Abstract
Computational models of hippocampal function propose that the hippocampus is capable of rapidly storing distinct representations through a process known as pattern separation. This prediction is supported by electrophysiological data from rodents and neuroimaging data from humans. Here, we test the prediction that damage to the hippocampus would result in pattern separation deficits by having memory-impaired patients with bilateral hippocampal damage study a series of objects or faces and then perform a modified recognition memory test. In the test phase, participants viewed true repetitions, novel foils, and lures that were perceptually and semantically related to the studied stimuli. Patients with hippocampal damage were unimpaired relative to matched controls in their baseline recognition memory. However, patients were less likely to uniquely identify lures as “similar” than matched controls, indicating an impairment in pattern separation processes following damage to the hippocampus.
Keywords: Memory, Amnesia, Pattern Separation
1. Introduction
Long-term declarative memory is critically dependent on the structures of the medial temporal lobe (MTL) including the hippocampus, and the perirhinal, entorhinal, and parahippocampal cortices. Damage to these structures results in declarative memory deficits (Squire et al., 2004), however there is considerable debate regarding the relative contributions of these structures to memory. Based on the neuroanatomical connections within the hippocampus, computational models propose that the hippocampus forms fast, distinct memories through a process of pattern separation, which is the process of establishing distinct, non-overlapping representations (Burgess & O’Keefe, 1996; Hasselmo & Wyble, 1997; McNaughton & Morris, 1987; Rolls, 1989; Rolls & Treves, 1998). Pattern separation is important to avoid catastrophic interference (McClelland, et al., 1995), particularly when memories are very similar (Rolls & Kesner, 2006). In particular, the computational models in the Complementary Learning Systems (CLS) framework propose that the major difference between the hippocampus and the MTL cortex is in the ability of the hippocampus to perform rapid pattern separation (McClelland et al., 1995; Norman & O’Reilly, 2003). This is not to suggest that the hippocampus alone performs pattern separation or that it does not perform pattern completion as well. Rather, according to this and other computational models, pattern separation and pattern completion are complementary processes that are carried out widely throughout the brain. The unique contribution of the hippocampus is that it has a strong bias toward pattern separation relative to cortical regions. As such, these models predict that selective damage to the hippocampus will result in disproportionate impairments in pattern separation.
Recent empirical evidence supports the predictions of the computational models regarding the role of the hippocampus in pattern separation and pattern completion. Electrophysiological studies in rodents (J. Leutgeb et al., 2007; S. Leutgeb, et al., 2005; S. Leutgeb et al., 2004), neuroimaging studies in humans (Bakker et al., 2008; Lacy et al., 2011), and neuropsychological studies with memory-impaired patients (Duff et al., 2011) have confirmed that the hippocampus is involved in pattern separation processes, consistent with the predictions of computational models. There is also a large body of literature demonstrating a role of the rodent hippocampus in pattern completion for spatial locations (Gold & Kesner, 2005; Kirwan et al., 2005) and contextual fear conditioning (Matus-Amat, et al., 2004; Rudy & O’Reilly, 1999).
The CLS model predicts that damage to the hippocampus should lead to a deficit in pattern separation and greater susceptibility to inter-item interference and an increased likelihood of mistakenly identifying related lures as having been previously encountered (due to intact MTL cortex-mediated pattern completion). It should be noted that while the model ascribes the function of pattern separation to the dentate gyrus subregion of the hippocampus, damage to other subregions including CA3 and CA1 would also lead to pattern separation deficits as these regions are the output channels for the dentate gyrus. Norman and O’Reilly (2003) explicitly predict from their model greater susceptibility to inter-item interference following hippocampal damage and note that behavioral testing with a patient with hippocampal damage (Patient Y.R.) has supported the model’s prediction (Holdstock et al., 2002; Mayes et al., 2002; but see Bayley et al., 2008). The model’s prediction is also consistent with the behavior of memory-impaired patients with hippocampal damage, who are more susceptible to interference than matched controls (Kinsbourne & Winocur, 1980; Lustig & Hasher, 2001; Mayes et al., 1987; Warrington & Weiskrantz, 1974, 1978; Winocur & Moscovitch, 1996; Winocur & Weiskrantz, 1976; for review, see Lustig & Hasher, 2001). Furthermore, healthy older participants are more prone to behavioral pattern completion than pattern separation for objects (Toner et al., 2009; Yassa, et al., 2010) and locations (S. Stark et al., 2010). There is evidence, however, that pattern separation for other stimuli such as faces may occur in cortical regions (Rotshtein et al., 2005).
