Abstract
The purpose of this study was to evaluate the effects of acute, oral modafinil (200 mg) exposure on daytime sleepiness in methamphetamine (Meth)-dependent individuals. Eighteen Meth-dependent subjects were enrolled in a 7-d inpatient study and were administered placebo or modafinil on day 6 and the counter-condition on day 7 (randomized) of the protocol. Subjects completed several subjective daily assessments (such as the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, Beck Depression Inventory and visual analogue scale) throughout the protocol as well as objective assessments on days 5–7, when the Multiple Sleep Latency Test was performed. The results of the current study suggest that short-term abstinence from Meth is associated with increased daytime sleepiness and that a single dose of 200 mg modafinil reduces daytime somnolence in this population. In addition, a positive correlation was found between subjective reporting of the likelihood of taking a nap and craving and desire for Meth, as well as the likelihood of using Meth and whether Meth would make the participant feel better. The results of this study should be considered when investigating candidate medications for Meth-dependence, especially in those individuals who attribute their Meth use to overcoming deficits resulting from sleep abnormalities.
Keywords: Methamphetamine, modafinil, sleep
Introduction
Methamphetamine (Meth) users self-report poor sleep quality, sleep disturbances and significant daytime sleepiness (Mahoney et al. unpublished observations; McGregor et al. 2005). Specifically, Mahoney et al. reported that, in a sample of 71 Meth-dependent individuals, the average score on the Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale (ESS) were 8.5±5.0 and 9.8±5.5 respectively, whereas the average scores in typical, healthy individuals are 2.7±1.7 for the PSQI (Buysse et al. 1989) and 5.9±2.2 for the ESS (Johns, 1991). These elevated scores raise the concern that sleep deficiencies can precipitate Meth use as a way to reduce daytime sleepiness and to increase alertness and attention. In fact, related studies reported that Meth administration is associated with reduced self-reported fatigue and daytime sleepiness (Shappell et al. 1996) and improved performance on measures of information-processing speed (Hart et al. 2003).
Poor sleep quality has also been reported in other stimulant users, such as cocaine-dependent individuals (Gawin & Kleber, 1986; Morgan & Malison, 2007; Weddington et al. 1990). For example, Mahoney et al. (unpublished observations) reported that, in a sample of 51 cocaine-dependent individuals, the average scores on the PSQI and ESS were 7.8±4.4 and 9.7±5.1, respectively. Thus, cocaine-dependent individuals also endorsed more sleep deficiencies when compared to healthy individuals and those sleep difficulties were comparable to those reported by Meth-dependent individuals. Moreover, this suggests that Meth-associated daytime sleepiness should be a target of treatment.
One possibility is that medications can be used to ameliorate these sleep difficulties and the wake-promoting agent, modafinil, is one such candidate. It has been reported that modafanil exposure is associated with increased daytime sleep latency and decreased daytime sleepiness in abstinent cocaine-dependent individuals (Morgan et al. 2010). Other characteristics of modafanil increase the viability of this compound as a treatment for Meth-associated sleep disturbances. For example, modafinil does not accentuate the stimulant effects produced by the administration of Meth in a laboratory setting (De La Garza et al. 2010). Furthermore, modafinil reduces daytime sleepiness in individuals with hypersomnolence due to narcolepsy and sleep apnea (Arnulf et al. 1997; Besset et al. 1993). Additionally, treatment with modafinil reduces fatigue, agitation and irritability in individuals experiencing Meth withdrawal (McGregor et al. 2008). Similarly, it has been reported that modafinil reduced sensory aspects of fatigue in Meth-dependent subjects (Ghahremani et al. 2011). Importantly, none of these studies reported serious adverse events as a result of modafinil exposure.
Each of the aforementioned factors supports the use of modafinil as a treatment for Meth-associated sleep disorders. To address this, we conducted a double-blind, placebo-controlled study to investigate the impact of modafinil administration on sleep characteristics among Meth-dependent individuals. Similar to the reported outcomes by Morgan et al. (2010) in abstinent cocaine users, we hypothesized that modafinil would increase daytime sleep latency and decrease daytime sleepiness in acutely abstinent Meth users.
