Abstract
The blood-brain barrier (BBB) has been considered as an important regulator of brain homeostasis, and its disturbance has been implicated in the onset and/or evolution of many pathological manifestations of neurodegenerative and inflammatory diseases [1, 2]. In particular, BBB breakdown has been closely associated with the primary insult, as well as the secondary cell death of stroke. Here, we review the pioneering contributions of leading scientists who have vested interest in advancing our understanding of the pivotal role of BBB in stroke, but also exploiting this knowledge in developing novel BBB-based therapeutic regimens to abrogate stroke symptoms. The study of BBB as a fundamental research theme and as a target for clinical applications in stroke can be approached in three main themes namely, basic science research, translational and clinical research, and emerging therapies for BBB repair in stroke. This mini-review captures cutting-edge discoveries establishing BBB as a central target for abetting neuroprotection and neurorestoration in stroke.
Introduction
This mini-review summarizes the frontier of basic, translational and clinical research on BBB as a core component of brain homeostasis and many neurological pathologic conditions, especially stroke [1,2]. A Special Volume of Current Pharmaceutical Design has been dedicated to this BBB study in an attempt to create a reference for future research in increasing our understanding of BBB and taking advantage of this scientific information to develop unique therapeutic strategies with BBB as a primary target for promoting neuroprotection and neurorestoration in stroke.
Cerebrovascular diseases are the fourth cause of death and the leading cause of long-term disability in the United States. Recent strategies to improve the neurologic outcome of stroke-affected patients include anti-inflammatory and neurorestorative approaches, aiming to repair function of the penumbral area and, in consequence, decrease neurologic disability. The BBB has a central role in stroke pathogenesis and may be considered a therapeutic target and a mediator of treatment strategies. This review addresses BBB dysfunction in stroke from multiple perspectives including molecular, cellular and system levels, and discusses their relevance for developing therapeutic approaches that may improve the outcome of stroke affected patients. In critically analyzing the milestone research themes of BBB in stroke, we subdivided this review into basic science research, translational and clinical research, and emerging therapies for BBB repair in stroke.
Basic Science Research
A very nice review about the role of each component of the neurovascular unit for the normal homeostasis of the BBB is provided by Drs. Patrick Ronaldson and Thomas Davis [3]. In sequence, functional and structural stroke-induced alterations of the BBB are described in detail, with an approach on how these changes can affect cell survival. The review also presents an updated discussion of how the BBB can be used as a therapeutic target, not only aiming restoration of its homeostasis, but also as a vehicle for drug delivery. For example, the protein transporter Oatp1a4 has been shown to display increased expression in inflammatory conditions, including stroke, suggesting that it may be used for drug transport across the BBB Fig. (1). In the authors' previous publications, upregulation of Oatp1a4 was opportunely used to deliver opioids into the CNS of rats with pain [4-6]. In stroke, similar upregulation of the protein transporter is described, through TGFβ/ALK5 signaling, possibly establishing an important therapeutic pathway.
Fig. (1). Control of drug permeation across the BBB.

Panel A) Under normal conditions, the intact BBB limits the movement of molecules into the brain. Panel B) Under pathologic conditions, the BBB becomes impaired, allowing molecules that would normally be prevented from crossing the BBB to enter the brain. Panel C) Under pathologic conditions, the endogenous channels that allow for the passage of neurotherapeutics are downregulated (i.e. Oatp1a4), limiting the efficacy of these therapies. Panel D) Pharmaceuticals aimed at upregulating these endogenous channels (i.e. Oatp1a4) are currently being investigated to improve BBB permeability to allow access of these therapeutic molecules into the brain.
Dr. Eng Lo and colleagues explore the specific roles of matrix metalloproteinases (MMPs) in BBB dysfunction, as biomarkers and therapeutic targets in stroke and other degenerative diseases [7]. Following injury, MMPs become activated and therefore contribute to further tissue damage, leading to edema, hemorrhage and cell death Fig. (2). Matrix metalloproteinase-2 and -9 are of special interest and the authors have previously shown that brain and serum MMP-9 levels correlate with infarct size [8]. Experimental research, with strategies that aim to restore MMP balance, either inhibiting MMP activation or increasing tissue inhibitor of metallo-proteinase (TIMP) expression, demonstrates successful neurological outcomes [9, 10]. In addition, in vitro brain endothelial cell survival is increased by drugs that decrease inflammatory mediators and MMP levels, suggesting a neuroprotective pathway [11]. Similarly, hemorrhagic transformation following tPA administration in humans may be associated with increased MMP-9 levels, indicating a possibly protective role of inhibitors of MMP-9 during thrombolysis [12]. Timing, however, is an important issue and must be accounted during management of MMP activity; in a more chronic phase of stroke MMPs seem to play a beneficial, rather than deleterious role, stimulating angiogenesis, tissue remodeling and repair.
Fig. (2). Matrix metalloproteinases (MMPs) and neurovascular unit in stroke.