Here, we directly tested the prediction that performance on yes-no recognition memory tests is impaired in the case of hippocampal damage when target-lure similarity is high but not when there is low similarity between targets and lures. The CLS model of Norman and O’Reilly (2003) makes the following prediction:
The difference in operating characteristics between the MTLC [MTL cortex] familiarity and hippocampal recall signals is most evident on yes-no (YN) related-lure recognition tests where lures are similar to studied items but studied items are dissimilar to one another…As target-lure similarity increases, lure familiarity increases steadily, but (up to a point) hippocampal pattern separation works to keep lure recall at floor. Very similar lures trigger recall, but when this happens the lure can often be rejected due to mismatch between retrieved information and the recall cue. (p. 614, emphasis in original)
Accordingly, we tested the hypothesis that the hippocampus is required for accurate pattern separation using a modified yes-no recognition memory test where we varied the target-lure similarity. We further tested whether changes in stimulus modality affected pattern separation processing demands by using both faces and objects. Memory-impaired patients and a group of matched controls incidentally encoded a series of objects and faces. After a short delay, we tested participants’ memory by showing them targets (stimuli from the study list), novel foils, or lure stimuli (related, but not identical to the target stimuli). Participants were informed of the three stimulus types and instructed to respond “old” if they recognized the stimulus as being exactly the same from the study phase, “new” if they thought the stimulus was new, or “similar” if they recognized the stimulus as being similar but not identical. We hypothesized that memory-impaired patients with hippocampal damage would have an impairment in pattern separation performance (similar judgments) relative to matched control participants and that this impairment would be evident as a reduction in the ability to correctly and uniquely identify lures as being similar to previously-studied targets. We hypothesized that patients would be more prone to pattern completion for these stimuli and would therefore falsely identify them as having been previously encountered.
2. Method
2.1. Participants
Three memory-impaired patients with damage to the hippocampal formation participated (RS, JRW, and CA). Patient RS became amnesic in 1998 following a drug overdose and associated respiratory failure. Patient JRW became amnesic in 1990 after an anoxic episode associated with cardiac arrest. Patient CA became amnesic following traumatic brain injury resulting from an automobile accident in 2000. Structural MTL volumes were calculated using quantitative MRI versus age- and sex-matched controls. RS, JRW, and CA have average bilateral reductions in hippocampal volume of 33, 44, and 42%, respectively (all values >3 SDs below the control mean), which appear to include reductions to all subregions of the hippocampus (dentate gyrus, CA3, and CA1). No other MTL region differed from controls. Hippocampal and MTL volumes for RS and JRW have been reported previously (Bayley, Gold, Hopkins, & Squire, 2005). Nine coronal MRI images of the MTL along with lesion descriptions of the memory-impaired patients are presented in Supplemental Materials. As noted previously (Bayley et al., 2005), Patient RS has an unusually small parietal lobe volume, though this appears to be due to natural variation as there is no evidence of parietal lobe damage on his MRI scans and he scores in the normal range for neuropsychological tests sensitive to parietal function. Patient CA as volume reductions in cerebral cortex volumes (0.76 and 1.22 SDs below control means for the left and right hemispheres, respectively). The mean age of the memory-impaired patients was 44.3 years, and the mean years of education was 14.33 (see Table 1).
Table 1.
| Initials | Age | Education | Gender | WAIS IQ | WMS General | WMS Delay |
|---|---|---|---|---|---|---|
| CA | 35 | 18 | F | 116 | 78 | 66 |
| JRW | 48 | 12 | M | 90 | 70 | <50 |
| RS | 50 | 12 | M | 99 | 82 | <50 |
| M | 44.3 | 14.0 | -- | -- | -- | -- |
WAIS scores for JRW and RS are WASI (Wechsler Abbreviated Scale of Intelligence); scores for CA are WAIS III. WMS scores for JRW and RS are WMS-R; scores for CA are WMS III.
The control group consisted of 11 participants (7 women) who were matched to the patients for age [mean (SD) = 37.4 (12.32); range = 25–56] and years of education [16.0 (2.67)].
2.2. Stimuli
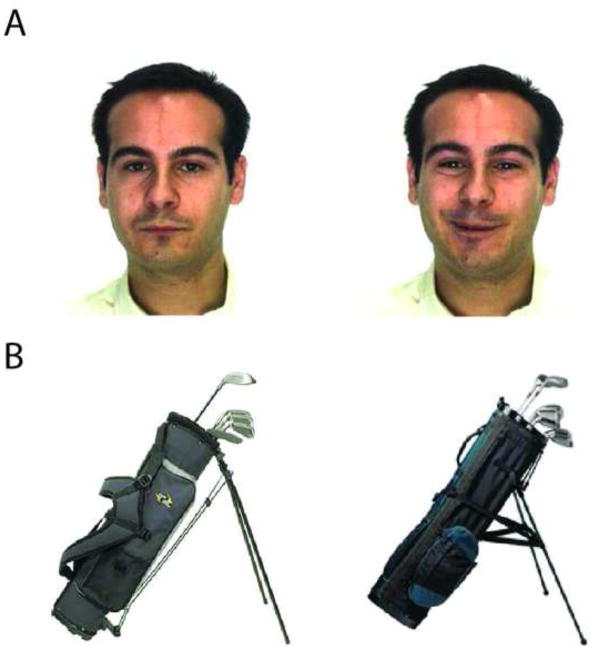
The face stimuli consisted of 823 color portrait-style photographs of 367 unique individuals. Each person was pictured in 2–4 different poses. There were a number of possible differences between pictures of the same person, such as gaze direction, lighting, expression, hairstyle, clothes, or a combination of any of these (see Figure 1A). All stimuli were obtained from freely available online databases (http://pics.psych.stir.ac.uk; http://www.lrv.fri.uni-lj.si/facedb.html; Martinez & Benavente, 1998; Nordstrom et al., 2004). Object stimuli consisted of 1,109 color photographs of common namable objects. Three hundred eighty-four of the stimuli were semantically related pairs, which were used for lure stimuli (see Behavioral Method below). Figure 1B depicts an example related lure pair. Each pair was chosen from a larger set of ~15–30 by selecting the two stimuli with the highest perceptual similarity rating based on independent normative ratings. The remaining 725 stimuli were unrelated random foils.