Method
Subjects
Eighteen Meth-dependent subjects were enrolled in a 7-d inpatient study. The Institutional Review Board of the University of California, Los Angeles approved this study and all subjects gave informed consent after being made aware of the possible risks of participation. All individuals were compensated for their participation in this study.
Individuals were recruited via newspaper and radio advertisements. All Meth-dependent subjects were non-treatment seeking and met DSM-IV-TR criteria for Meth-dependence, as assessed by the Mini International Neuropsychiatric Interview (Sheehan et al. 1997). Other inclusion criteria included being aged between 18 and 45 yr, at least twice-weekly use of Meth (smoked or i.v.), positive urine toxicology for Meth prior to admission, and normal vital signs and electrocardiogram. Exclusion criteria included diagnosis of any other Axis I psychiatric disorder, dependence on any other drugs aside from nicotine, a history of seizure disorder, prior head trauma, or concomitant use of any psychotropic medication. Additionally, a previous diagnosis with any primary sleep disorder (narcolepsy, insomnia, rapid eye movement sleep behaviour disorder) or any sleep disordered breathing, such as apnea, was exclusionary.
Upon intake to the UCLA General Clinical Research Center (GCRC), Meth-dependent individuals completed a comprehensive drug use history form, the PSQI (Buysse et al. 1989), to assess the quality and patterns of sleep, the ESS (Johns, 1991) to assess self-perceived sleep quality and excessive daytime sleepiness over the past month, the Beck Depression Inventory (BDI-II; Beck et al. 1996) to assess presence of depressive symptoms, sleep quality and craving visual analogue scales (VAS), and the American National Adult Reading Test to assess for verbal IQ. Subjects spent days 1–4 acclimatizing to GCRC and ‘washing out’ from any recent Meth use and no major procedures were performed on those days; however, the aforementioned daily written assessments (e.g. PSQI, ESS and VAS) were conducted. Subjects were required to be in bed with ‘lights out’ at 23:00 hours and were awoken at 07:00 hours every morning for daily assessments. Other than these times (23:00–07:00 hours), subjects were not allowed to nap or lie down unless they participated in the Multiple Sleep Latency Test (MSLT) sessions.
Drugs
On day 1, Meth-dependent subjects were randomized to receive one dose (200 mg) of modafinil (Provigil®; Cephalon, USA) on either the sixth or seventh day of the study, with the counter-condition presented the following day (i.e. if the subject received modafinil on day 6, he/she received placebo on day 7 and vice versa). Modafinil was encapsulated to match placebo in appearance and subjects were administered the drug at 08:00 hours.
Daytime sleep assessment
On days 5–7, at 09:00, 11:00, 13:00 and 15:00 hours, the MSLT, a procedure used to assess sleep onset latency and measure physiological sleep tendency in the absence of alerting stimuli (Carskadon & Dement, 1982), was performed. Standard electroencephalography electrodes were attached to the scalp and face according to the international 10–20 system. Five minutes prior to each test, subjects were asked to get into bed and were led through a standardized diagnostic series of movements (eyes left, eyes right, eyes up, eyes down, blink) to ensure electrode connectivity. Afterward, subjects completed the Stanford Sleepiness Scale (SSS) to assess self-perceived somnolence. Immediately following completion of the SSS, lights in the room were turned off, shades and curtains were drawn and the subjects were asked to try to fall asleep (these sessions are referred to as ‘naps’ throughout the remainder of this paper). As per clinical research standards for the MSLT, subjects were given 20 min to fall asleep. If they did not fall asleep after 20 min, the test was concluded and the lights were turned back on. Individuals were permitted to sleep if it occurred during the MSLT, meaning that the absolute maximum of sleep during the day would have been 80 min (four naps × 20 min each); however, they were not permitted to lie down at times other than the MSLT sessions. Between test sessions, subjects were instructed to remain out of bed and no napping was permitted on those days. To prevent confounds due to the arousing effects of nicotine, exercise and caffeine, each was restricted during the inpatient stay (Prosise et al. 1994).
