Abstract
Pancreatic cancer is the fourth leading cause of cancer related deaths in North America. The poor survival statistics are due to the fact that there are no reliable tests for early diagnosis and no effective therapies once metastasis has occurred. Surgical resection is the only curative treatment for pancreatic cancer; however, only less than 15% of the patients are eligible for surgery at diagnosis. New therapies are urgently needed for this malignant disease. And combinational therapy including surgery, chemotherapy and molecular targeted therapy may further improve the efficacy of individual therapies. However, a reliable mouse model which mimics the human disease and can be used for testing the surgical treatment and surgery-based combinational therapy is not available. In this study, we have established a mouse model for curative surgical resection of pancreatic cancer. Human pancreatic cancer cells were used to create orthotopic xenografts in nude mice, distal pancreatectomy was performed using imaging-guided technology to remove the pancreatic tumors, and sham surgery was performed in the control group. All mice survived the operation and no complication was observed. Surgical resection at early stage improved the survival rate and quality of life of the mice compared with the sham surgery and surgical resection at the late stage. If combined with other therapies such as chemotherapy and molecular targeted therapy, it could further improve the outcome of pancreatic cancer. This mouse model is a useful tool to study the surgical therapy and the tumor recurrence of pancreatic cancer, and could potentially impact the therapeutic choices for this deadly disease.
Keywords: surgical resection, imaging, pancreatic cancer, mouse model, distal pancreatectomy, combinational therapy
1. Introduction
Earlier diagnoses and improvements in current treatments for most cancers have led to great advancements in the 5-year survival rate in the past few decades. However, pancreatic cancer has shown little improvement in survival compared with other major cancers [1]. Pancreatic cancer is the fourth leading cause of cancer related deaths in North America. The overall 5-year survival rate is less than 5%. The poor survival statistics are due to the fact that there are no reliable tests for early diagnosis and no effective therapies once metastasis has occurred. Surgical resection is the only curative treatment for pancreatic cancer; however, patients with pancreatic cancer usually present with locally advanced, unresectable or metastatic disease; even for patients with resectable disease, most of them will relapse. Standard chemo- and radiation therapies do not offer significant improvement of survival. New treatments targeting known oncogenes or growth factors in pancreatic cancer such as K-Ras, VEGF, and EGF/EGFR have mostly failed, and do not provide survival benefit. Therefore, it is important to identify novel molecular markers and therapeutic targets in pancreatic cancer that could lead to more effective treatment or enhancement of standard chemo- and radiation therapy for this malignant disease [2]. And combinational therapy including molecular targeted therapy, surgical resection and chemotherapy may further improve the efficacy of individual therapies.
However, a reliable mouse model which mimics the human pancreatic cancer disease and can be used to test the surgical treatment and combinational therapy is currently not available. The widely used Kras transgenic mouse models provide an excellent tool to test preclinical anti-cancer drugs, but the lengthy time in which it takes to develop pancreatic cancer and the unpredicted sites for the primary tumor growth make it challenging to perform surgical resection and to test the efficacy of the surgery-based combinational therapy [3; 4; 5]. Orthotopic xenograft mouse model has a unique advantage in establishing a resectable pancreatic cancer model and is easy to test the efficacy of surgical therapy. Previous studies have also used the orthotopic xenograft models to investigate the perineural invasion and tumor recurrence [6; 7]. In this study, we have established a reliable orthotopic xenograft mouse model for curative surgical resection of pancreatic cancer (distal pancreatectomy), and compared the survival rate, tumor progress and recurrence between the distal pancreatectomy and the sham surgery group. This mouse model is a useful tool to study the surgical therapy and surgery-based combinational therapy of pancreatic cancer, and could potentially impact the development of therapeutic choices for this deadly disease.
2. Materials and Methods
2.1 Cell culture and chemicals
Human pancreatic cancer cell lines ASPC-1 and MIA PaCa-2 were purchased from the American Type Culture Collection (ATCC, Rockville, MD), and was cultured in RPMI 1640 and DMEM (with 2.5% horse serum) medium, respectively, with 10% fetal bovine serum (FBS) as previously described [8; 9; 10]. The ASPC-1 cells were transfected with green fluorescent protein (GFP) plasmid using Lipofectamine 2000 (Invitrogen Corporation, Carlsbad, CA), and stable cells (ASPC-GFP) were selected with puromycin. Other chemicals were from Sigma (St. Louis, MO).
2.2 Orthotopic implantation of pancreatic cancer cells in nude mice
Subconfluent cells were harvested by trypsinization, and resuspended in DMEM. The cells (3×106) were inoculated into the tail of the pancreas of 6- to 8-week-old male nude mice (NCI-Charles River). All mice were cared for in accordance with the Office for Protection from Research Risks (OPRR) and Animal Welfare Act Guidelines under an animal protocol approved by Animal Welfare Committee at the University of Texas Health Science Center at Houston. Systemic anesthesia was administered and the mice were covered with surgical towels. A small incision (0.5–1 cm) was made in the left subcostal region, and the spleen was gently exteriorized to fully expose the pancreas tail and body. The tumor cells (3×106) in a volume of 50 µl were injected into the tail of the pancreas with a 27-Gauge needle. The peritoneum and skin were closed with a 4.0 surgical suture.
