each IE’s first 20 assessments and 10% of all subsequent assessments (n = 11) were rated by a second rater. Internal consistency reliability and interrater agreement on the RAID were good (Cronbach’s alpha = .75; average weighted kappa = .71).
Secondary outcomes for patients
Secondary patient outcomes included patient self-ratings of worry, anxiety, depression, and quality of life. Worry and anxiety were assessed with the Penn State Worry Questionnaire-Abbreviated (PSWQ-A) (47) and the Geriatric Anxiety Inventory (GAI). (48) The PSWQ-A is an eight-item measure of worry severity with strong psychometric properties among older adults. (47) A mean score of 26 is characteristic of older adults with GAD without cognitive impairment. (32) Among patients with dementia, mean scores of 20 and 15 have been reported for patients with and without GAD, respectively, (4) and the measure was used in our prior open trial to evaluate outcomes following Peaceful Mind. (35) Internal consistency of the PSWQ-A here was .87. The GAI includes 20 agree/disagree items and was developed to assess anxiety severity in older adults. A score of 2.7 is average for older adults in the community; scores greater than 4 indicate mild anxiety, and scores greater than 11 indicate severe anxiety. (49) The GAI also was used in our prior open trial. (35) Internal-consistency reliability of the GAI here was .92. Patient depressive symptoms were measured by the Geriatric Depression Scale (GDS), (50) a 30-item yes/no questionnaire assessing mood over the past week. Internal consistency and test-retest reliability are good, (51) and the measure has been used to assess depression in other late-life anxiety studies. (15) A score at or above 11 indicates possible depression. (52) Patient quality of life was assessed using the Quality of Life in Alzheimer’s disease (QOL-AD). (11, 53) The 13-item scale asks the patient to score aspects of physical health, energy level, mood, living situation, memory, family, marriage, friends, self, ability to do chores and things for fun, money, and life as a whole. A score of 38 represents an average score for community samples of patients with dementia. (53, 54) Internal consistency in prior work has been high (alpha = .87 to .91), and ratings correlate inversely with self-rated depression (54) and positively with day-to-day functioning and frequency of pleasant events. (53)
Secondary outcomes for collaterals
Collateral distress was measured by the distress item from the NPI-A, which asks caregivers to rate their distress related to the patient’s anxiety symptoms on a scale of 0 (not at all) to 5 (very severely or extremely). Collateral depression was assessed with the Patient Health Questionnaire (PHQ-9), a nine-item measure based on diagnostic criteria for major depression. (55) The PHQ-9 has been used with older adults (55) and diverse populations. (56) A score of 4 represents an average score for caregivers of elderly dementia patients; (57) and scores of 5-9 indicate mild depression, 10-14 moderate depression, and 15-19 moderately severe depression. (55) Internal consistency of the PHQ-9 here was .85.
Program Satisfaction
Program satisfaction was assessed with three questions from the Client Satisfaction Questionnaire (CSQ), (58) administered to collaterals to rate overall satisfaction, quality of care, and helpfulness. Ratings were on a scale of 1 (quite dissatisfied/poor/made things worse) to 4 (very satisfied/excellent/helped a great deal). Collaterals were also queried, using standardized questions about the positive and negative impact of the program and with respect to their communication and interaction with their loved one. One open-ended question asked collaterals to offer suggestions for improvement.
Procedures
All outcome measures were administered in-person by trained Masters-level IEs who were unaware of treatment assignment, except the treatment-satisfaction survey administered at 6 months by a research assistant. Measures were administered at baseline, 3 and 6 months. IEs administered the patient self-report inventories orally to eliminate any potential impact of reading problems; all patients and collaterals had a copy of assessment items and possible response choices to read during the administration. Following completion of baseline assessments, patients were randomly assigned to Peaceful Mind (n = 16) or UC (n = 16).
Treatment Description
Peaceful Mind was provided by two Master’s-level graduate-student clinicians and a predoctoral intern supervised by clinical psychologists (MS, CK) and a geriatric social worker (NW). All sessions were audiotaped, and a random 20% were reviewed by an independent treatment integrity rater (AP) for adherence (0 [no adherence] to 8 [optimal adherence]) and competence (0 [no competency] to 8 [excellent competency]). The treatment integrity rater helped develop the treatment (35) but did not provide clinical care in this study. Ratings suggest adequate adherence (5.6 [SD = 1.5]) and competency (5.4 [SD = 1.08]).
Peaceful Mind was offered over 6 months and included up to 12 weekly in-home sessions over the initial 3 months and up to eight brief telephone booster appointments during months 3 to 6. Skills were presented and practiced during the weekly sessions; and telephone booster appointments allowed skills review, reinforcement of skills practice, questions and answers, and problem-solving to integrate skills into daily life. Collaterals were involved in weekly skill learning and served as a coach for the patients’ practice between sessions. The collateral’s role as a coach was determined jointly by the patient, collateral, and clinician, based on the patient’s and collateral’s level of understanding, patient preferences, and collateral availability.
Initial skills sessions included self-monitoring for symptoms of anxiety and deep breathing. The clinician, along with the patient and collateral, could decide to add other skills (coping self-statements, behavioral activation, and sleep management), depending on patients’ symptoms, abilities, and interest. Modification from traditional CBT to enhance learning included repeated instructions, more in-session practice, spaced retrieval (i.e., structured, repeated rehearsal of information until success is consistently achieved in consecutive trials), reminder cues (note cards, calendars), and simplified session summaries and instructions for between-session practice. A large-font workbook with study materials was provided to patients and collaterals;(see Paukert et al. (35) for more details) and handouts to address communication (with healthcare providers and patients with dementia), dementia education, and stress reduction for collaterals were available for use as needed.