Panel A) Under normal conditions, the BBB is selectively permeable to molecules. Panel B) Under pathologic conditions i.e., acute ischemic stroke, MMPs are upregulated causing the disruption of BBB permeability. Panel C) Novel pharmacologic agents afford MMP inhibitory action, resulting in restoration of the leaky BBB.
In an effort to further understand the major components of BBB, Drs. Toru Yamashita and Koji Abe describe the relevance of endothelial progenitor cells (EPCs) in repair and remodeling of the neurovascular unit [13]. The authors have extensive previous experience with neuroprotective strategies to improve outcome of stroke, especially concerning BBB dysfunction [14, 15]. In the present issue, the authors highlight the role of EPCs in vascular repair following stroke, as well as very promising therapeutic strategies, which aim to increase such repair Fig. (3).
Fig. (3). Endogenous endothelial cells, BBB, and stroke.

Panel A) Under non-pathologic conditions, the intact BBB restricts the movement of platelets, fibrinogen, and other small molecules into the brain parenchyma. Panel B) Under ischemic conditions, the loss of tight junction proteins allows for the movement of platelets, fibrinogen, and other small molecules into the brain. Panel C) Smoking, hypertension, elevated serum cholesterol, and diabetes all decrease the quantity of circulating endogenous endothelial progenitor cells. The low numbers of endothelial progenitor cells restricts the repair of the broken BBB. Panel D) A higher quantity of endogenous endothelial progenitor cells allows for the repair of the broken BBB via the paracrine secretion of VEGF and IGF-1. Endogenous EPCs also repair the broken vasculature by incorporating into the newly formed vessels.
In addition to endothelial cells, pericytes represent another major component of the neurovascular unit as described by Dr. Mark Fisher and co-workers [16]. In particular, the close interaction between pericytes and endothelial cells has been implicated in the homeostasis of the blood-brain barrier Fig. (4). Among the many structural roles of pericytes in coordination with endothelial cells, they maintain the structure and function of the basement membrane and endothelial tight junction, both of which directly relevant to BBB integrity [17]. Following experimental stroke, pericytes have been shown to alter BBB permeability, regulate capillary blood flow, and restore the neurovascular unit [17, 18]. Accumulating evidence demonstrating novel features of pericytes such as their scavenging action and stem cell-like phenotypic characteristics can be exploited for therapeutic regimens against stroke [16].
Fig. (4). Pericytes, BBB, and stroke.