2.3 Mouse Distal Pancreatectomy and Imaging
The mice were randomly selected and divided into two groups. One group underwent distal pancreatectomy and the other group underwent sham surgery on either the 10th or 20th day after tumor implantation. Mice were anesthetized and opened by laparotomy. The tail and the majority of the body of pancreas harboring the pancreatic tumors and the whole spleen were resected by ligating the pancreas proximal of the tumors with a 4-0 suture to prevent from bleeding and transecting the pancreas distal of the suture with a sterile scissor. Intraoperative GFP imaging was used to ensure a negative margin after the resection. Sham surgery involved laparotomy and mobilization of the distal pancreas. Imaging was also performed to ensure the presence of the primary tumor. No resection was performed and the peritoneum and skin were closed with a 4.0 surgical suture as described above. Necropsy was performed when mice died or after euthanization. GFP imaging was used to identify locally recurrent and distal metastasis. The primary tumors and locally recurrent and metastasized tumors were assessed and collected. The mice body weight and general health of each mouse were monitored and recorded daily. Fluorescent imaging was performed using the Illumatool fluorescence imaging system (Lightools Research, Encinitas, CA), and pictures were taken using a Nikon D5100 digital camera with 18–55 mm lens.
2.4 Immunohistochemical staining
The orthotopic pancreatic tumors and metastasized tissues were collected and fixed in 4% PFA. The paraffin sections were stained with H&E and Ki-67 as previously described [9]. Stained slides were assessed using a phase contract microscopy.
2.5 Statistical analysis
The mouse body weight index (daily body weight/body weight on the day of tumor implantation) was measured and recorded every day. Quantitative results are shown as means ± SD. The statistical analysis was done by Log-rank (Mantel-Cox) Test between different groups. The Kaplan-Meier method was used to plot survival curves for the treatment and control groups. A value of P <0.05 was considered statistically significant.
3. Results
Establish a resectable pancreatic cancer mouse model
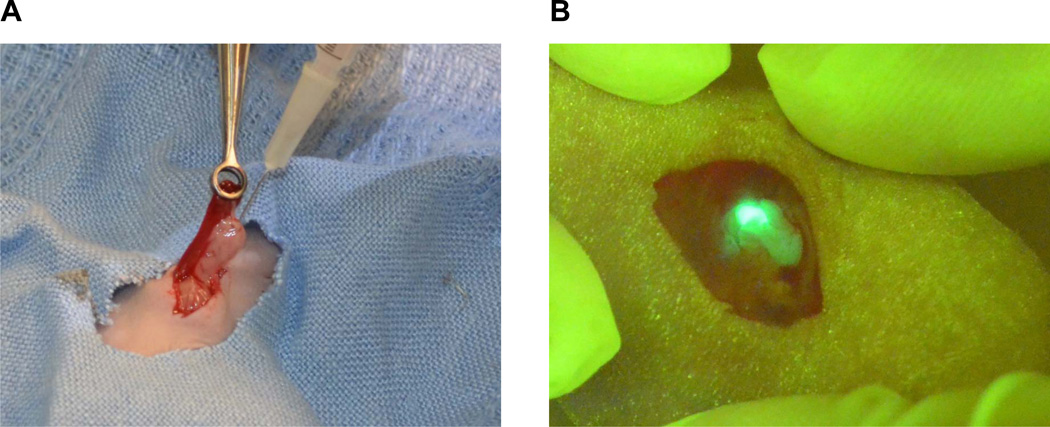
A reliable pancreatic cancer mouse model which mimics human pancreatic cancer and can be used to test the combinational therapy including surgical resection is urgently needed. Because of the physiological relevance, short growth curve, and ease to reproduce, orthotopic pancreatic cancer model was selected to establish a resectable pancreatic cancer mouse model. We have used the orthotopic model in our previous studies to characterize the in vivo functions of many key molecules in pancreatic cancer such as ZIP4 [8; 9; 10; 11], which showed great consistency. In this study, we injected the ASPC-GFP and MIA PaCa-2 cells into the pancreas tail of nude mice, to establish a resectable pancreatic cancer mouse model. As shown in Fig. 1A, 50 ul of human pancreatic cancer cells (3×106) were carefully injected into the tail of the pancreas using a 27-gauge needle. A picture was taken after the mouse was injected with ASPC-GFP cells, which showed the presence of fluorescent tumor cells in the tail of the pancreas, and lack of leakage in the abdominal cavity (Fig. 1B). The take rate of tumor growth for both pancreatic cancer cells are 100%. Because of the advantage of the fluorescence in ASPC-GFP cells which allows for real time monitoring of the tumor progress, we focused on this cell line for our consequent experiments in this study. All mice were in excellent conditions during the first week after the tumor implantation without any noticeable complications.