Figure 1.
Example stimuli. For the faces section of the experiment (A), related lures varied from studied stimuli in aspects such as facial expression or gaze direction. For the objects section of the experiment (B), related lures were visually similar objects from the same sematic category as studied stimuli.
2.3. Behavioral Method
All behavioral testing was conducted on a computer as the participant was seated comfortably at the keyboard. Stimuli were presented using the Cogent 2000 toolbox (www.fil.ion.ucl.ac.uk) for Matlab (The MathWorks, Natick, MA). Behavioral testing consisted of a baseline recognition memory task followed by a pattern separation task for either the objects or the faces (order of stimulus type counterbalanced across participants). In the baseline task, participants were shown a series of 30 randomly selected stimuli and asked to rate the stimuli as either “pleasant” or “unpleasant” by pressing one of two keys on the keyboard. Participants were informed that their memory for the stimuli would be tested after a short delay. Task instructions were displayed at the bottom of the screen during each trial. Stimuli were displayed for 3000 ms and participants were encouraged to respond during the stimulus presentation. A blank screen separated trials for 500 ms. A 3-minute conversation-filled delay separated the study and test phases of each block. The test phase of the baseline blocks consisted of 60 trials with 30 targets from the study phase and 30 novel foils. Participants were instructed to press one button on the keyboard for “old” stimuli (targets) and a second button for “new” stimuli (foils). Trials were self-paced and separated by a 500 ms inter-trial interval. The study phase of the experimental (pattern separation) blocks was identical to that of the baseline (recognition) blocks with 30 stimuli presented while participants made pleasant/unpleasant judgments. In the test phase of the experimental blocks there were again 60 total trials. Half the study items from each block (15) were randomly assigned to be repeated (targets), while items from the respective sets to which the remaining 15 study stimuli belonged were presented as lures. Participants were informed that some items from the study phase would repeat and were instructed to respond “old” to these stimuli. Participants were also informed that some of the items would be similar to the study items, e.g. the same person but a different picture of that person, or the same kind of object as at study, but a different example of that kind. Participants were instructed to respond “similar” if they thought this was the case for a given stimulus. Finally, participants were instructed to respond “new” if they had not seen the stimulus during the preceding study phase. Trials for the test phase of the experimental blocks were also self-paced. The baseline and experimental study-test blocks were then repeated with the other set of stimuli (either faces or objects).
3. Results
Memory-impaired patients and age-matched controls were tested once on the baseline blocks and twice each for the experimental blocks for both faces and objects using different stimuli for each test block (Patient JRW only contributed one test block each for faces and objects). Where two test blocks were obtained, the data from the two experimental runs were averaged for analysis. Patients RS and CA performed similarly in the two testing blocks (correlations of r=0.51 for Patient RS and r=0.50 for Patient CA across stimulus types).