A note on full study design
Participants in the current study also completed neurocognitive assessments following modafinil administration and these null findings will be published separately. Also, a subset of these individuals (n=9) were also exposed to temazepam during the evenings of days 5 and 6 and the null findings from that study will also be reported separately.
Statistical analysis
Sleep onset during the MSLT was defined as the first 30-s epoch of any stage of sleep and sleep stages were scored using a standardized set of criteria (Rechtschaffen & Kales, 1968). For baseline (day 5 when no medication was administered) and on days in which placebo or modafinil were administered, only three naps were utilized to calculate mean daytime sleep latency (the first nap was excluded from the analysis). The rationale for this approach was as follows: modafinil was administered at 08:00 hours on the scheduled days and peak plasma levels are not achieved for approximately 2 h following dosing (Cephalon, 2004; Wong et al. 1999). Thus, the 09:00 hours nap on that day would not be an accurate representation of modafinil’s effects. As such, only the naps at 11:00, 13:00 and 15:00 hours were used to calculate mean sleep latency at baseline and on placebo days for consistency purposes.
Paired t tests were used to determine the relationship between subjective reporting of sleep quality, mood and variables associated with Meth use (e.g. craving, desire, etc.). The variables night-time sleep, bedtime, ‘how well did you sleep last night’, ‘how likely would you be to take a nap’, ‘how much better would meth make you feel ‘ and ‘how likely would you be to use meth’ were included as part of the sleep quality VAS, which was administered on days 2–7 of the protocol. The other variables (ESS, BDI, craving and desire) were all obtained on day 1 during admission and were completed daily to day 7. We decided to compare days 1 or 2 to day 5 because it provided a valuable and important comparison between baseline (when they first arrived at the GCRC) and after becoming acclimatized to the GCRC, but prior to any modafinil administration (day 5). In addition, correlations between sleep and Meth variables were determined using Pearson product-moment correlations. Statistical significance was set at p<0.05. All analyses were performed using PASW 18.0 (SPSS, USA).
Results
Characteristics of Meth users
Demographic and sleep characteristics
Demographic and drug use information for subjects is provided in Table 1. Subjects were primarily male (89%) and either Caucasian (44%) or Hispanic (44%). The average age was ~35 yr, average years of education were ~12 and the average verbal IQ was ~110. On average, subjects reported using Meth for ~12 yr, used ~18 d out of the past 30 and ~10 g per week. In addition, the majority (83%) of the subjects reported smoking cigarettes.
Table 1.
Demographic and drug use characteristicsa
| Methamphetamine users (n=18) | |
|---|---|
| Gender (n) | |
| Male | 16 (89%) |
| Female | 2 (11%) |
| Ethnicity (n) | |
| Caucasian | 8 (44%) |
| Hispanic | 8 (44%) |
| African–American | 1 (6%) |
| Other | 1 (6%) |
| Age (yr) | 34.8±7.7 |
| Education (yr) | 12.1±1.2 |
| Verbal IQ (AMNART) | 110.4±5.4 |
| Nicotine use | 15 (83%) |
| Methamphetamine use | |
| Years of use | 12.4±7.4 |
| Recent useb | 18.3±8.9 |
| g/week | 9.5±9.1 |
| Amount spent/week ($) | 219.6±158.0 |
| Days abstinent prior to enrolment | 6.2±1.3 |
| Sleep quality (day 1) | |
| PSQI | 6.4±4.3 |
| ESS | 8.9±4.8 |
AMNART, American National Adult Reading Test; PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale.
Values reflect mean±S.D.
All data obtained during screening (0–30 d prior to enrolment).
Recent use equals the number of days in which methamphetamine was used in the 30 d prior to the interview.