Fig. 1. Establish a resectable orthotopic pancreatic cancer mouse model.
A. Pancreatic cancer cells were injected orthotopically into the tail of the pancreas. B. A fluorescence picture was taken after the tumor cell inoculation. Green fluorescence showed the presence of tumor cells in the tail of the pancreas, and lack of leakage in the abdominal cavity.
Imaging-Guided Surgical Resection
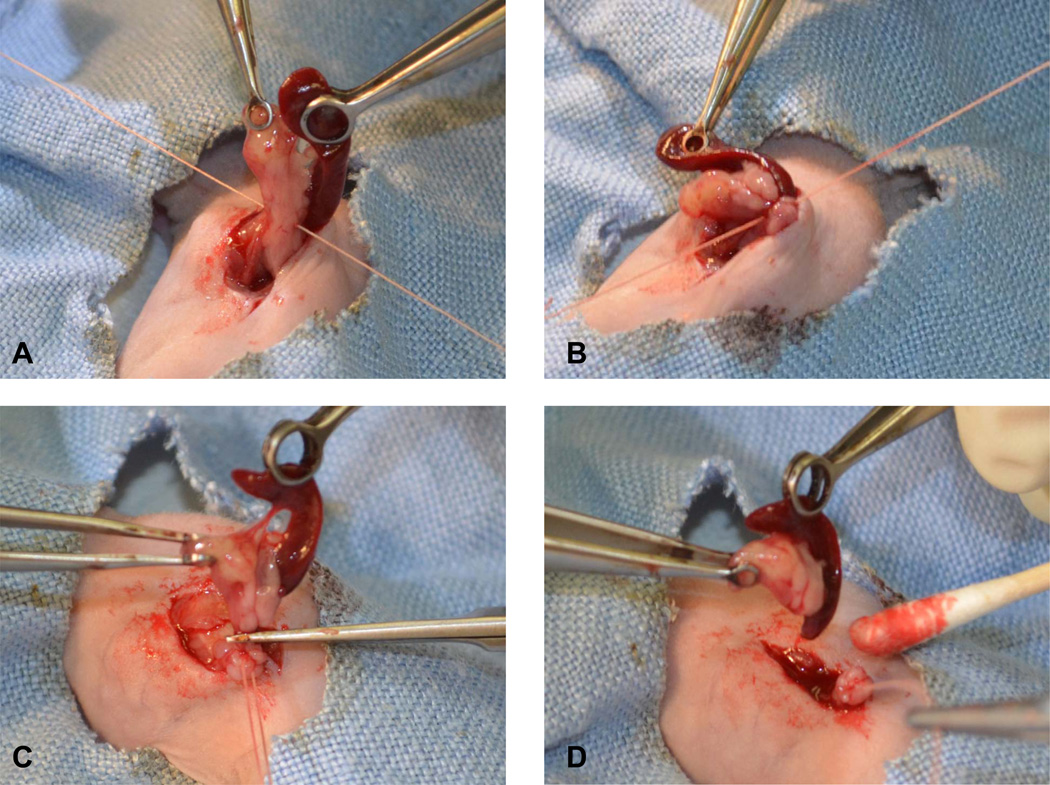
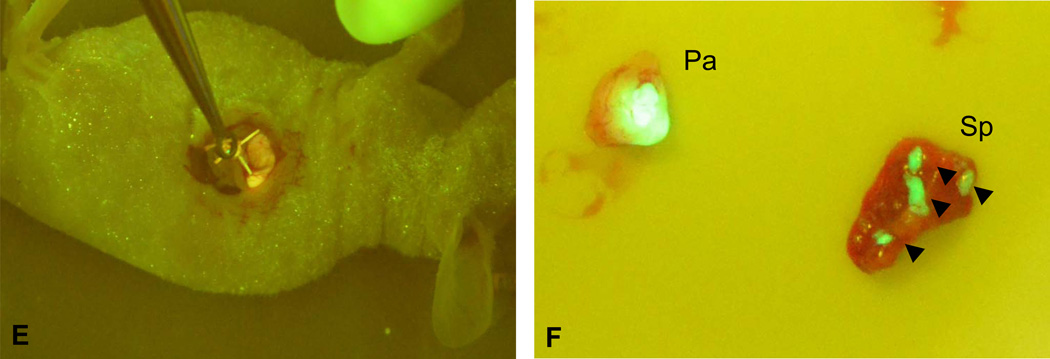
Based on our previous studies and the real time monitoring of the tumor progress, we chose two time points to perform the distal pancreatectomy, day 10 and day 20 after the initial tumor implantation. Mice were randomly divided into two groups, one group underwent distal pancreatectomy, and the other group underwent sham surgery. In the early surgery group on the 10th day after the tumor implantation, the tail and the majority of the body of pancreas harboring the pancreatic tumors and the whole spleen were resected (Fig. 2A-D). Every caution has been taken to avoid unnecessary bleeding and leakage of the pancreas juice. Intraoperative GFP imaging was used to ensure a negative margin after the resection (Fig. 2E). Any visible fluorescent tissue residues were removed. Postoperative tissue assessment showed tumor invasion into the spleen (Fig. 2F), which was confirmed by further histological staining (data not shown), suggesting the necessity of resecting the spleen along with the distal pancreatectomy. Imaging was also performed in the sham surgery group to ensure the presence of the primary tumors (100% take rate of tumor growth). All mice that underwent the distal pancreatectomy and sham surgery survived the operation and no complication was observed in the following week. In the late surgery group on the 20th day after the tumor implantation, similar procedure was performed for the distal pancreatectomy and the sham surgery. However, local metastasis has occurred for most mice, which mimics the late stage pancreatic cancer in human, and the tumors were not completely resectable (data not shown).