Panel A) After hypoxic injury, some pericytes migrate away from endothelial cells contributing to BBB breakdown. Panel B) In hypoxic-ischemic states, filamentous pericytes are responsible for constricting brain microvessels, exacerbating hypoxic-ischemic injury.
An intriguing topic is also addressed by Dr. Harry van Loveren and colleagues, who discuss the possible contribution of cerebrovascular aneurysms to the incidence or aggravation of ischemic stroke [19]. Unexpected rates of association between stroke and pre-existing aneurysms are reported, along with several mechanisms of injury and aneurysm formation. Interestingly, possible therapeutic strategies with stem cells are discussed, attempting to increase vessel resistance, and therefore decrease aneurysm rupture, or promote tissue neovascularization, thus improving tissue viability after stroke Fig. (5).
Fig. (5). Cerebral aneurysm and stroke.
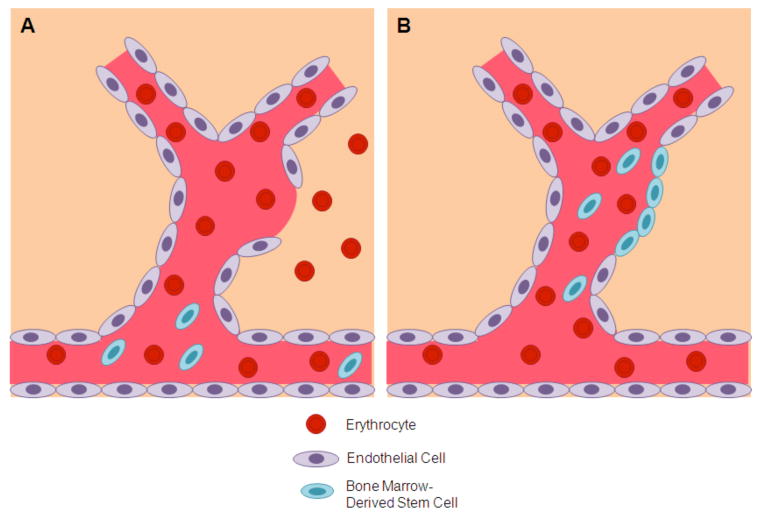
Panel A) Bone marrow-derived stem cells are mobilized from the circulation, and migrate to the site of injury after aneurysm rupture. Panel B) The endogenous bone marrow-derived stem cells aid in the repair of the ruptured blood vessel.
Translational and Clinical Research
Dr. David Hess and collaborators thoroughly describe the preclinical evidence of cell transplantation across intact and injured BBB, and the effects of delivery agents, such as mannitol, upon cell migration into the CNS [20]. The method, successfully described before [4, 21], is hampered by the BBB leakage that follows stroke-induced inflammation. Attempting to circumvent such hindrance, the authors discuss a challenging and bold strategy of stimulating cell influx with mannitol, while blocking BBB leakage with NF-kβ decoy Fig. (6).
Fig. (6). Combination therapy of BBB permeation, stem cell transplantation, and anti-inflammatory treatment for stroke.

The administration of mannitol allows the BBB to become permeable to stem cells or other therapeutic drugs in stroke. However, stroke also increases BBB permeability thereby allowing inflammatory cells to enter the ischemic brain possibly exacerbating tissue damage. NF-kB decoys block the entry of proinflammatory cells and cytokines into the brain, suggesting that a combination therapy of stem cells, mannitol and NF-kB decoys may be ideal for post-stroke therapy.
Dr. Fagan and colleagues get closer to the clinical relevance of BBB and discuss vascular damage and neuronal toxicity secondary to thrombolytic therapy [22]. It is well known that although tissue plasminogen activator (tPA) is the best available therapy for ischemic stroke, several complications may follow, most of which associated with endothelial or perivascular damage [23]. The authors discuss mechanisms of thrombolytic-associated vascular damage and, importantly, potential therapeutic targets, aiming to increase integrity of the BBB and prevent hemorrhagic transformation Fig. (7). Recent preclinical studies showed that the association of tPA and minocycline decreased levels of MMP-2 and MMP-9, reduced the incidence of hemorrhagic complications and improved neurological outcome [24, 25], indicating that clinical studies are forthcoming.
Fig. (7). Vascular protection for stroke.

Panel A) tPA therapy for ischemic stroke has been known to increase the risk of hemorrhagic transformation and increase edema. Panel B) The administration of tPA in combination with pharmacologic agents designed ofr vascular protection may reduce the incidence of hemorrhagic transformation and decrease edema in ischemic stroke patients.
The topic of cell tracking also could not be missed as a novel clinical tool for BBB research. Drs. Nathan Manley and Gary Steinberg, who are experts on the subject, provide an elegant and complete review about the methods used for in vivo tracking of transplanted cells Fig. (8) [26]. The article addresses benefits and drawbacks of different imaging modalities, as well as potential applications in the clinic. Concerning human studies, cell tracking is an indispensable non-invasive tool, and will contribute to the progress and development of cell-based therapy [27].
Fig. (8). Imaging of stem cell grafts in stroke.

Panel A) Experimental stroke is induced in an animal model. Panel B) Post-stroke, stem cells are transplanted into the injured area. Panel C) Various imaging techniques, including SPECT, PET, and MRI are utilized to track the migration of the transplanted stem cells.
Emerging Therapies for BBB Repair in Stroke
Dr. Paul Lapchak has extensive background on neuroprotective approaches using a novel screening method to identify therapeutic compounds [28], such as flavonoid drugs [29]. In the present review, the role of these compounds in the inflammatory post-ischemic injury of stroke is provided in detail, and flavonoids are presented as pleiotropic molecules, with multiple pharmacological activities, therefore able to interact with the different targets of the ischemic injury [30]. The author provides a precise step by step description on how to identify, isolate and characterize a compound, and then synthesize new molecules based on the isolated substance Fig. (9), allowing the understanding of this unique and unconventional therapy.
Fig. (9). Synthesis of new BBB-penetrating compounds for stroke therapeutics.