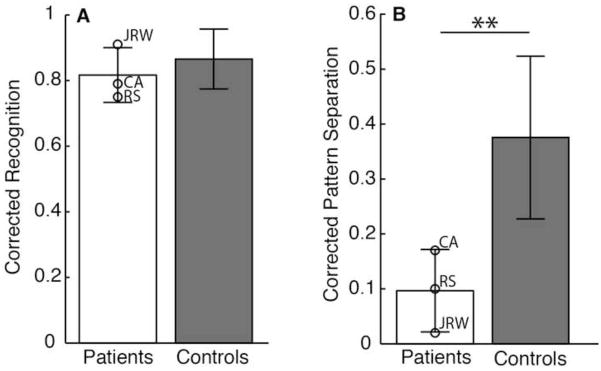
The specific hypothesis under investigation was that damage limited to the hippocampus would disproportionately affect pattern separation ability while leaving recognition memory performance relatively intact. Accordingly, we first assessed recognition memory performance by computing a corrected recognition memory score for the baseline condition by subtracting the false alarm rate (the proportion of “old” responses to unrelated foils) from the hit rate (the proportion of correct “old” responses to targets). Figure 2A depicts the mean corrected recognition scores for the patients and controls collapsed across stimulus type (objects and faces), which were 0.81 (SD=0.08) and 0.87 (0.09), respectively. Considered separately, the patient group and control group did not differ in their hit rate for faces [patients=0.89 (0.09), controls=0.86 (0.11)], false alarm rates for faces [patients=0.16 (0.06), controls=0,08 (0.10)], hit rates for objects [patients=0.92 (0.08), controls=0.97 (0.04)], or false alarm rates for objects [patients=0.16 (0.06), controls=0.09 (0.09)]. We calculated t-scores using the method of Crawford and Howell (1998), which allows for the comparison of a single patient against a control group. The patient group’s pattern separation performance was on the whole less impaired for the face stimuli [modified t(10)=0.79, p=0.23; t(10)=1.49, p=0.08; t(10)=1.8, p=0.05 one-tailed for Patients CA, RS, and JRW, respectively] than for the object stimuli [modified t(10)=1.58, p=0.07; t(10)=1.83, p=0.05; t(10)=2.43, p=0.02 one-tailed for Patients CA, RS, and JRW, respectively].
Figure 2.
Recognition memory performance. Patients were not impaired relative to controls in recognition memory performance on the baseline task (A). The corrected recognition score was calculated as the hit rate minus the false alarm rate; i.e., p(“old”|target) – p(“old”|foil). Patients were impaired relative to controls in their pattern separation performance (B). The pattern separation index was calculated as the proportion of lure trials eliciting a correct response of “similar” minus the proportion of novel foil trials eliciting a response of “similar”. I.e.: p(“similar”|lure) – p(“similar”|foil). Error bars indicate standard deviations. **p<0.01.
We next considered the response rates for the two groups in the experimental task. To quantify the difference in response rates between the two groups (see Table 2), we performed 3×3×2 ANOVAs for the face and object conditions separately with stimulus condition (repeat, lure, and foil) and response (“old”, “similar”, and “new”) as within subjects factors and group as a between subjects factor. For the face stimuli, only the stimulus condition by response interaction was significant [F(2,24)=15.50, p<0.001], indicating that both groups modified their behavioral responses in accordance with the stimulus condition. For the objects, the main effect of response [F(2,24)=21.28, p<0.001], the stimulus condition by response interaction [F(4,48)=55.81, p<0.001], and the stimulus condition by response by group interaction [F(4,48)=3.97, p<0.01] were significant. Follow-up t-tests on the three-way interaction revealed that the patient and control groups differed in their proportions of “new” responses to lures [t(12) = 4.75, p< 0.001] and in their proportions of “similar” responses to foils [t(12)=2.80, p<0.05].
Table 2.
| Trial Type Response | Targets | Lures | Foils | ||||
|---|---|---|---|---|---|---|---|
| “old” | “similar” | “new” | “old” | “similar” | “new” | “old” | |
| Faces | |||||||
| CA | 0.30 | 0.50 | 0.20 | 0.03 | 0.63 | 0.33 | 0.10 |
| RS | 0.40 | 0.17 | 0.43 | 0.33 | 0.07 | 0.60 | 0.25 |
| JRW | 0.64 | 0.36 | 0.00 | 0.29 | 0.71 | 0.00 | 0.10 |
| Patient Mean (SD) | 0.45 (0.18) | 0.34 (0.17) | 0.21 (0.22) | 0.22 (0.16) | 0.47 (0.35) | 0.31 (0.30) | 0.15 (0.09) |
| Control Mean (SD) | 0.64 (0.19) | 0.25 (0.16) | 0.11 (0.10) | 0.28 (0.20) | 0.47 (0.21) | 0.24 (0.08) | 0.17 (0.17) |
| Objects | |||||||
| CA | 0.87 | 0.13 | 0.00 | 0.57 | 0.33 | 0.10 | 0.40 |
| RS | 0.87 | 0.03 | 0.10 | 0.60 | 0.20 | 0.20 | 0.35 |
| JRW | 0.67 | 0.33 | 0.00 | 0.47 | 0.33 | 0.20 | 0.10 |
| Patient Mean (SD) | 0.80 (0.12) | 0.17 (0.15) | 0.03 (0.05) | 0.54 (0.07) | 0.29 (0.08) | 0.17 (0.06) | 0.28 (0.16) |
| Control Mean (SD) | 0.91 (0.07) | 0.07 (0.07) | 0.03 (0.06) | 0.46 (0.18) | 0.51 (0.18) | 0.03 (0.04) | 0.25 (0.22) |
| Combined | |||||||
| CA | 0.58 | 0.32 | 0.10 | 0.30 | 0.48 | 0.22 | 0.25 |
| RS | 0.63 | 0.10 | 0.27 | 0.47 | 0.13 | 0.40 | 0.30 |
| JRW | 0.66 | 0.34 | 0.00 | 0.38 | 0.52 | 0.10 | 0.10 |
| Patient Mean (SD) | 0.62 (0.04) | 0.25 (0.13) | 0.12 (0.13) | 0.38 (0.08) | 0.38 (0.21) | 0.24 (0.15) | 0.22 (0.10) |
| Control Mean (SD) | 0.77 (0.11) | 0.16 (0.11) | 0.07 (0.06) | 0.37 (0.17) | 0.49 (0.18) | 0.14 (0.05) | 0.21 (0.19) |
The raw response rates do not take into account overall response bias. Accordingly, we next assessed pattern separation performance by examining participants’ responses to the similar lure stimuli in the experimental condition. We hypothesized that the proportion of “similar” responses to lures (lure correct rejections) would be reduced for the memory-impaired patients relative to controls. To measure this difference and account for any overall bias to respond “similar”, we calculated a corrected pattern separation score by subtracting the proportion of “similar” responses to unrelated foils from the proportion of “similar” responses to lures (see Table 2). The corrected pattern separation score can range from −1 to 1, with a value of 0 reflecting chance performance and 1 being perfect performance for both lure and foil trials. The pattern separation scores for the control group and the memory-impaired patients are summarized in Figure 2B (again collapsed across stimulus type). The mean pattern separation score for the patients was 0.10 (0.07) and for the controls was 0.37 (0.15).