Patients assigned to UC received diagnostic feedback. With additional consent, their providers were informed of inclusion status. Patients did not, however, receive any additional study contact other than scheduled assessments. Following 6-month assessments, patients and collaterals in the UC group were offered Peaceful Mind skill sessions.
Data Analytic Plan
Non-completers were those missing an assessment and never returning to the study. Patients in the two treatment conditions (as well as completers versus non-completers) were compared on pretreatment demographic variables, clinical characteristics, and medication use, using Fisher’s Exact test and the non-parametric Wilcoxon-Mann-Whitney U test. Initial outcome analyses were Intention-to-Treat (ITT) and used the multiple imputation procedures Proc MI and MINANALYZE in SAS to address missing data. (59) Treatment-group comparisons were repeated with another set of analyses that included only observed data, using an Analysis of Covariance (ANCOVA). Respective baseline scores were included as a covariate in all models, and a second set of models also included characteristics that differed between treatment conditions at baseline as covariates for relevant outcomes (e.g., baseline patient differences as covariates for patient outcomes; baseline collateral differences for collateral outcomes). All analyses involved two-sided significance testing and were conducted in SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina). To control for inflated experiment-wise error rates due to multiple comparisons, the Bonferroni correction was used within clusters of measures (primary outcomes: 2/.05, p < .025; secondary patient outcomes: 4/.05, p < .0125; secondary collateral outcomes: 2/.05; p < .025) .
Results
Patient and Caregiver Characteristics
Sample characteristics are in Table 1. Baseline scores suggested clinically significant anxiety on primary measures (NPI-A, RAID) and mild symptoms on secondary measures (PSWQ-A, GAI). The majority (65%) had at least one psychiatric diagnosis. GAD was the most frequent principal diagnosis, and comorbid anxiety and depressive disorders were common. Most patients (75%) were taking psychotropic medication, most frequently antidepressants (59%). Patients self-reported minimal depressive symptoms (GDS) and slightly low quality of life (QOL-AD). Collaterals were mildly depressed (PHQ-9). Because collaterals in Peaceful Mind had lower scores than those in UC, the PHQ-9 was included as a covariate in the second set of analyses of collateral self-report ratings. Collaterals were primarily spouses (N = 16, 50.0%) and children (N = 13, 40.6%), and most were women (N = 28, 87.5%), with a mean age of 63.0 (SD = 12.9).
Table 1.
Baseline demographic and clinical comparisons
| Overall (N = 32) |
Peaceful Mind (n = 16) |
UC (n= 16) | p Value* | ||
|---|---|---|---|---|---|
|
|
|||||
| Age (patient), mean (SD) | 78.6 (9.68) | 77.6 (10.54) | 79.6 (8.97) | 0.92 | |
|
|
|||||
| Female (patient), N (%) | 19 (59.4) | 10 (62.5) | 9 (56.3) | 1.00 | |
| Education (patient), N (%) | |||||
| High school or less | 15 (46.9) | 6 (37.5) | 9 (56.3) | 0.48 | |
| College | 17 (53.1) | 10 (62.5) | 7 (43.8) | ||
| Race (patient), N (%)† | |||||
| White | 21 (65.6) | 12 (75.0) | 9 (56.3) | ||
| Black | 7 (21.9) | 1 (6.3) | 6 (37.5) | 0.46 | |
| Multiracial | 1 (3.1) | 0 (0.0) | 1 (6.3) | ||
| Other | 3 (9.4) | 3 (18.8) | 0 (0.0) | ||
| Hispanic (patient), N (%)‡ | 4 (13.3) | 4 (26.7) | 0 (0.0) | 0.10 | |
| Marital status (patient), N (%) |
1.00 | ||||
| Married | 17 (53.1) | 9 (56.3) | 8 (50.0) | ||
| Widowed/divorced | 15 (46.9) | 7 (43.8) | 8 (50.0) | ||
| Dementia diagnosis, N (%)¶ | |||||
| Alzheimers | 20 (62.5) | 9 (56.3) | 11 (68.8) | ||
| Lewy body | 1 (3.1) | 1 (6.3) | 0 (0.0) | .72 | |
| Vascular | 3 (9.4) | 2 (12.5) | 1 (6.3) | ||
| Dementia NOS | 8 (25.0) | 4 (25.0) | 4 (25.0) | ||