Panel A) Flavonoid and their derivatives may possess neuroprotective properties. Panel B) These compounds are then characterized in in vitro assays using MDCK cell assay and mouse HT22 hippocampal cells. Panel C) To improve their neuroprotective and BBB-penetration properties, the compounds can structurally altered by adding various function groups. Panel D) The newly synthesized molecules are subsequently tested for their ability to penetrate a simulated BBB in vitro, even including experimental stroke models such as oxygen glucose deprivation (OGD).
As an additional emerging therapy, our research group introduce two very important translational aspects of cell therapy, namely safety and efficacy of stem cell transplantation for stroke with emphasis on endothelial progenitor cells for BBB repair [31]. We highlight the main steps involved in cell-based therapy as well as the important caveats to consider during analysis of pivotal preclinical data for proceeding with future clinical trials of cell therapy in stroke [32, 33] Fig. (10). The readers are referred to recent lab-to-clinic guidelines of Stem cell Therapeutics as an Emerging Paradigm for Stroke or STEPS [34, 35, 36] in improving the successful translation of cell-based therapies into clinical applications.
Fig. (10). Endothelial progenitor cell therapy for BBB repair in stroke.

Following brain injury, such as stroke, endogenous endothelial progenitor cells or EPCs migrate from the periphery to the site of injury, which can be enhanced by exogenous EPC delivery either from the periphery or direct intracerebral transplantation. This EPC therapy can promote restoration of BBB via repair of the vasculature and/or anti-inflammatory effects.
Next Dr. Jun Chen and his research team review the modern topic of neurotherapeutics across the BBB [37]. The therapeutic potential of cell penetrating peptide is explored and its function as an important mediator of transduction is discussed, drawing from a series of preclinical studies demonstrating that arresting the apoptotic pathway via genetic manipulationpromotes neuroprotection against stroke [38, 39, 40]. Such unique peptide properties, with an effective gene fabrication technique, and a solid mechanism of action directly relevant to stroke cell death cascade make these novel compounds as appealing therapeutic candidates for clinical application in stroke Fig. (11).
Fig. (11). Cell penetrating peptides with improved BBB bioavailability as novel therapeutics for stroke.

Panel A) Traditional neurotherapeutics are not able to penetrate the BBB. Panel B) Neurotherapeutics designed as cell penetrating peptides or CPPs are able to penetrate the BBB via direct internalization, endocytosis, and inverse micelle formation. Panel C) CPPs allow neurotherapeutics to penetrate the BBB; however, they are non-specific and also migrate to peripheral tissue. Panel D) Future research and development of CPPs with sequence modifications will allow for CNS-specific penetration of neurotherapeutics.
Finally, Dr. Sunghee Cho exploits the theme of peripheral immune system contributing to stroke pathology [41]. In particular, the demonstration of a clear-cut role of CD-36 in the systemic immune response opens new avenues for developing novel strategies targeting the peripheral immune system. In stroke, multiple injury mechanisms may lead to common pathways of CD36 activation, leading to inflammation and endothelial dysfunction [42, 43]. The current state of the art describes the effects of CD36 activation upon endothelial cell function angiogenesis and tissue inflammation and proposes CD36 targeting by different agents as therapeutic alternative following stroke Fig. (12) [41-43].
Fig. (12). CD36 as a therapeutic target for endothelial dysfunction in stroke.