A 2×2×2 ANOVA with task (baseline and experimental) and stimulus type (objects and faces) as within subjects factors and group (patients and controls) as between subjects factors a main effect of group [F(1,12) = 7.86, p < 0.05], a main effect of task [F(1,12) = 155.04, p < 0.001], and a main effect of stimulus type [F(1,12)=27, p < 0.001], indicating that the control group overall performed better than the patient group, that baseline recognition memory performance was overall better than pattern separation task performance collapsed across groups, and that performance was better for objects than for faces collapsed across tasks and groups, respectively. The stimulus type by group interaction was not significant [F(1,12)=0.92, p=0.36], indicating that the patient and control groups did not perform differentially with either stimulus type. The stimulus type by task interaction also was not significant [F(1,12)=0.35, p=0.57]. Similarly, the three-way interaction of stimulus type by task by group failed to reach significance [F(1,12)=0.47, p=0.51]. Critically, however, the task by group interaction was significant [F(1,12)=5.50, p<0.05]. Post-hoc t-tests confirmed that overall the patient group was differentially impaired in the pattern separation measure relative to the control group [t(12) = 3.18, p < 0.01], but was not impaired in the baseline recognition measure [t(12) = 0.83, p = 0.42]. Thus, consistent with our first hypothesis, patients were impaired relative to controls in their pattern separation ability as measured by their ability to uniquely identify lures as “similar”.
To further characterize our data, we also calculated corrected “old” scores [p(“old”|lure) - p(“old”|foil)] and a corrected “new” scores [p(“new”|foil) - p(“new”|lure)]. Mean corrected “old” scores for the face stimuli were 0.11 (0.13) and 0.07 (0.13) for the control and patient groups, respectively [t(12) = 0.54, p=0.60]. Mean corrected “old” scores for the object stimuli were 0.21 (0.21) and 0.26 (0.10) for the control and patient groups, respectively [t(12) = 0.45, p = 0.66]. Mean corrected “new” scores for the face stimuli were 0.39 (0.21) and 0.12 (0.04) for the control and patient groups, respectively [t(12) = 2.13, p=0.06]. Mean corrected “new” scores for the object stimuli were 0.67 (0.22) and 0.40 (0.04) for the control and patient groups, respectively [t(12) = 2.11, p=0.06]. Thus, contrary to our second prediction, patients did not respond “old” to similar stimuli more often than control participants. Further, the marginal significance of the corrected “new” scores indicates that the control group was better able to discriminate lures from foil stimuli.
4. Discussion
In this experiment, we tested the hypothesis that memory-impaired patients with bilateral hippocampal damage are impaired relative to controls under conditions requiring increased pattern separation. In an explicit recognition memory task, memory-impaired patients with damage to the hippocampus had significantly reduced corrected pattern separation scores compared to age-matched controls, indicating an impairment in pattern separation performance. Recognition memory scores in the baseline memory task were well above chance for the patients and were not different from the scores of controls. This intact performance in the baseline recognition task may have been due to lower pattern separation demands in this task, which used unique and non-overlapping stimuli. These results indicate that the hippocampus does support pattern separation, which is necessary to correctly recognize and identify the related lures.
The effects of reduced pattern separation ability might be manifested at the time of retrieval by an overall increase in false alarms (or “old” responses) to similar lures, indicating an overall bias toward pattern completion. Alternately, at the time of encoding a pattern separation deficit may be manifested as a failure to form an orthogonalized, pattern separated representation of the stimulus. The patient group’s responses to similar lures (particularly to the objects stimuli) are consistent with a pattern separation failure at the time of encoding (Table 2).