With regard to sleep characteristics, upon enrolment subjects reported an average score of 6.4±4.3 on the PSQI and 8.9±4.8 on the ESS. On day 2 and day 5, when subjects were asked to report the previous night’s sleep, subjects reported sleeping significantly less on night 4 (7.6±2.1 h) in comparison to night 1 (9.3±2.1 h; t15=3.6, p<0.005, η2=0.5). Moreover, subjects’ bedtime was significantly later on night 4 relative to night 1 (t15=−4.3, p<0.001, η2=0.6). There were no significant correlations between days abstinent prior to admission and total hours of sleep on night 1 (r=0.1, p=0.9) or bedtime on night 1 (r=−0.3, p=0.3). In addition, BDI-II scores decreased significantly between day 1 and day 5 (Table 2). Likelihood of taking a nap on day 5 was not different from day 2 (t16=1.0, p=0.3) nor was there a difference between quality of sleep between night 1 and night 4 when asked ‘how well did you sleep last night?’ (t16=1.5, p=0.2).
Table 2.
Baseline subjective mood and sleep characteristics for methamphetamine users
| Variable | Mean±S.D. |
|---|---|
| Night-time sleep | |
| Day 2 (h)a | 9.3±2.1 |
| Day 5 (h) | 7.6±2.1* |
| Bedtime | |
| Day 2 | 21:13±3.0 hours |
| Day 5 | 22:54±1.5 hours* |
| VAS – ‘How well did you sleep last night?’ (range 0–100) | |
| Day 2 | 66.5±33.2 |
| Day 5 | 53.5±39.8 |
| VAS – ‘How likely would you be to take a nap today?’ | |
| Day 2 | 74.1±28.3 |
| Day 5 | 64.7±30.9 |
| ESS score (range 0–24) | |
| Day 1 | 9.6±4.6 |
| Day 5 | 10.1±5.0 |
| Daytime sleep latency | |
| Day 5 (min) | 11.9±3.7 |
| BDI-II score (range 0–63) | |
| Day 1 | 11.0±12.3 |
| Day 5 | 3.6±5.8* |
| VAS – ‘Craving for methamphetamine’ | |
| Day 1 | 46.3±29.2 |
| Day 5 | 40.6±33.0 |
| VAS – ‘Desire for methamphetamine’ | |
| Day 1 | 40.0±31.4 |
| Day 5 | 44.4±38.3 |
| VAS – ‘How much better would methamphetamine make you feel?’ | |
| Day 2 | 81.2±20.3 |
| Day 5 | 71.2±37.1 |
| VAS – ‘If you had access to methamphetamine, how likely would you be to use it?’ | |
| Day 2 | 83.1±25.5 |
| Day 5 | 65.0±34.8* |
VAS, Visual analogue scale; ESS, Epworth Sleepiness Scale; BDI-II, Beck Depression Inventory.
Information reported on day 2 and day 5 is regarding the sleep patterns on the evening of day 1 and day 4, respectively.
p<0.5.
Drug use characteristics
As mentioned previously, subjects in the current study were asked to provide a self-report of their craving for Meth once daily, at 11:45 hours. Data from four items of interest on the sleep quality and craving VAS forms were analysed to examine changes across 4 d abstinence from Meth (Table 2).
When asked, ‘ If you had access to methamphetamine right now, how likely would you be to use it, ‘ subjects’ ratings decreased significantly from 83.1 on day 2 to 65.0 on day 5 (t15=2.4, p<0.03, η2=0.3). Subjects’ rating of ‘How much better would methamphetamine make you feel right now,’ did not differ across days 2 and 5 t16=1.2, p=0.2). On the craving VAS form, there were no significant differences between day 1 and day 5 for either ‘craving’ (t15=0.5, p=0.7, η2=0.0) or ‘desire’ for Meth (t15=−0.5, p=0.6, η2=0.0).