| CDR | 0.48 | ||||
| .5 or 1 | 15 (46.9) | 6 (37.5) | 9 (56.3) | ||
| 2 | 17 (53.1) | 10 (62.5) | 7 (43.8) | ||
| Medications | |||||
| At least one psychotropic medication, N (%) |
24 (75.0) | 11 (68.8) | 13 (81.3) | 0.69 | |
| Number of psychotropic medications, mean (SD) |
1.31 (1.0) | 1.13 (1.2) | 1.50 (0.8) | 0.14 | |
| Anti-anxiety medication, N (%) |
4 (12.5) | 2 (12.5) | 2 (12.5) | 1.00 | |
| Anti-depressant medication, N (%) |
19 (59.4) | 10 (62.5) | 9 (56.3) | 1.00 | |
| Anti-psychotic medication, N (%) |
6 (18.8) | 3 (18.8) | 3 (18.8) | 1.00 | |
| Hypnotic medication,N (%) |
5 (15.6) | 1 (6.3) | 4 (25.0) | 0.33 | |
| Principal DSM-IV Diagnoses |
|||||
| GAD, N (%) | 14 (43.84) | 2 (12.5) | 1 (6.3) | 1.00 | |
| Other anxiety, N (%) | 6 (18.8) | 1 (6.3) | 2 (12.5) | 1.00 | |
| Depression, N (%) | 1 (3.1) | 0 (0.0) | 0 (0.0) | 1.00 | |
| Comorbid anxiety and depression, N (%) |
14 (43.8) | 7 (43.8) | 7 (43.8) | 1.00 | |
| No diagnosis | 11 (34.4) | 6 (37.5) | 5 (31.3) | 1.00 | |
|
|
|||||
| Assessment Scores, mean (SD) |
Possible Range of Scores |
Overall (N = 32) |
PM (n= 16) | UC (n = 16) | p Value |
|
|
|||||
| NPI Anxiety -- Total |
0-12 l | 4.7 (3.63) | 4.8 (4.16) | 4.6 (3.11) | 1.00 |
| RAID | 0-54 | 15.1 (7.57) | 13.9 (6.90) | 16.2 (8.24) | 0.46 |
| PSWQ-A | 8-40 | 17.4 (7.39) | 16.0 (7.14) | 18.8 (7.59) | 0.26 |
| GAI | 0-20 | 5.8 (5.58) | 5.0 (5.07) | 6.7 (6.10) | 0.54 |
| GDS | 0-30 | 10.1 (6.80) | 9.4 (7.19) | 10.7 (6.457) | 0.53 |
| QOL-AD | 13-52 | 36.0 (7.12) | 36.8 (6.37) | 35.1 (7.92) | 0.67 |
| NPI Anxiety -- Caregiver Distress |
0-5 | 2.3 (1.81) | 2.3 (1.96) | 2.4 (1.71) | 0.95 |
| PHQ-9 | 0-24 | 5.7 (5.25) | 3.6 (3.56) | 7.8 (5.91) | 0.02 |
p Value compares Peaceful Mind and UC, using Wilcoxon-Mann-Whitney-U test or Fisher’s exact test.
Black, multiracial, and other categories were infrequently endorsed and were therefore collapsed into one group before conducting Fisher’s exact test.
Two patients did not provide data on ethnicity.
Lewy body, Vascular, and Dementia NOS were infrequently endorsed and were therefore collapsed into one group before conducting Fisher’s exact test.
UC = usual care; NOS = not otherwise signified; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; NPI = Neuropsychiatric Inventory, RAID = Rating Anxiety in Dementia scale; PSWQ-A = Penn State Worry Questionnaire-Abbreviated; GAI = Geriatric Anxiety Inventory; GDS = Geriatric Depression Scale; QOL-AD = Quality of Life in Alzheimer’s disease; PHQ = Patient Health Questionnaire
Feasibility: Success of Recruitment Strategies and Attrition
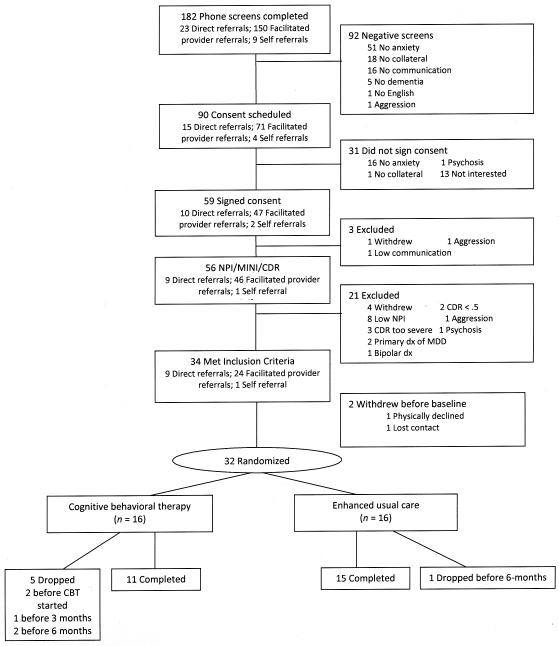
A total of 182 potential patients were recruited and screened (Direct provider referral: 23; Facilitated provider referral: 150; Self-referral: 9) (see Figure 1). Of these, 90 (49%) were eligible to schedule consent, and 56 were assessed with the NPI, CDR, and MINI. Of the 34 who met inclusion criteria, 39% (n= 9) were from direct provider referral, 16% (n = 24), and 11% (n = 1) from self referral. Thirty-two completed baseline assessment and were randomized to Peaceful Mind (N = 16) or UC (N = 16). Six dropped out (five [31.3%] from Peaceful Mind [two moved, two lost contact, and one thought treatment was unhelpful] and one [6.3%] from UC [passed away], leaving 26 who completed the 3-month assessment and 26 who completed the 6-month assessment [N = 11 in Peaceful Mind and 15 in UC]). Treatment condition was not significantly associated with study completion (Fisher’s Exact Test p = .17). Completers (n = 26) did not differ from non-completers (n = 6) on demographics, clinical characteristics, or medication use, except for baseline RAID scores, suggesting that completers had less anxiety (Mean = 13.30, SD = 6.52) than non-completers (Mean = 22.67, SD = 7.58), p = .02.
Figure 1.
Flow of participants through the study.