Fig. 2. Mouse distal pancreatectomy.
A and B. The tail and the majority of the body of pancreas harboring the pancreatic tumors and the whole spleen were resected by ligating the pancreas proximal of the tumors with a 4-0 suture. C and D. The pancreatic tail and spleen were removed with a sterile scissor. E. Intraoperative GFP imaging was used to ensure a negative margin after the resection. F. Tumor presence in the resected pancreatic tail and spleen. Pa, pancreas; Sp, spleen. Black arrow head, tumor presence in spleen.
Surgical resection at early stage improves the survival rate and quality of life of the mice
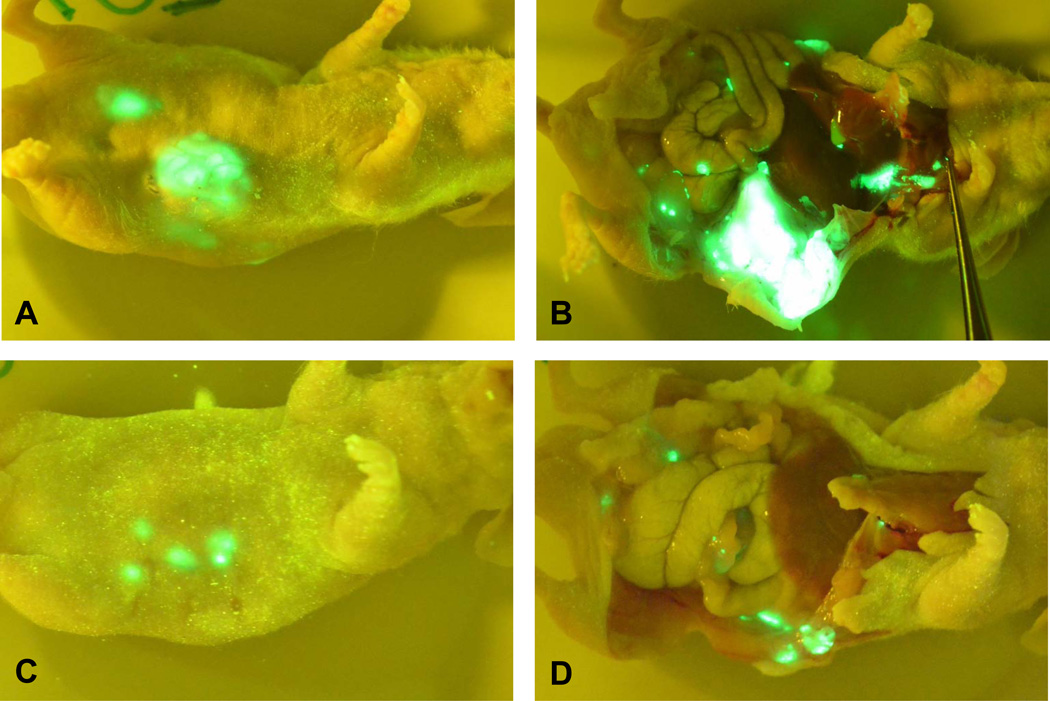
To examine the tumor growth, metastasis, and recurrence in mice with or without surgical resection, mice were euthanized at four weeks after the initial tumor implantation. Necropsy was performed and GFP imaging was used to identify locally recurrence and distal metastasis. The sham surgery group showed big primary tumors and extensive metastasis to peritoneum, spleen, liver, and lymph nodes (Fig. 3A and 3B). Histological evaluations revealed extensive cancer cell invasion to peritoneum, spleen, liver, and local omentum (Fig. 4B-E), and showed tumor presence in pancreas. Further immunohistochemical staining of Ki-67 confirmed the aggressive proliferation of the tumor cells (Fig. 5B-E). In the early surgery group, there were various degrees of local recurrent tumors including mild peritoneal invasion, but no liver metastasis was found (Fig. 3C and 3D). Histological evaluations revealed cancer cell invasion to peritoneum (Fig. 4A), which was Ki-67 positive (Fig. 5A). In the late surgery group, all mice had extensive metastatic disease (data not shown), similar to the sham surgery group, indicating the importance of the choice for resection time.