Panel A) White blood cells are attracted to the injured tissue via CD36 signaling pathway. This promotes inflammation and induces proinflammatory cytokines and chemokines, possibly contributing to larger infarct volumes. Panel B) CD36 receptor antagonists are currently being investigated as a possible way to decrease inflammation following stroke and possible decrease infarct volume.
Conclusions
Altogether, these thought-provoking and clinically relevant studies offer genuine points of view about the critical role of BBB in stroke pathology. We believe that further basic and translational investigations into the signaling pathways and downstream processes altered by BBB compromise after stroke will optimize BBB-based therapeutics. In the same vein, stroke-related neurodegenerative and inflammatory diseases similarly characterized by BBB breakdown will benefit from the successful advancement of this field, not only deciphering the contribution of BBB to the disease manifestations but also acting as a highly potent therapeutic target for the development of new treatment strategies.
Acknowledgments
The authors extend appreciation to Ms. Loren E. Glover and Ms. Lourdes Cortes who provided excellent technical assistance in the final preparation of the manuscript.
Footnotes
Disclosures/Conflict of Interests: CVB is supported by NIH NINDS R01 NS071956-01 and James and Esther King Foundation for Biomedical Research Program Research Grant 6129101700, and receives research grant support for his projects on stem cell therapy for stroke from SanBio Inc., Celgene Cellular Therapeutics, KMPHC, and NeuralStem Inc.
References
- 1.Miyakawa T. Vascular pathology in Alzheimer's disease. Ann N Y Acad Sci. 2002;977:303–5. doi: 10.1111/j.1749-6632.2002.tb04830.x. [DOI] [PubMed] [Google Scholar]
- 2.Monahan AJ, Warren M, Carvey PM. Neuroinflammation and peripheral immune infiltration in Parkinson's disease: an autoimmune hypothesis. Cell Transplant. 2008;17:363–72. [PubMed] [Google Scholar]
- 3.Ronaldson TR, Davis TP. Blood-Brain Barrier Integrity and Glial Support: Mechanisms that can be targeted for Novel Therapeutic Approaches in Stroke. Curr Pharm Design. 2012 doi: 10.2174/138161212802002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuhara T, Hara K, Maki M, et al. Mannitol facilitates neurotrophic factor upregulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14:914–921. doi: 10.1111/j.1582-4934.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronaldson PT, Finch JD, DeMarco KM, Quigley CE, Davis TP. Inflammatory Pain Signals an Increase in Functional Expression of Organic Anion Transporting Polypeptide 1a4 at the Blood-Brain Barrier. J Pharm Exp Ther. 2011;336:827–39. doi: 10.1124/jpet.110.174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronaldson PT, DeMarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor signaling alters substrate permeability and tight junction protein expression at the blood, brain barrier during inflammatory pain. J Cereb Blood Flow Metab. 2009;29:1084–98. doi: 10.1038/jcbfm.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo JH, Guoa S, Lok J, Navaratnan D, Kim KW, Lo EH. Neurovascular Matrix Metalloproteinases and the Blood-Brain Barrier. Curr Pharma Design. 2012 doi: 10.2174/138161212802002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park KP, Rosell A, Foerch C, et al. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke. 2009;40:2836–2842. doi: 10.1161/STROKEAHA.109.554824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Jung JC, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–42. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tejima E, Guo S, Murata Y, et al. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma. 2009;26:1935–41. doi: 10.1089/neu.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo S, Stins M, Ning M, Lo E. Amelioration of inflammation and cytotoxicity by dipyridamole in brain endothelial cells. Cerebrovasc Dis. 2010;30:290–6. doi: 10.1159/000319072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–7. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita A, Abe K. Mechanisms of endogenous endothelial repair in stroke. Curr Pharma Design. 2012 doi: 10.2174/138161212802002832. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita T, Deguchi K, Nagotani S, Abe K. Vascular protection and restorative therapy in ischemic stroke. Cell Transplant. 2011;20:95–7. doi: 10.3727/096368910X532800. [DOI] [PubMed] [Google Scholar]
- 15.Takamiya M, Miyamoto Y, Yamashita T, Deguchi K, Ohta Y, Ikeda Y, Matsuura T, Abe K. Neurological and pathological improvements of cerebral infarction in mice with platinum nanoparticles. J Neurosci Res. 2011 doi: 10.1002/jnr.22622. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Agalliu D, Chuanhui Y, Fisher M. The Roles of Pericytes in Blood-Brain Function and Stroke. Curr Pharma Design. 2012 doi: 10.2174/138161212802002706. [DOI] [PubMed] [Google Scholar]