Feasibility: Treatment Characteristics
The average number of in-person Peaceful Mind sessions was 8.7 (SD = 2.27), and average duration of completed sessions was 47.3 minutes (SD = 10.31 minutes). All patients completed self-monitoring for anxiety and deep breathing to manage anxiety. Thirteen patients (92.9%) learned behavioral activation, nine (64.3%) learned coping self statements, and four (28.6%) learned sleep-management skills. Patients completed an average of 3.5 (SD = 2.15) homework exercises per week and spent an average of 81.3 hours (SD = 63.19 hours) per week with the collateral. Four patient-collateral dyads (25%) received handouts to address communication, stress reduction for collaterals, and/or dementia education. Between months 3 and 6, dyads received an average of 5.4 (SD = 3.16) of a possible eight telephone booster calls (66%). During 81% of these calls, collaterals reported that they were using at least one program skill. Breathing was used most often (58%), followed by behavioral activation (50%) and calming thoughts (41%).
Primary Outcomes
Mean observed scores at baseline, 3 months, and 6 months, as well as tests of primary and secondary outcomes, are included in Table 2. Intention to treat (ITT) analyses revealed significantly greater improvements from baseline to 3 months on the RAID for patients completing Peaceful Mind relative to those completing UC. The effect size for this difference was large (d = .99). Group differences from baseline to 3 months on the NPI-A were not statistically significant, and no significant differences manifested between Peaceful Mind and UC on either the RAID or NPI-A from baseline to 6 months. Completer analyses revealed the same patterns. Among patients who received Peaceful Mind, amount of homework and collateral-patient contact were not associated with baseline to 3-month change in the NPI-A (β = −.10, p = .79 and β = .18, p = .61, respectively) or RAID (β = .43, p = .10 and β = −.06, p = .83).
Table 2.
Intent-to-Treat Outcome Analyses for Baseline to 3 Months and Baseline to 6 Months
| Baseline (N = 32) |
3-Mo (n = 26) |
6-Mo (n = 26) |
BL-3-month Treatment Effect |
BL-6-month Treatment Effect |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
|
F (df)* |
p | d (95% CIs) |
F (df)* |
p |
d (95 % CIs) |
|||||
| Primary Outcomes | ||||||||||
|
| ||||||||||
| NPI Anx (total) |
PM | 4.8 (4.16) | 1.2 (1.47) | 1.5 (2.84) | 3.80 | .69 | 2.40 | .55 | ||
|
|
.067 | .15 | ||||||||
| UC | 4.6 (3.11) | 3.9 (3.81) | 3.9 (3.83) | (1, 18.1) | (−.05, 1.41) | (1, 12.9) | (−.19, 1.26) | |||
|
| ||||||||||
| RAID | PM | 13.9 (6.90) | 10.3 (6.23) | 11.9 (6.92) | 7.84 | .99 | .48 | .24 | ||
|
|
.014 | .50 | ||||||||
| UC | 16.2 (8.24) | 19.1 (8.89) | 17.2 (9.89) | (1, 13.8) | (.19, 1.76) | (1, 11.6) | (−.46, .94) | |||
|
| ||||||||||
| Secondary Outcomes for Patients | ||||||||||
|
| ||||||||||
| PSWQ-A | PM | 16.0 (7.14) | 15.8 (6.79) | 15.3 (7.65) | .01 | .03 | 1.06 | .36 | ||
|
|
.93 | .32 | ||||||||
| UC | 18.8 (7.59) | 18.2 (8.18) | 16.2 (7.87) | (1, 24.7) | (−.66, .72) | (1, 14.0) | (−.35, 1.06) | |||
|
| ||||||||||
| GAI | PM | 5.0 (5.07) | 4.4 (3.83) | 3.9 (3.57) | .07 | .10 | 1.42 | .42 | ||
|
|
.79 | .25 | ||||||||
| UC | 6.7 (6.10) | 5.2 (5.25) | 4.2 (5.20) | (1, 24.8) | (−.60, .79) | (1, 16.6) | (−.29, 1.12) | |||
|
| ||||||||||
| GDS-30 | PM | 9.4 (7.19) | 7.9 (4.36) | 8.2 (2.86) | .04 | .07 | .77 | .31 | ||
| .84 | .39 | |||||||||
| UC | 10.7 (6.57) | 9.2 (6.31) | 7.8 (5.95) | (1, 14.9) | (−.62, .76) | (1, 15.5) | (−.40, 1.01) | |||
|
| ||||||||||
| QOL-AD | PM | 36.8 (6.37) | 38.8 (5.74) | 36.5 (4.28) | 8.82 | 1.05 | .12 | .12 | ||
|
|
.007 | .74 | ||||||||
| UC | 35.1 (7.92) | 32.6 (7.43) | 35.1 (6.64) | (1, 21.9) | (.28, 1.80) | (1, 10.8) | (−.57, .82) | |||
|
| ||||||||||
| Secondary Outcomes for Collaterals | ||||||||||
|
| ||||||||||
| NPI Anx (distress) |
PM | 2.3(1.96) | .8 (1.17) | 1.2 (1.81) | 7.08 | .94 | 1.14 | .38 | ||
|
|
.017 | .30 | ||||||||
| UC | 2.4 (1.71) | 2.6 (1.88) | 2.3 (1.83) | (1, 15.8) | (.16, 1.69) | (1, 16.2) | (−.33, 1.08) | |||
|
| ||||||||||
| PHQ-9 | PM | 3.6 (3.56) | 3.9 (3.21) | 2.9 (2.70) | .48 | .77 | ||||
|
|
.50 | .24 (−.46, .94) |
.39 | .31 (−.39, 1.01) |
||||||
| UC | 7.8 (5.91) | 6.2 (5.95) | 6.2 (4.74) | (1, 16.8) | (1, 22.4) | |||||
Note: Means are observed means at each assessment. Analyses controlled for respective baseline scores. Error/denominator degrees of freedom are adjusted, based on number of imputations and relative increase in variance due to non-response. Complete error degrees of freedom is 29.