Fig. 3. Surgical resection at early stage reduced tumor growth and metastasis.
A and B. Sham surgery. C and D. Surgical resection at early stage. Mice were euthanized at four weeks after the initial tumor implantation. Necropsy was performed and GFP imaging was used to identify locally recurrence and distal metastasis.
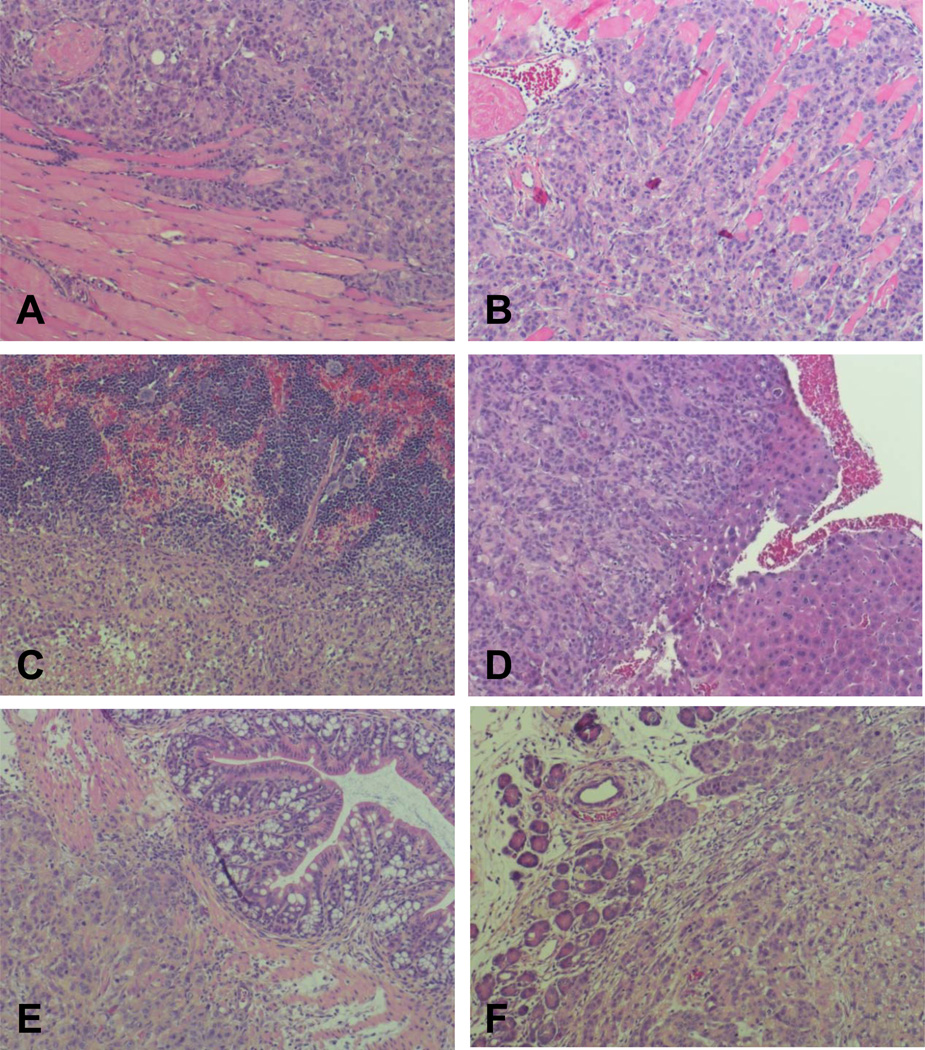
Fig. 4. H&E staining.
A. Tumor recurrence on peritoneum in early surgery group. B. Tumor recurrence on peritoneum in sham surgery group. In the sham surgery group, tumor invasion was also found in spleen (C), liver (D), omentum and intestine (E); and tumor presence was found in pancreas (F).