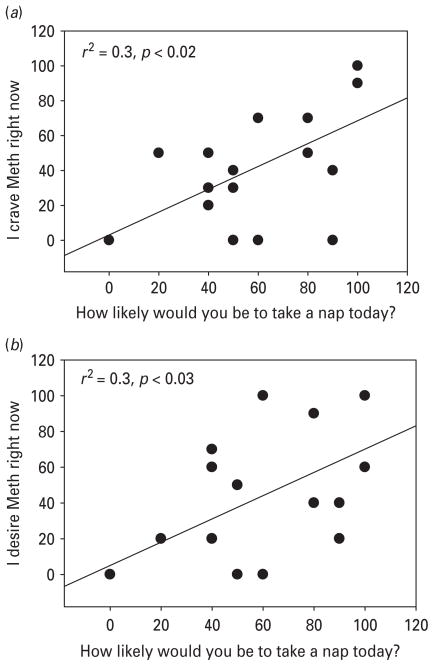
Responses to the craving variables of interest (‘I crave methamphetamine right now’) was positively correlated with the question ‘How likely are you to take a nap today’ (r=0.6, r2=0.3, p<0.02; Fig. 1a). Similarly, ‘I desire methamphetamine right now’ was also positively correlated with the question ‘How likely are you to take a nap today’ (r=0.5, r2=0.3, p≤0.03; Fig. 1b). Other drug use questions such as ‘If you had access to methamphetamine, how likely would you be to use it’ (r=0.5, r2=0.2, p<0.01) and ‘How much better would methamphetamine make you feel right now’ (r=0.7, r2=0.5, p<0.05) were also strongly correlated with the question ‘How likely are you to take a nap today’.
Fig. 1.
(a) Correlation between likelihood of taking a nap and craving for methamphetamine (Meth); (b) correlation between likelihood of taking a nap and desire for Meth.
Efficacy of modafinil
On average, sleep latency (min) was 12.1±3.5 at baseline and 16.0±4.6 following modafinil administration (t14=−4.0, p<0.001, η2=0.9). Follow-up analyses revealed that the sleep latencies differed significantly between baseline and the second (11.1±5.6 vs. 14.6±6.1; t13=−2.4, p<0.03, η2=0.9) and third (13.1±4.2 vs. 16.2±5.3; t13=−2.5, p<0.03, η2=1.0) naps and a trend towards significance during baseline and the fourth nap (12.9±5.9 vs. 16.2±5.3; t14=−1.8, p=0.1, η2=0.9) (Table 3). There were no significant differences between baseline and post-placebo administration with regard to MSLT on any of the three naps (as well as the MSLT average). When comparing days where modafinil was administered vs. placebo, there were no significant differences with regard to sleep latency.
Table 3.
Baseline sleep latency vs. sleep latency post-modafinil administration
| Baseline (day 5) (min) | Post-modafinil (min) | p | |
|---|---|---|---|
| MSLT 2 | 11.1±5.6 | 14.6±6.1 | 0.03* |
| MSLT 3 | 13.1±4.2 | 17.4±5.0 | 0.03* |
| MSLT 4 | 12.9±5.9 | 16.2±5.3 | 0.10 |
| MSLT averagea | 12.1±3.5 | 16.0±4.6 | 0.001* |
Only three naps (excluding the first nap) were utilized to calculate mean daytime sleep latency, since peak plasma levels are not achieved for approximately 2 h following dosing. For consistency purposes, only three naps were used to calculate the mean Multiple Sleep Latency Test (MSLT) in the baseline and placebo conditions as well.
p<0.05.
Discussion
The results of the current study suggest that short-term abstinence from Meth is associated with increased daytime sleepiness and that a single dose of 200 mg modafinil reduced daytime somnolence in this population. In addition, a positive correlation was found between subjective reporting of the likelihood of taking a nap and craving and desire for Meth, as well as the likelihood of using Meth and whether Meth would make the participant feel better.