BL = baseline; NPI Anxi = Neuropsychiatric Inventory-Anxiety subscale; RAID = Rating Anxiety in Dementia scale; PSWQ-A = Penn State Worry Questionnaire-Abbreviated; GAI = Geriatric Anxiety Inventory; GDS = Geriatric Depression Scale; QOL-AD = Quality of Life in Alzheimer’s disease; PHQ = Patient Health Questionnaire
Secondary Outcomes for Patients
According to ITT analyses, there were significantly greater improvements on the QOL-AD from baseline to 3 months for patients completing Peaceful Mind relative to those completing UC (see Table 2); but group differences on the PSWQ-A, GAI, and GDS from baseline to 3 months were not significant. From baseline to 6 months, there were no group differences in change on any patient self-report outcomes. The same pattern of statistical findings emerged for completer analyses.
Secondary Outcomes for Collaterals
Collaterals of patients receiving Peaceful Mind reported significantly greater decreases in NPI anxiety distress from baseline to 3 months than collaterals of patients receiving UC (see Table 2). Treatment groups did not differ, however, in collateral ratings on the PHQ-9 during this interval. Peaceful Mind and UC did not differ significantly from baseline to 6 months on any collateral measures. Completer analyses revealed the same pattern of results. Analyses were repeated, including baseline collateral PHQ-9 as a covariate, and revealed similar findings, except that differences were no longer significant between Peaceful Mind and UC from baseline to 3 months for NPI Anxiety distress, F (1, 15.72) = 2.25, p = .15. Amount of homework completed and amount of collateral-patient contact were not associated with any secondary outcomes for patients/collaterals completing Peaceful Mind (all p values > .05).
Program Satisfaction
Ten (90.9%) collaterals from the 11 dyads completing Peaceful Mind rated the quality of the program. Collaterals thought the service quality was excellent (M = 3.9, SD = .32), and Peaceful Mind helped them a great deal to manage their problems more effectively (M = 3.7, SD = .48). Overall, collaterals were very satisfied with the service they received (M = 4.00, SD = 0.00); all reported that the program helped them know how to respond to their loved one’s anxiety, and all but one noted positive effects on communication. No consistent negative impacts were noted. Fifty percent of collaterals had no suggestions for changing the program, two recommended longer treatment, two had suggestions for altering materials for patients, and one mentioned a need to adapt the program further, as many patients cannot retain information.
Discussion
Feasibility of the Peaceful Mind intervention was demonstrated in a number of ways. First, the inclusion rate from direct provider referral (39%) was comparable to studies of depression treatment among older adults with executive dysfunction (43%), (24, 27, 28) and inclusion via facilitated provider referral (16%) was comparable to our own prior work using a similar recruitment strategy (14%). (32) In addition, attrition from Peaceful Mind was within the range of rates reported in clinical trials of late-life anxiety treatment among patients without cognitive impairment, (60) although patients with greater anxiety according to the RAID were less likely to complete the intervention. Most patients with dementia and their collaterals were able to participate together in the intervention for an average of nine sessions over 3 months. This finding is of particular note, given that the majority of patients included here had dementia of moderate severity; and skills training was directed toward patients rather than collaterals/caregivers. Patients with any diagnosis of dementia or with dementia of moderate severity have been excluded from prior trials of CBT-based interventions for depression in patients with cognitive impairment, (24, 27, 28) and many interventions offered to patients with a wider range of dementia severity are caregiver-focused. (26) Elective skills most frequently used with Peaceful Mind included coping self-statements and behavioral activation. Patients and/or collaterals were able to complete home practice assignments, and collaterals reported high satisfaction and improved communication. The importance of dyadic interventions for patients with dementia and their collaterals/caregivers has been highlighted in other dementia research (23) and in discussions of intervention development. (61)
Symptom outcomes following Peaceful Mind also were positive. At 3 months, after skills-training sessions, patients who received the intervention were rated by clinicians as less anxious; and they rated themselves as having higher quality of life. Collaterals who participated in the intervention reported less distress related to their loved ones’ anxiety. Contrary to predictions, however, no positive effects of Peaceful Mind were noted on patient self-reported worry, anxiety, or depression or on collateral depression. The lack of findings on patient self-report measures may result from underreporting of symptoms among patients relative to collateral ratings (4) and general difficulties inherent in self-report measures for patients with dementia. (62) Lack of findings related to collateral depression may result from the relatively low level of depression in this subgroup. Samples of caregivers often report higher rates of anxiety and depression than the sample in this study; but, here, many participating collaterals were not primary caregivers, and range of time in dyadic interaction varied widely across dyads. Nevertheless, more attention to collateral needs and the nature of the coaching role may be important in future revisions to Peaceful Mind. Additional modifications also may be needed for patients with more severe anxiety, as those who failed to complete the intervention had significantly higher anxiety according to the RAID.