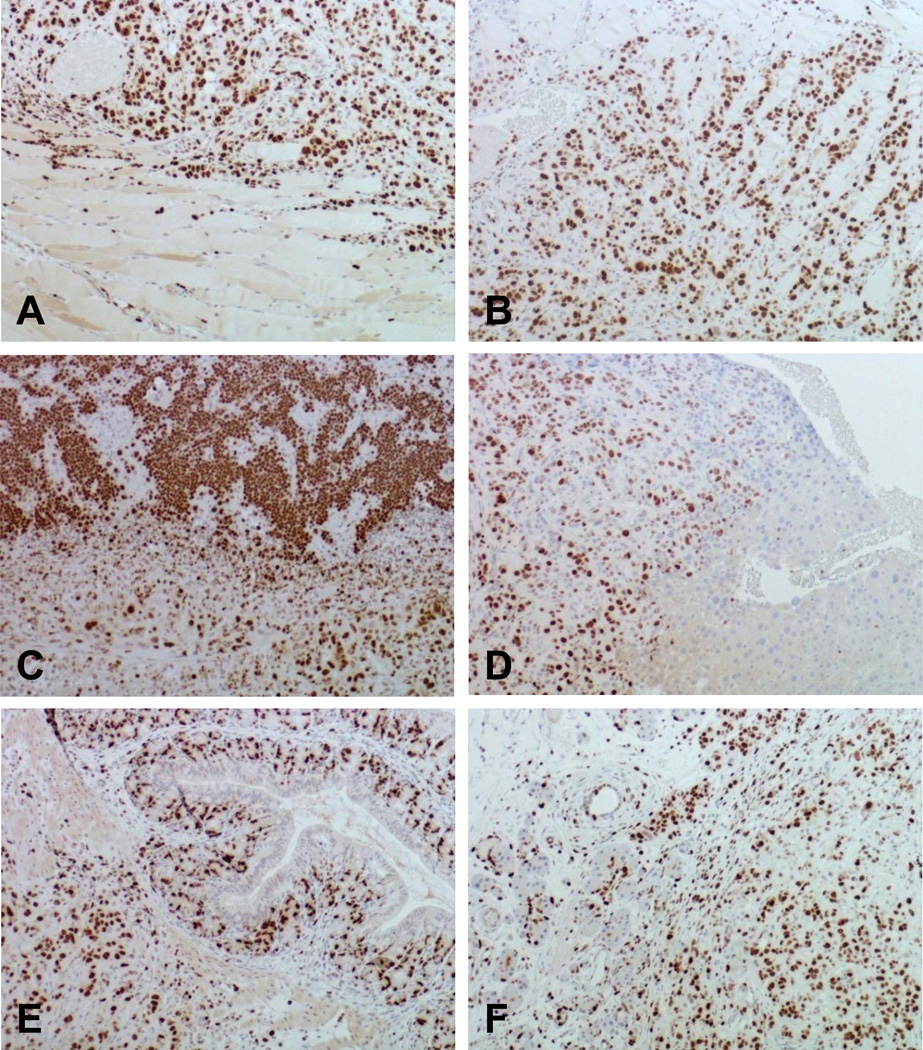
Fig. 5. Ki-67 staining.
A. Tumor cell proliferation in peritoneum in early surgery group. B. Tumor cell proliferation in peritoneum in sham surgery group. In the sham surgery group, tumor cell proliferation was also found in spleen (C), liver (D), omentum and intestine (E), and pancreas (F).
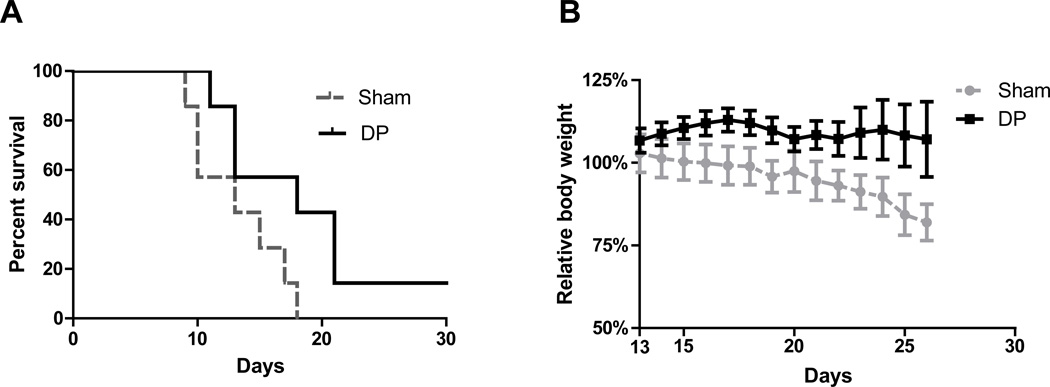
In a separate experiment, the overall survival rate was recorded for mice with or without distal pancreatectomy. Although not statistically significant, the surgical resection at early stage did improve the overall survival compared with the sham surgery (P= 0.054). The mean survival of mice was increased from 13 days (sham) to 18 days (distal pancreatectomy, DP) (Fig. 6A). The surgical resection at late stage did not provide any survival benefit.
Fig. 6. Surgical resection at early stage improves the survival rate and quality of life of the mice.
A. Kaplan–Meier analysis of survival showed improved survival of early distal pancreatectomy group (DP) vs. sham group (Sham) (P=0.054). B. Log-rank (Mantel-Cox) Test showed significant loss of body weight in the sham surgery group vs. early distal pancreatectomy group (P<0.05).