Since depressive symptoms have been linked to sleep abnormalities (Rothschild, 1988), it is important to rule these out as a potential confound. BDI-II scores were not associated with any sleep variables, indicating that self-reported levels of depression did not modulate sleep. A frequently reported symptom of acute abstinence/withdrawal from Meth is hyper-somnolence (for review, see Meredith et al. 2005). This symptom was observed in the subjects in the current study. Subjects initially slept just over 9 h the first night following abstinence initiation. By the fourth night of study participation, participant sleep levels declined to<8 h, which is consistent with the average weeknight sleep duration reported by healthy adults of a similar age (NSF, 2009). In addition to the difference in time spent asleep, subjects went to bed, on average, earlier on their first night of enrolment than their fourth night. Despite normalization of night-time sleep, subjects still reported daytime somnolence, as the mean ESS score was unchanged between day 1 and day 5. In addition to the unchanged ESS score, subjects did not report reductions in likelihood of taking naps between admission (day 1) and day 5. Mean sleep latency for the four naps on day 5 was ~12 min. Importantly, mean sleep latencies for comparably aged control individuals are in the range of 15.5 min (Johnson et al. 1990), suggesting mild daytime sleepiness in this cohort of Meth-dependent individuals.
It is also important to evaluate modafinil, Meth and their impact on sleep from a neurochemical perspective. The precise mechanism of action for modafinil is currently unknown; however, rodent and primate models of modafinil exposure are associated with the modulation of several neurotransmitter systems, including dopamine (Madras et al. 2006; Volkow et al. 2009), norepinephrine (Madras et al. 2006) and orexin/hypocretin (Tobin, 2007). Notably, dopamine, serotonin and norepinephrine systems are affected as a consequence of Meth-dependence (Sofuoglu & Sewell, 2009).
Another possible explanation for excessive daytime sleepiness in this population involves the recently emerging literature concerning the relationship between Meth use and compromised immune system functioning. Immune function plays a critical role in the regulation and maintenance of sleep, so it would not be unexpected to see alterations in levels of immune system makers and function in a population with disturbed sleep. Perhaps the most interesting and relevant data concerning immune function, sleep and Meth deal with pro-inflammatory cytokines, such as interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumour necrosis factor-α. Daytime IL-6 plasma concentrations are negatively correlated with quality of night-time sleep (Vgontzas et al. 1997) and sleep deprivation results in increased IL-6 levels (Shearer et al. 2001; Vgontzas et al. 1999). We did not collect serum as part of this protocol and future studies might obtain these data to determine the degree to which levels of proinflammatory cytokines co-vary with sleep in Methdependent individuals.
As the current results show, abstinence from Meth is associated with sleepiness ratings of about 10 (on a scale of 0–24), which generally remain stable over 10 d from last use. ESS scores of 10 reflect normal levels of sleepiness (Johns, 1991). The mean length of abstinence for subjects in the current study was approximately 6 d. Given that the half-life of Meth is 10–12 h (Cho et al. 2001) and that baseline MSLT observations did not occur until the fifth day of the study, not only would Meth no longer be in the subjects’ body, any initial withdrawal symptoms that occur during acute abstinence would have resolved (Newton et al. 2004). However, the longer in duration the symptoms last, the more appropriate it is to refer to the phenomenon as an abstinence syndrome. Resolution of the abstinence syndrome is further illustrated by scores on the BDI-II and the self-reported estimates of length of night-time sleep. Specifically, both measures were significantly lower on day 5 than on day 1 of the study and were in the subclinical range (<10), suggesting that daytime sleep latency on day 5 was not due to the ‘crash’ and rebound hypersomnia often associated with withdrawal from Meth (Gawin et al. 1994).
The results of the current study show that a single dose of 200 mg modafinil significantly lengthens daytime sleep latency. The implications of lengthening sleep latency include improving alertness, which may affect Meth usage patterns. In other words, if an individual is more alert, he/she may be able to avoid triggers associated with continued Meth use. It was not known whether modafinil would increase wakefulness in Meth users, who have been shown to have reduced dopamine transporter (DAT) availability as compared to healthy controls (McCann et al. 1998, 2007; Sekine et al. 2001, 2003; Volkow et al. 2001). A number of studies have shown reduced DAT availability in the prefrontal cortex of Meth users and this may play a role in the lack of an effect seen in this study.