The general lack of positive Peaceful Mind effects at 6 months for all outcomes may underscore a suggestion made by two collaterals that the intervention may require a longer treatment interval. Although dyads participated in an average of 66% of the available booster sessions and reported some skills practice during the 3-month follow-up interval, spacing skill sessions over a longer interval of time may facilitate increased learning and practice. Increased duration of treatment, however, would need to take into account continued changes in the patient’s cognitive ability, the changing nature of the dyadic interaction, the potential for even higher attrition, (61) and cost effectiveness. The intervention as currently delivered is time-intensive and potentially expensive. However, if the same number of skills sessions were offered over a longer treatment interval, cost would not be enhanced significantly. Further, traditional insurance coverage is not the most likely source of reimbursement. People with dementia and their caregivers receive services through a range of settings, including many community agencies, and in diverse living environments, such as assisted living. In these settings, funding is braided from several different public and private sources (Older American’s Act, Medicare, Medicaid, private philanthropy, etc.); and community agency service providers have demonstrated interest and capacity to implement evidence-based practices within current services, sometimes replacing programs without evidence. (63, 64)
The primary limitation of this pilot study is the use of a UC control condition that failed to control for non-specific treatment factors, such as time and attention. Future trials will need to test outcomes of Peaceful Mind relative to alternative interventions that control for non-specific effects (e.g., supportive counseling, relaxation training) and are potentially effective and less expensive. (15) Future trials also require much larger samples that will provide sufficient power to detect smaller, but meaningful, clinical outcomes and facilitate testing of potential moderating and mediating variables (e.g., homework adherence, patient-collateral contact, medication use, etc.). The current sample had 80% power to detect very large effects (d = 1.05) but only 57% and 27% power to detect the large (d = .80) and moderate effects (d = .50) that are characteristic of late-life anxiety trials for patients without cognitive impairment. (32) Future trials also may test the value of Peaceful Mind offered by providers with greater expertise in home-based treatments and interventions for patients with dementia and their families. Although less experienced providers can be taught to deliver mental health interventions with adequate competence and outcomes, ratings of clinician adherence and competence in this pilot study were lower than in prior trials of late-life anxiety in patients without cognitive impairment. (32) Future studies that include clinic- and community-based providers also would better represent real-world care. The Peaceful Mind intervention also could be tested in alternative treatment settings, such as assisted living and community care settings, where staff could serve in collateral roles. The current preliminary data support the readiness of Peaceful Mind for future comparative clinical trials of these types.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (NIMH) (R34-MH078925) to the first author and by the VA HSR&D Houston Center of Excellence (HFP90-020). The content is solely the responsibility of the author and does not necessarily represent the official views of the NIMH, the National Institutes of Health, the Veterans Administration or Baylor College of Medicine. The NIMH had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; or the preparation, review or approval of the manuscript. The authors do not have any financial conflicts of interest to report.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: A critical review. Clinical Psychology Review. 2008;28:1071–82. doi: 10.1016/j.cpr.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferretti L, McCurry SM, Logsdon R, et al. Anxiety and Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2001;14:52–8. doi: 10.1177/089198870101400111. [DOI] [PubMed] [Google Scholar]
- 3.Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15:42–9. doi: 10.1097/01.JGP.0000229664.11306.b9. [DOI] [PubMed] [Google Scholar]
- 4.Calleo J, Kunik ME, Reid D, et al. Characteristics of generalized anxiety disorder in patients with dementia. Am J Alzheimers Dis Other Dement. 2011 doi: 10.1177/1533317511426867. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qazi A, Spector A, Orrell M. User, carer and staff perspectives on anxiety in dementia: a qualitative study. J Affect Disord. 2010;125(1-3):295–300. doi: 10.1016/j.jad.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Neville C, Teri L. Anxiety, anxiety symptoms, and associations among older people with dementia in assisted-living facilities. Int J Ment Health Nurs. 2011;20(3):195–201. doi: 10.1111/j.1447-0349.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 7.Schultz SK, Hoth A, Buckwalter K. Anxiety and impaired social function in the elderly. Ann Clin Psychiatry. 2004;16:47–51. doi: 10.1080/10401230490281429. [DOI] [PubMed] [Google Scholar]
- 8.Porter VR, Buxton WG, Fairbanks LA, et al. Frequency and characteristics of anxiety among patients with Alzheimer’s disease and related dementias. Journal of Neuropsychiatry and Clinical Neuroscience. 2003;15:180–6. doi: 10.1176/jnp.15.2.180. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons LE, Teri L, Logsdon RG, et al. Anxiety symptoms as predictors of nursing home placement in patients with Alzheimer’s disease. Journal of Clinical Geropyschology. 2002;8:335–42. [Google Scholar]