The current finding is consistent with findings from other groups of memory-impaired patients and from healthy aging populations. In a recent study by Duff and colleagues (Duff et al., 2011), patients with bilateral hippocampal damage were shown to have difficulty forming rapid, distinct representations for similar tangram images, whereas they were unimpaired relative to controls for dissimilar tangrams. The authors interpreted this finding in terms of a pattern separation deficit as predicted by the CLS framework. Similarly, using a continuous recognition version of the current task, we have demonstrated that healthy older adults are more likely to mistakenly identify related lures as “old”, indicating a bias toward pattern completion and away from pattern separation (Toner, et al., 2009; Yassa, et al., 2010). Also, using high-resolution fMRI and diffusion tensor imaging (DTI), Yassa and colleagues (Yassa et al., 2011) recently demonstrated age-related changes in activity within the hippocampus as well as hippocampal input that predicted performance on tasks requiring pattern separation. Cermack and Butters (1972) used a continuous recognition task developed by Underwood (Underwood, 1965) to demonstrate that Korsakoff patients had greater false recognition of related lures compared to controls, indicating that amnesic patients are more susceptible to interference. This is somewhat in contrast to our current results where we observed both an increase in “old” responses as well as “new” responses to similar lures.
Lesion studies with rodents have also demonstrated that damage to the hippocampus results in pattern separation deficits. In a study by Gilbert and colleagues (Gilbert et al., 2001), rats were tested on a spatial pattern separation task in which rats had to discriminate between two locations in order to receive a food reward. The distance between the two locations was parametrically varied. Rats with lesions to the dentate gyrus performed as well as controls for large spatial separations, but the lesion group’s performance decreased linearly with smaller spatial separations between the rewarded and unrewarded locations. The same graded performance decreases were not seen for rats with lesions to the CA1 subregion of the hippocampus. The authors interpreted these results as a deficit in spatial pattern separation following dentate gyrus damage. It should be noted that the lesions in this study were complete for the subregions in the dorsal hippocampus, which leaves open the possibility for the pattern-separated representations to be communicated back to the cortex via the intact ventral CA1. In a recent study, Goodrich-Hunsaker and colleagues (Goodrich-Hunsaker et al., 2008) demonstrated that rats with dorsal dentate gyrus lesions were impaired relative to controls in a metric spatial memory task that placed high demands on spatial pattern separation. Lesions to the dorsal CA3 and dorsal CA1 also produced behavioral impairments, but to a lesser degree than the dentate gyrus lesions. Recent neuroimaging studies have suggested that in the human hippocampus, early processing stages (dentate gyrus/CA3) respond differentially to “mnemonic similarity” when compared with later stages (CA1) (Lacy et al., 2011). This seems to be analogous to how the rat hippocampus seems to respond to spatial similarity. It should be noted that the lesions in the patient group in the current study are not localized to one hippocampal subregion. Thus it is difficult to determine if the observed deficits are differentially due to impaired processing in one subregion or due to the reduction of overall hippocampal function.
The current finding, on the other hand, is in apparent contrast to another body of literature examining “gist” memory in Alzheimer’s disease (AD; Budson et al., 2000; Budson et al., 2001) and amnesia (Koutstaal et al., 2001; Schacter et al., 1998; Verfaellie et al., 2004). These studies typically use one of several variants of the DRM paradigm (Deese, 1959; Roediger & McDermott, 1995) in which participants study a list of related stimuli that lacks a critical central associate. When normal participants’ recognition memory for the list is tested, false recognition rates for the omitted critical lure are exceptionally high. A consistent finding with AD (Budson, et al., 2000) and amnesia of mixed etiologies (Schacter, et al., 1998) is that memory-impaired patients do not have comparable false alarm rates, once corrected for baseline false alarm rates. The lack of false alarm rates in memory-impaired patients is taken as evidence of a “gist” memory impairment, or an inability to generalize (pattern complete) in the memory impaired patients. This is in apparent contrast to the findings of the current research that memory-impaired patients are impaired in their pattern separation abilities.
The above two sets of findings are reconcilable when considering the computational processes needed to solve the two tasks. There are a number of important differences between the DRM paradigm and the paradigm employed here. The DRM paradigm employs a list of related stimuli to elicit the implicit activation of a central, critically omitted stimulus presumably through pattern completion processes. The authors (cf. Koutstaal, et al., 2001) postulate that control participants form and retain a strong and well-organized representation for the entire set of associated stimuli, thus making it difficult to reject the critical lure. Amnesic and AD patients are only able to form a weak version of this representation. From the point of view of the computational principles involved, this is an excellent demonstration of pattern separation giving way to pattern completion as a way to efficiently represent a set of information. On the other hand, in the case of the current experiment, the lures were only related to one of the study items and not an entire set. Normal performance, therefore, depends much more on pattern separation. Since both pattern completion and pattern separation in this context depend on medial temporal lobe (MTL) processes, hippocampal amnesic patients perform differently than controls in both tasks.