We also examined the quality of life of all the mice after the surgical resection. The mice in the sham group showed significant loss of body weight after tumor implantation, and most of the mice died after they lost more than 20% of their initial body weight. While the body weight index in the early surgical resection group was stable, no significant loss of body weight was observed within two weeks after the distal pancreatectomy (Fig. 6B).
4. Discussion
Surgical resection is the only curative treatment for human pancreatic cancer; however, a reliable mouse model is not available to test the surgical resection and surgery-based combinational therapies for pancreatic cancer. In this study, we have established an orthotopic xenograft mouse model for imaging-guided curative surgical resection of pancreatic cancer. We found that surgical resection at early stage improves overall survival and quality of life in mice. If combined with other standard therapy and molecular targeted therapy, it may further increase the overall survival of pancreatic cancer.
Two poorly differentiated pancreatic cancer cells, ASPC-1 and MIA PaCa-2, were used in this study to establish orthotopic xenografts in nude mice. Those two cells were derived from ascites and primary pancreatic adenocarcinoma, respectively, and exhibited aggressive features including a great metastatic potential [12; 13; 14]. Both cell lines had a 100% take rate of tumor growth in an orthotopic xenograft mouse model. The MIA PaCa-2 cells tend to grow into bigger primary tumors, and are less lethal. ASPC-1 cells are more metastatic and cause the mice to die rapidly. Because of the advantage of the fluorescence in ASPC-GFP cells which allows for real time monitoring of the tumor progress, we focused on this cell line for most of our experiments in this study. Our previous data have also indicated that ASPC-1 orthotopic tumors are a reliable model system to study pancreatic cancer growth, metastasis, and survival [8; 10]. Previous studies have used those pancreatic cancer cell lines to examine the perineural invasion and tumor recurrence, respectively, and different red fluoresence protein (RFP) was used to label the cells [6; 7]. With the help of the fluorescence imaging, radical surgical resection was performed to remove the tail and the majority of the body of the pancreas, which contains pancreatic tumors. Any visible fluorescent tissue residues were also removed whenever possible. Local tumor invasion into the spleen was observed using both fluorescent imaging and histological staining, suggesting the necessity of removing the spleen along with the distal pancreatectomy. Those data strongly indicate the importance of molecular imaging in surgical resections of pancreatic cancer, and suggest that future combinational therapies should also include imaging as a critical component to guide the therapy and to monitor the tumor progress.
Pancreatectomy can be curative when the tumor is in early stage. Our results suggest that the early distal pancreatectomy on the 10th day after tumor implantation improves the overall survival rate and the quality of life of the mouse, which closely mimics human disease when the human pancreatic cancer patient receives surgery at an early stage. The mice in the distal pancreatectomy group showed better health status with good appetite, moving ability, and stable body weight index compared with the sham surgery group. The distal pancreatectomy at the late stage (20th day after tumor implantation) did not provide any survival benefit except for palliative effect. Local metastasis has occurred for most mice in this group, which also mimics the late stage pancreatic cancer in human, and the tumors were not completely resectable. These results suggest the importance of the selection of surgery time, and provide direct evidence that surgical resection is only effective for patients with early stage pancreatic cancer.
Neoadjuvant has been widely used for gastric and rectal cancer treatment to downstage the disease [15]. A number of patients received neoadjuvant therapy showed improvement of quality of life and overall survival especially in rectal cancer [16; 17; 18; 19; 20]. Neoadjuvant therapy is less used in pancreatic cancer patients because of the resistance to conventional chemo and radiation therapy. However, novel chemotherapy or molecular targeted therapy with less toxicity and better efficacy are still considered to be an important therapeutic strategy for human pancreatic cancer, and a reliable mouse model to mimic the human disease which can be used to test the novel neoadjuvant and molecular targeted therapy is urgently needed. The surgical resection mouse model we established in this study could be a useful tool to test the above mentioned therapies and the surgery-based combinational therapy. Recent reported novel molecular targeted therapies such as shRNAs, small molecules, microRNAs, and inhibitors for tumor initiating cells (TICs) may also be included in the combinational therapy to further improve the overall survival for pancreatic cancer patients [8; 21; 22; 23]. Our previous studies have shown that zinc transporter ZIP4 can serve as a novel therapeutic target in pancreatic cancer, silencing of ZIP4 significantly inhibited pancreatic cancer growth and increased the survival of mice with pancreatic cancer xenografts [8; 10; 11]. PDX-1 can also act as a potential molecular target for treatment of human pancreatic cancer, it has been shown that down-regulation of PDX-1 expression inhibits pancreatic cancer cell growth in vitro and in vivo [21; 24]. The molecular targeted therapies along with appropriate delivery system and new strategies to over drug resistance [25; 26], could potentiate the standard care and increase the efficacy of the combinational therapy as well.