- 10.King-Kallimanis B, Gum AM, Kohn R. Comorbidity of depressive and anxiety disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatr. 2009;17(9):1157–64. doi: 10.1097/JGP.0b013e3181ad4d17. [DOI] [PubMed] [Google Scholar]
- 11.Teri L, Ferretti LE, Gibbons LE, et al. Anxiety of Alzheimer’s disease: prevalence, and comorbidity. J Gerontol A Biol Sci Med Sci. 1999;54:M348–M352. doi: 10.1093/gerona/54.7.m348. [DOI] [PubMed] [Google Scholar]
- 12.Hoe J, Hancock G, Livingston G, et al. Quality of life of people with dementia in residential care homes. Br J Psychiatry. 2006;188:460–4. doi: 10.1192/bjp.bp.104.007658. [DOI] [PubMed] [Google Scholar]
- 13.McClive-Reed KP, Gellis ZD. Anxiety and related symptoms in older persons with dementia: directions for practice. J Gerontol Soc Work. 2011;54(1):6–28. doi: 10.1080/01634372.2010.524284. [DOI] [PubMed] [Google Scholar]
- 14.Pinquart M, Duberstein PR. Treatment of anxiety disorders in older adults: a meta-analytic comparison of behavioral and pharmacoligical interventions. Am J Geriatr Psychiatry. 2007;15(8):639–51. doi: 10.1097/JGP.0b013e31806841c8. [DOI] [PubMed] [Google Scholar]
- 15.Thorp SR, Ayers CR, Nuevo R, et al. Meta-analysis comparing different behavioral treatments for late-life anxiety. Am J Geriatr Psychiatry. 2009;17:105–15. doi: 10.1097/JGP.0b013e31818b3f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wetherell JL, Lenze EJ, Stanley MA. Evidence-based treatment of geriatric anxiety disorders. Psychiatr Clin North Am. 2005;28(871):896. doi: 10.1016/j.psc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Wolitzky-Taylor KB, Castriotta N, Lenze EJ, et al. Anxiety disorders in older adults: a comprehensive review. Depress Anxiety. 2010;27(2):190–211. doi: 10.1002/da.20653. [DOI] [PubMed] [Google Scholar]
- 18.Mintzer J, Faison W, Street JS, et al. Olanzapine in the treatment of anxiety symptoms due to Alzheimer’s disease: a post hoc analysis. Int J Geriatr Psychiatry. 2001;16(Suppl 1):571–6. doi: 10.1002/1099-1166(200112)16:1+<::aid-gps568>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Koder DA. Treatment of anxiety in the cognitively impaired elderly: can cognitive-behavior therapy help? Int Psychogeriatric. 1998;10:173–82. doi: 10.1017/s1041610298005286. [DOI] [PubMed] [Google Scholar]
- 20.Qazi A, Shankar K, Orrell M. Managing anxiety in people with dementia. A case series. J Affect Disord. 2003;76:261–5. doi: 10.1016/s0165-0327(02)00074-5. [DOI] [PubMed] [Google Scholar]
- 21.Cooke ML, Moyle W, Shum DH, et al. A randomized controlled trial exploring the effect of music on agitated behaviours and anxiety in older people with dementia. Aging Ment Health. 2010;14(8):905–16. doi: 10.1080/13607861003713190. [DOI] [PubMed] [Google Scholar]
- 22.Guetin S, Portet F, Picot MC, et al. Effect of music therapy on anxiety and depression in patients with Alzheimer’s type dementia: randomised, controlled study. Dement Geriatr Cogn Disord. 2009;28(1):36–46. doi: 10.1159/000229024. [DOI] [PubMed] [Google Scholar]
- 23.Gitlin LN, Winter L, Burke J, et al. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. Am J Geriatr Psychiatr. 2008;16(3):229–39. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiosses DN, Arean PA, Teri L, et al. Home-delivered problem adaptation therapy (PATH) for depressed, cognitively impaired, disabled elders: a preliminary study. Am J Geriatr Psychiatr. 2010;18(11):988–98. doi: 10.1097/JGP.0b013e3181d6947d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCurry SM, Gibbons LE, Logdon RG, et al. Training caregivers to change the sleep hygiene practices of patients with dementia: the NITE-AD project. J Am Geriatr Soc. 2003;51(10):1455–60. doi: 10.1046/j.1532-5415.2003.51466.x. [DOI] [PubMed] [Google Scholar]
- 26.Teri L, McCurry SM, Logsdon R, et al. Training community consultants to help family members improve dementia care: A randomized controlled trial. Gerontologist. 2005;45:802–11. doi: 10.1093/geront/45.6.802. [DOI] [PubMed] [Google Scholar]
- 27.Aréan PA, Raue P, Mackin RS, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am J Psychiatry. 2010;167(11):1391–8. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexopoulos GS, Raue PJ, Kiosses DN, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry. 2011;68(1):33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Institute for Health and Clinical Excellence, Social Care Institute for Excellence . Supporting people with dementia and their carers in health and social care. National Insistute for Health and Clinical Excellence; London: 2006. Dementia. Report No. NICE clinical guidelines 42. [Google Scholar]
- 30.Camp CJ, Koss E, Judge K. Cognitive assessment in late-stage dementia. In: Lichtenberg PA, editor. Handbook of assessment in clinical gerontology. John Wiley & Sons, Inc.; Hoboken, NJ: 1999. pp. 442–67. [Google Scholar]
- 31.Quijano L, Stanley MA, Petersen NJ, et al. Healthy IDEAS. A depression intervention delivered by community-based case managers serving older adults. J Applied Gerontol. 2007;26:139–56. [Google Scholar]
- 32.Stanley MA, Wilson N, Novy DM, et al. Cognitive behavior therapy for older adults with generalized anxiety disorder in primary care: A randomized clinical trial. JAMA. 2009;301(14):1460–7. doi: 10.1001/jama.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wetherell JL, Ayers CR, Sorrell JT, et al. Modular psychotherapy for anxiety in older primary care patients. Am J Geriatr Psychiatr. 2009;17(6):483–92. doi: 10.1097/JGP.0b013e3181a31fb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snow AL, Powers D, Liles D. Cognitive-behavioral therapy for long-term care patients with dementia. In: Hyer L, Intrieri RC, editors. Geropsychological Interventions in Long-Term Care. Springer Publishing Company, Inc.; New York NY: 2006. pp. 265–93. [Google Scholar]