Another important difference between the task in the current experiment and the traditional DRM paradigm is the addition of the third response option of “similar” in addition to the more traditional “old” and “new” options. In the current research, the critical finding was that amnesic patients were impaired in their ability to uniquely identify related lure stimuli as such by utilizing the “similar” response option. It is unclear what predictions the gist memory account would make regarding the “similar” response option.
In contrast to our use of an explicit memory task, prior neuroimaging studies that have indicated a role of the hippocampus in pattern separation have relied on implicit memory paradigms (e.g., Lacy et al., 2011). For example, in the study by Bakker and colleagues (2008) in which it was demonstrated that the CA3/DG areas are preferentially involved in pattern separation, participants viewed objects and made an indoor/outdoor judgment and were not required to make explicit recognition memory decisions. In a study where neurologically intact participants performed an explicit recognition memory judgment, we demonstrated that the pattern of MTL responses to pattern separation demands is more complicated (Kirwan and Stark; 2007). Both pattern separation and pattern completion are involved in an explicit memory task. According to the CLS model (Norman and O’Reilly, 2003), however, the performance of patients with hippocampal damage on a yes/no recognition memory test with related lures should be differentially impaired because of the increased reliance on MTL cortex-mediated pattern completion. Our study was a direct test of this prediction and our results in general are consistent with the predictions of the model, i.e., pattern separation as indexed by the ability to uniquely identify similar lures as “similar” was impaired in the patients. The exact pattern of responding by the patient group, however, was not consistent with the model’s prediction. We hypothesized that patients’ pattern separation deficit would manifest as an increased likelihood to respond “old” to similar lures (i.e., pattern complete). While there was an increased rate of “old” responses to these stimuli for the patients relative to the controls, there was also an increased proportion of “new” responses to similar lures for the patients, particularly for the face stimuli.
While there was evidence for a deficit in the patient group’s pattern separation scores with face stimuli, this difference was driven by a different pattern of responding than the difference observed for object stimuli (Table 2). This may be due to several factors. One possibility is that the inter-item similarity for the face stimuli was overwhelming for the patients with their reduced pattern separation ability. This may have resulted in their shift in criterion to call more unrelated foils “similar”. A similar shift was observed for the control group, who responded “similar” to unrelated face foils significantly more than for unrelated object foils [t(10)=3.31, p<0.01]. Unlike the patient group, however, the control group was still able to discriminate between novel foils and related lures. A second possibility is that the orthogonalization of the representation necessary to complete the task is being carried out some place other than the MTL for the faces. Indeed, this interpretation is consistent with our previous fMRI findings which showed hippocampal activity that was consistent with pattern separation for objects, but a less clearly identifiable pattern of activity for faces (Kirwan & Stark, 2007). A recent study by Rotshtein and colleagues (2005) confirms that cortical regions including the fusiform face area (FFA) show a non-linear response as faces smoothly morph from one to another. These results are consistent with the FFA establishing a pattern-separated representation of the different faces. This residual ability may be spared in the patients tested here, thus preserving their ability to perform this task.
In considering the patient group’s responses as presented in Table 2, it is apparent that Patient RS responded “similar” to lures and foils less often than the other two patients, particularly for the face stimuli. Volumetric analysis of Patient RS’s MRI scans revealed parietal lobe reductions, which may have influenced performance on this memory task (Wagner, et al., 2005). It is unclear, however, whether this reflects an overall response bias concerning the “similar” response, or parietal-dependent memory-processing deficit as his responding on the recognition memory test (baseline) was within the normal range.
In conclusion, we found evidence that damage to the hippocampus impairs pattern separation. These results are consistent with the predictions of computational models of MTL function and a growing body of electrophysiological and neuroimaging data.