5. Conclusion
The reproducible surgical resection mouse model presented in this study could be a great asset to investigate the role of surgery in pancreatic cancer treatment, and to test the efficacy of surgery-based combinational therapy including neoadjuvant and molecular targeted therapy in pancreatic cancer. This concept is important, since it will most likely require multiple treatments to have an impact on survival in patients with metastatic pancreatic cancer.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) grant R01CA138701, R21CA133604, and the William and Ella Owens Medical Research Foundation (Li M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
Reference
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Shi S, Yao W, Xu J, Long J, Liu C, Yu X. Combinational therapy: new hope for pancreatic cancer? Cancer letters. 2012;317:127–135. doi: 10.1016/j.canlet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 3.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA, Hingorani SR. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11:229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31:258–262. doi: 10.1097/01.mpa.0000175176.40045.0f. [DOI] [PubMed] [Google Scholar]
- 7.Torgenson MJ, Shea JE, Firpo MA, Dai Q, Mulvihill SJ, Scaife CL. Natural history of pancreatic cancer recurrence following "curative" resection in athymic mice. J Surg Res. 2008;149:57–61. doi: 10.1016/j.jss.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Zhang Y, Bharadwaj U, Zhai QJ, Ahern CH, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res. 2009;15:5993–6001. doi: 10.1158/1078-0432.CCR-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Bharadwaj U, Logsdon CD, Chen C, Yao Q, Li M. ZIP4 Regulates Pancreatic Cancer Cell Growth by Activating IL-6/STAT3 Pathway through Zinc Finger Transcription Factor CREB. Clin Cancer Res. 2010;16:1423–1430. doi: 10.1158/1078-0432.CCR-09-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Chen C, Yao Q, Li M. ZIP4 upregulates the expression of neuropilin-1, vascular endothelial growth factor, and matrix metalloproteases in pancreatic cancer cell lines and xenografts. Cancer Biol Ther. 2010;9:235–241. doi: 10.4161/cbt.9.3.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan MH, Shimano T, Chu TM. Differential localization of human pancreas cancer-associated antigen and carcinoembryonic antigen in homologous pancreatic tumoral xenograft. J Natl Cancer Inst. 1981;67:563–569. [PubMed] [Google Scholar]
- 13.Tan MH, Chu TM. Characterization of the tumorigenic and metastatic properties of a human pancreatic tumor cell line (AsPC-1) implanted orthotopically into nude mice. Tumour Biol. 1985;6:89–98. [PubMed] [Google Scholar]
- 14.Chen WH, Horoszewicz JS, Leong SS, Shimano T, Penetrante R, Sanders WH, Berjian R, Douglass HO, Martin EW, Chu TM. Human pancreatic adenocarcinoma: in vitro and in vivo morphology of a new tumor line established from ascites. In Vitro. 1982;18:24–34. doi: 10.1007/BF02796382. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Participants MT. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Hur H, Kim NK, Kim YW, Cho SY, Kim JY, Min BS, Ahn JB, Keum KC, Kim H, Sohn SK, Cho CH. Oncologic outcomes after radical surgery following preoperative chemoradiotherapy for locally advanced lower rectal cancer: abdominoperineal resection versus sphincter-preserving procedure. Ann Surg Oncol. 2009;16:1266–1273. doi: 10.1245/s10434-009-0338-3. [DOI] [PubMed] [Google Scholar]
- 17.Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ, Wolmark N. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, Bessell E, Griffiths G, Thompson LC, Parmar M. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 20.Wong RK, Tandan V, De Silva S, Figueredo A. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD002102.pub2. CD002102. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Ballian N, Belaguli NS, Patel S, Li M, Templeton NS, Gingras MC, Gibbs R, Fisher W, Brunicardi FC. PDX-1 acts as a potential molecular target for treatment of human pancreatic cancer. Pancreas. 2008;37:210–220. doi: 10.1097/MPA.0b013e31816a4a33. [DOI] [PubMed] [Google Scholar]
- 22.Ni X, Long J, Cen P, Chen L, Yang J, Li M. Pancreatic cancer tumour initiating cells: the molecular regulation and therapeutic values. J Cell Mol Med. 2012;16:988–994. doi: 10.1111/j.1582-4934.2011.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, Fearon ER, Lawrence TS, Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu SH, Patel S, Gingras MC, Nemunaitis J, Zhou G, Chen C, Li M, Fisher W, Gibbs R, Brunicardi FC. PDX-1: demonstration of oncogenic properties in pancreatic cancer. Cancer. 2011;117:723–733. doi: 10.1002/cncr.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Zhang Y, Chen C, Yao Q, Li M. Targeted drug delivery in pancreatic cancer. Biochim Biophys Acta. 2010;1805:97–104. doi: 10.1016/j.bbcan.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long J, Zhang Y, Yu X, Yang J, LeBrun DG, Chen C, Yao Q, Li M. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets. 2011;15:817–828. doi: 10.1517/14728222.2011.566216. [DOI] [PMC free article] [PubMed] [Google Scholar]