- 35.Paukert AL, Calleo J, Kraus-Schuman C, et al. Peaceful Mind: An open trial of cognitive-behavioral therapy for anxiety in persons with dementia. Int Psychogeriatr. 2010;22(6):1012–21. doi: 10.1017/S1041610210000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraemer HC, Mintz J, Noda A, Tinklenberg J, et al. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63(5):484–9. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- 38.Cummings JL, Mega MS, Gray K, et al. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 39.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 40.Mattis S. Dementia Rating Scale-2. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- 41.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 42.Hashimoto H, Monserratt L, Nguyen P, et al. Anxiety and regional cortical glucose metabolism in patients with Alzheimer’s Disease. J Neuropsychiatry Clin Neurosci. 2006;18(4):521–8. doi: 10.1176/jnp.2006.18.4.521. [DOI] [PubMed] [Google Scholar]
- 43.Shankar KK, Walker M, Frost D, et al. The development of a valid and reliable scale for rating anxiety in dementia (RAID) Aging Ment Health. 1999;3:39–49. [Google Scholar]
- 44.Cheston R, Jones K, Gilliard J. Group psychotherapy and people with dementia. Aging Ment Health. 2003;7:452–61. doi: 10.1080/136078603100015947. [DOI] [PubMed] [Google Scholar]
- 45.Twelftree H, Qazi A. Relationship between anxiety and agitation in dementia. Aging Ment Health. 2006;10(4):362–7. doi: 10.1080/13607860600638511. [DOI] [PubMed] [Google Scholar]
- 46.Snow AL, Huddleston C, Robinson C, et al. Psychometric properties of a structured interview guide for the Rating for Anxiety in Dementia (RAID-SI) Aging Ment Health. 2011 doi: 10.1080/13607863.2011.644518. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crittendon J, Hopko DR. Assessing worry in older and younger adults: Psychometric properties of an Abbreviated Penn State Worry Questionnaire (PSWQ-A) J Anxiety Disord. 2006;20:1036–54. doi: 10.1016/j.janxdis.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Pachana NA, Byrne GJA, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19:103–14. doi: 10.1017/S1041610206003504. [DOI] [PubMed] [Google Scholar]
- 49.Andrew DH, Dulin PL. The relationship between self-reported health and mental health problems amoung older adults in New Zealand: Experiential avoidance as a moderator. Aging Ment Health. 2007;11(5):596–603. doi: 10.1080/13607860601086587. [DOI] [PubMed] [Google Scholar]
- 50.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 51.Stiles PG, McGarrahan JF. The Geriatric Depression Scale: A comprehensive review. J Clin Geropsychology. 1998;4(2):89–110. [Google Scholar]
- 52.Brink TL, Yesavage JA, Owen L, et al. Screening tests for geriatric depression. Clin Gerontol. 1982;1(1):37–43. [Google Scholar]
- 53.Logsdon RG, Gibbons LE, McCurry SM, et al. Assisting quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64:510–9. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Snow AL, Kunik ME, Molinari VA, et al. Accuracy of self-reported depression in persons with dementia. J Am Geriatr Soc. 2005;53:389–96. doi: 10.1111/j.1532-5415.2005.53154.x. [DOI] [PubMed] [Google Scholar]
- 55.Kroenke K, Spitzer RL, Williams JB. The PHQ-P: Validity of a brief depression severity measure. J Gen Intern Medicine. 2001;16(1):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang FY, Chung H, Kroenke K, et al. Using Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21(6):547–52. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schubert CC, Boustani M, Callahan CM, et al. Acute care utilization by dementia caregivers within urban primary care practices. J Gen Intern Med. 2008;23(11):1736–40. doi: 10.1007/s11606-008-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larsen DL, Attkisson CC, Hargreaves WA, et al. Assessment of client/patient satisfaction: Development of a general scale. Eval Program Plann. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 59.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–90. [Google Scholar]
- 60.Wetherell JL, Hopko DR, Diefenbach GJ, et al. Cognitive-behavioral therapy for late-life generalized anxiety disorder: Who gets better? Behav Ther. 2005;36:147–56. [Google Scholar]
- 61.Zarit SH, Leitsch SA. Developing and evaluating community based intervention programs for Alzheimer’s patients and their caregivers. Aging Ment Health. 2001;5:S84–S98. doi: 10.1080/13607860120044864. [DOI] [PubMed] [Google Scholar]
- 62.Snow AL, Cook KF, Lin P-S, et al. Proxies and other external raters: methodological considerations. Health Serv Res. 2005;40(5 Pt 2):1676–93. doi: 10.1111/j.1475-6773.2005.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabiner DJ, Brown D, Osber D, et al. Executive summary. Prepared for the US Administration on Aging; Washington, D.C.: 2006. Implementing evidence-based models and promising practices: the experience of Alzheimer’s disease demonstration grants to states (ADDGS) programs. Research Triangle Institute International Project No. 0209351.001.008. [Google Scholar]
- 64.Tilly J, Wiener J, Gould E. Building dementia capability into the long-term services and support system; Presented at “Improving Lives,” 2011 Administration on Aging Health and Dementia Grantee Meeting; Crystal City, VA. June 14, 2011; Available at http://222.adrc-tae.org/tiki-searchresults.php?words=2011+grantee+meeting. [Google Scholar]