Supplementary Material
Memory-impaired patients and control participants
Patients had intact recognition memory for objects and faces
Patients had impaired pattern separation performance
Acknowledgments
We would like to acknowledge Dr. Barry Gordon for assistance with recruitment. Grant Sponsor: NSF BCS-0544959.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Gold JJ, Hopkins RO, Squire LR. The Neuroanatomy of Remote Memory. Neuron. 2005;46:799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Wixted JT, Hopkins RO, Squire LR. Yes/no recognition, forced-choice recognition, and the human hippocampus. The Journal of Cognitive Neuroscience. 2008;20:505–512. doi: 10.1162/jocn.2008.20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: Gist-based memory distortion in Alzheimer’s disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Desikan R, Daffner KR, Schacter DL. Perceptual false recognition in Alzheimers disease. Neuropsychology. 2001;15:230–243. [PubMed] [Google Scholar]
- Burgess N, O’Keefe J. Neuronal computations underlying the firing of place cells and their role in navigation. Hippocampus. 1996;6:749–762. doi: 10.1002/(SICI)1098-1063(1996)6:6<749::AID-HIPO16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Butters N. The role of interference and encoding in the short-term memory deficits of Korsakoff patients. Neuropsychologia. 1972;10:89–95. doi: 10.1016/0028-3932(72)90045-0. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. The Clinical Neuropsychologist. 1998;12:482–486. [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. Journal of Experimental Psychology. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- Duff MC, Warren DE, Gupta R, Vidal JP, Tranel D, Cohen NJ. Teasing apart tangrams: Testing hippocampal pattern separation with a collaborative referencing paradigm. Hippocampus. doi: 10.1002/hipo.20967. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: A double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15:808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: How the dentate gyrus, CA3 and CA1 process spatial information. Behavioral Neuroscience. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble B. Free recall and recognition in a network model of the hippocampus: Simulating effects of scopolamine on human memory function. Behavioral Brain Research. 1997;89:1–34. doi: 10.1016/s0166-4328(97)00048-x. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O’Reilly RC, Norman KA. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M, Winocur G. Response competition and interference effects in paired-associate learning by Korsakoff amnesics. Neuropsychologia. 1980;18:541–548. doi: 10.1016/0028-3932(80)90155-4. [DOI] [PubMed] [Google Scholar]
- Kirwan BC, Gilbert PE, Kesner RP. The role of the hippocampus in the retrieval of a spatial location. Neurobiology of Learning and Memory. 2005;83:65–71. doi: 10.1016/j.nlm.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learning & Memory. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W, Verfaellie M, Schacter DL. Recognizing identical versus similar categorically related common objects: further evidence for degraded gist representation in amnesia. Neuropsychology. 2001;15:268–289. [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learning & Memory. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M, Moser EI. Pattern Separation in Dentate Gyrus and CA3 of the Hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent Codes for Spatial and Episodic Memory in Hippocampal Neuronal Ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser M, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L. Implicit memory is not immune to interference. Psychological Bulletin. 2001;127:618–628. doi: 10.1037/0033-2909.127.5.618. [DOI] [PubMed] [Google Scholar]
- Martinez AM, Benavente R. CVC Technical Report #24. 1998. The AR Face Database. [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. Journal of Neuroscience. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12:325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Pickering A, Fairbairn A. Amnesic sensitivity to proactive interference: its relationship to priming and the causes of amnesia. Neuropsychologia. 1987;25:211–220. doi: 10.1016/0028-3932(87)90132-1. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences. 1987;10:408–415. [Google Scholar]
- Nordstrom MM, Larsen M, Sierakowski J, Stegmann MB. The IMM Face Database: An Annotated Dataset of 240 Face Images. Vol. 2005. Informatics and Mathematical Modelling, Technical University of Denmark; 2004. [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary learning systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology Learning, Memory, and Cognition. 1995;12:803–814. [Google Scholar]
- Rolls ET. Functions of neuronal networks in the hippocampus and neocortex in memory. In: Byrne JH, Berry WO, editors. Neural models of plasticity: Experimental and theoretical approaches. San Diego, CA: Academic Press; 1989. [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Treves A. Neural networks and brain function. Oxford: Oxford University Press; 1998. [Google Scholar]
- Rotshtein P, Henson RNA, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissocates physical and identity face representations in the brain. Nature Neuroscience. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Anes MD, Racine C. When true recognition supresses false recognition: evidence from amnesic patients. Journal of Cognitive Neuroscience. 1998;10:668–679. doi: 10.1162/089892998563086. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learning & Memory. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & Memory. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Underwood BJ. False recognition produced by implicit verbal responses. Journal of Experimental Psychology. 1965;70:122–129. doi: 10.1037/h0022014. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Rapcsak SZ, Keane M, Alexander MP. Elevated false recognition inpatients with frontal lobe damage is neither a general nor a unitary phenomenon. Neuropsychology. 2004;18:94–103. doi: 10.1037/0894-4105.18.1.94. [DOI] [PubMed] [Google Scholar]
- Wagner A, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Science. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Warrington E, Weiskrantz L. The effect of prior learning on subsequent retention in amnesic patients. Neuropsychologia. 1974;12:419–428. doi: 10.1016/0028-3932(74)90072-4. [DOI] [PubMed] [Google Scholar]
- Warrington E, Weiskrantz L. further analysis of the prior learning effect in amnesic patients. Neuropsychologia. 1978;16:169–177. doi: 10.1016/0028-3932(78)90104-5. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. Heightened interference on implicit, but not explicit, tests of negative transfer: Evidence from patients with unilateral temporal lobe lesions and normal old people. Brain and Cognition. 1996;30:44–58. doi: 10.1006/brcg.1996.0004. [DOI] [PubMed] [Google Scholar]
- Winocur G, Weiskrantz L. An investigation of paired-associate learning in amnesic patients. Neuropsychologia. 1976;14:97–110. doi: 10.1016/0028-3932(76)90011-7. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.