Another interesting finding is that subjective ratings for Meth ‘craving’ and ‘desire’ did not significantly increase after subjects had been abstinent for several days, which may have been expected among nontreatment- seeking individuals; yet, this is consistent with previously published research (Galloway et al. 2010). In addition, when subjects were asked to rate the likelihood that they would use if they had access to Meth, there was a significant decrease between day 2 and day 5. We expected that the likelihood of use would be higher after a period of prolonged abstinence from Meth, but the opposite effect was observed. One potential explanation for this would be that, as subjects remained abstinent over several days, their interest in using if given access would decrease since withdrawal effects would expectedly subside over time. In other words, the subjects would become acclimatized to not using Meth and would then be less likely to report using Meth if they had access to the drug.
It is important to concede some limitations with this study. First, a higher dose of modafinil and longer duration of treatment may increase daytime sleep latency and further reduce daytime sleepiness. Specifically, since it takes approximately 72 h for modafinil to reach steady-state peak levels (Robertson & Hellriegel, 2003), the efficacy of a single dose of modafinil is unlikely to reveal the full effectiveness of the compound. A second limitation, night-time sleepiness, was not assessed using polysomnographic sleep measurement, which would have provided a quantifiable measure of night-time sleep. In lieu of this, the current study relied on self-report for assessing night-time sleep characteristics. Since several other symptoms of stimulant withdrawal seem to be improving, and total sleep seems to be declining, it would be of great interest to know whether sleep latency during daytime naps is increasing or declining. Unfortunately, we did not measure sleep latency prior to day 5 to make this comparison. In addition, while the subset of subjects (n=9) receiving temazapam did not differ on any of the subjective or objective measurements when compared to those receiving placebo, we still concede the lack of uniformity in procedures as a potential confound. Also, it would have been very informative to assess craving levels (as well as the other subjective variables) more than once daily to determine whether these subjective reports changed throughout the day. Finally, we did not administer modafinil to a control group to investigate differences in sleep characteristics between Meth-dependent individuals and healthy controls.
In addition to the ability of modafinil to reduce objective symptoms of excessive daytime sleepiness, modafinil also reduced subjective ratings, specifically self-perceived likelihood of taking a nap. As likelihood of taking a nap correlated positively to a number of the craving questions (‘access’ and ‘better’) both prior to and after treatment with modafinil, we report indirect evidence that self-perceived symptoms of excessive daytime sleepiness may be a trigger for drug use and that treating these symptoms may improve treatment outcomes and reduce relapse.
Acknowledgments
The authors acknowledge grants from the National Institutes of Health DA 18185, DA 014593, DA 017754 and RR-00865. In addition, the authors acknowledge the UCLA General Clinical Research Center nursing staff for their support.
Footnotes
Statement of Interest
None.