- 17.Fisher M, French S, Ji P, Kim RC. Cerebral Microbleeds in the Elderly. Stroke. 2010;41:2782–5. doi: 10.1161/STROKEAHA.110.593657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40:S13–5. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajiri N, Glover LE, Shinozuka K, Kaneko Y, van Loveren H, Borlongan CV. Cerebral Aneurysm as an Exacerbating Factor in Stroke Pathology and a Therapeutic Target for Neuroprotection. Curr Pharma Design. 2012 doi: 10.2174/138161212802002724. [DOI] [PubMed] [Google Scholar]
- 20.Hess DC, Glover LE, Sanberg PR, Borlongan CV. Permeating the blood brain barrier and abrogating the inflammation in stroke: Implications for stroke therapy. Curr Pharma Design. 2012 doi: 10.2174/138161212802002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borlongan CV, Hadman M, Davis Sanberg C, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–9. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 22.Ishrat T, Soliman S, Guan W, Saler M, Fagan S. Vascular Protection to Increase the Safety of Tissue Plasminogen Activator for Stroke. Curr Pharma Design. 2012 doi: 10.2174/138161212802002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Group Nt-PSS. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–18. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 24.Switzer JA, Hess DC, Ergul A, et al. Matrix Metalloproteinase-9 in an Exploratory Trial of Intravenous Minocycline for Acute Ischemic Stroke. Stroke. 2011;42:2633–5. doi: 10.1161/STROKEAHA.111.618215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machado LS, Sazonova IY, Kozak A, et al. Minocycline and tissue-type plasminogen activator for stroke. Stroke. 2009;40:3028–33. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manley NC, Steinberg GK. Tracking Stem Cells for Cellular Therapy in Stroke. Curr Pharma Design. 2012 doi: 10.2174/138161212802002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gera A, Steinberg GK, Guzman R. In vivo neural stem cell imaging: current modalities and future directions. Regen Med. 2010;5:73–86. doi: 10.2217/rme.09.79. [DOI] [PubMed] [Google Scholar]
- 28.Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–25. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15 lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150:585–91. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 30.Lapchak PA. A Series of Novel Neuroprotective Blood Brain Barrier Penetrating Flavonoid Drugs to Treat Acute Ischemic Stroke. Curr Pharma Design. 2012 doi: 10.2174/138161212802002652. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko Y, Tajiri N, Shinozuka K, Glover LE, Weinbren N, Cortes L, Borlongan CV. Cell Therapy for Stroke: Emphasis on Optimizing Safety and Efficacy Profile of Endothelial Progenitor Cells. Cur Pharma Design. 2012 doi: 10.2174/138161212802002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The Great Migration of Bone Marrow-Derived Stem Cells Toward the Ischemic Brain: Therapeutic Implications for Stroke and Other Neurological Disorders. Prog Neurobiol. 2011 doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borlongan C. Bone marrow stem cell mobilization in stroke: a bonehead may be good after all! Leukemia. 2011 doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chopp M, Steinberg GK, Kondziolka D, et al. Who's in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant. 2009;18:691–693. doi: 10.3727/096368909X470883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borlongan CV. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 2009;40:S146–8. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borlongan CV, Weiss MD. Baby STEPS: a giant leap for cell therapy in neonatal brain injury. Pediatr Res. 2011;70:3–9. doi: 10.1203/PDR.0b013e31821d0d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hua X, Zhang M, Leak RK, Gana Y, Li P, Gao Y, Chen J. Delivery of Neurotherapeutics across the Blood Brain Barrier in Stroke. Cur PharmaDesign. 2012 doi: 10.2174/138161212802002715. [DOI] [PubMed] [Google Scholar]
- 38.Cao G, Pei W, Ge H, et al. In vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J Neurosci. 2002;22:5423–31. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F, Signore AP, Zhou Z, Wang S, Cao G, Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J Neurosci Res. 2006;83:1241–51. doi: 10.1002/jnr.20816. [DOI] [PubMed] [Google Scholar]
- 40.Yin W, Cao G, Johnnides MJ, et al. TAT-mediated delivery of Bcl-xL protein is neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and AIF. Neurobiol Dis. 2006;21:358–71. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Cho S. CD36 as a therapeutic target for endothelial dysfunction in stroke. CurrPharma Design. 2012 doi: 10.2174/138161212802002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem. 2007;282:4634. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- 43.Cho S, Kim E. CD36: A multiÄêmodal target for acute stroke therapy. J Neurochem. 2009;109:126–32. doi: 10.1111/j.1471-4159.2009.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