References
- Arnulf I, Homeyer P, Garma L, Whitelaw WA, et al. Modafinil in obstructive sleep apnea-hypopnea syndrome: a pilot study in 6 patients. Respiration. 1997;64:159–161. doi: 10.1159/000196661. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Besset A, Tafti M, Villemin E, Billiard M. The effects of modafinil (300 mg) on sleep, sleepiness and arousal in narcoleptic patients. Neurophysiologie Clinique. 1993;23:47–60. doi: 10.1016/s0987-7053(05)80282-5. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Journal of Psychiatric Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. The multiple sleep latency test: what does it measure? Sleep. 1982;2:67–72. doi: 10.1093/sleep/5.s2.s67. [DOI] [PubMed] [Google Scholar]
- Cephalon . Provigil Product Information and Medication Guide. Frazer, PA: Cephalon; 2004. [Google Scholar]
- Cho AK, Melega WP, Kuczenski R, Segal DS. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse. 2001;39:161–166. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- De La Garza R, II, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug and Alcohol Dependence. 2010;106:173–180. doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway GP, Singleton EG, Buscemi R, Baggott MJ, et al. Methamphetamine treatment project corporate authors: an examination of drug craving over time in abstinent methamphetamine users. American Journal on Addictions. 2010;6:510–514. doi: 10.1111/j.1521-0391.2010.00082.x. [DOI] [PubMed] [Google Scholar]
- Gawin F, ME K, Ellinwood E. Stimulants. In: Galanter M, Kleber H, editors. Textbook of Substance Abuse Treatment. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers: clinical observations. Archives of General Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann, et al. Effect of modafinil on learning and task-related brain activity in methamphetaminedependent and healthy individuals. Neuropsychopharmacology. 2011;36:950–959. doi: 10.1038/npp.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Nasser J, et al. Methamphetamine attenuates disruptions in performance and mood during simulated night-shift work. Psychopharmacology (Berlin) 2003;169:42–51. doi: 10.1007/s00213-003-1464-4. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johnson LC, Spinweber CL, Gomez SA. Benzodiazepines and caffeine: effect on daytime sleepiness, performance, and mood. Psychopharmacology (Berlin) 1990;101:160–167. doi: 10.1007/BF02244120. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2007;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, et al. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. Journal of Neuroscience. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Mitchell A, Wickes W, et al. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: a comparison of mirtazapine and modafinil with treatment as usual. Journal of Substance Abuse Treatment. 2008;35:334–342. doi: 10.1016/j.jsat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. Journal of Pharmacology and Experimental Therapeutics. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harvard Review of Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Malison RT. Cocaine and sleep: early abstinence. Scientific World Journal. 2007;7:223–230. doi: 10.1100/tsw.2007.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, et al. Normalizing effects of modafinil on sleep in chronic cocaine users. American Journal of Psychiatry. 2010;3:331–340. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, Vansluis N, et al. Methamphetamine abstinence syndrome: preliminary findings. American Journal on Addictions. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- NSF. [Accessed December 2011];Aging and sleep – poll data. 2009 ( http://www.sleepfoundation.org/article/topics/aging-andsleep-poll-data)
- Prosise GL, Bonnet MH, Berry RB, Dickel MJ. Effects of abstinence from smoking on sleep and daytime sleepiness. Chest. 1994;105:1136–1141. doi: 10.1378/chest.105.4.1136. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda: National Institute for Neurological Disorders and Blindness; 1968. [Google Scholar]
- Robertson P, Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clinical Pharmacokinetics. 2003;42:123–137. doi: 10.2165/00003088-200342020-00002. [DOI] [PubMed] [Google Scholar]
- Rothschild AJ. Biology of depression. Medical Clinics of North America. 1988;72:765–790. doi: 10.1016/s0025-7125(16)30744-1. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Ouchi Y, Takei N, et al. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. American Journal of Psychiatry. 2003;160:1699–1701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Archives of General Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Shappell SA, Kearns GL, Valentine JL, Neri DF, et al. Chronopharmacokinetics and chronopharmacodynamics of dextromethamphetamine in man. Journal of Clinical Pharmacology. 1996;36:1051–1063. doi: 10.1177/009127009603601109. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. Journal of Allergy and Clinical Immunology. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, et al. Reliability and validity of the MINI International Neuropsychiatric Interview (M.I.N.I.): according to the SCID-P. European Psychiatry. 1997;12:232–241. [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addiction Biology. 2009;14:119–29. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin ML. Why choose modafinil for excessive daytime sleepiness? Issues in Mental Health Nursing. 2007;28:313–317. doi: 10.1080/01612840601174110. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. Journal of Clinical Endocrinology & Metabolism. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. Journal of the American Medical Association. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, et al. Changes in mood, craving and sleep during acute abstinence reported by male cocaine addicts. NIDA Research Monograph. 1990;105:453–454. [PubMed] [Google Scholar]
- Wong YN, King SP, Simcoe D, Gorman S, et al. Open-label, single-dose pharmacokinetic study of modafinil tablets: influence of age and gender in normal subjects. Journal of Clinical Pharmacology. 1999;39:281–288. [PubMed] [Google Scholar]