Abstract
Cell therapy has been shown as a potential treatment for stroke and other neurological disorders. Human umbilical cord blood (HUCB) may be a promising source of stem cells for cell therapy. The most desired outcomes occur when stem cells cross the blood brain barrier (BBB) and eventually reach the injured brain site. We propose, from our previous studies, that mannitol is capable of disrupting the BBB, allowing the transplanted cells to enter the brain from the periphery. However, when the BBB is compromised, the inflammatory response from circulation may also be able to penetrate the brain and thus may actually exacerbate the stroke rather than afford therapeutic effects. We discuss how an NF-kB decoy can inhibit the inflammatory responses in the stroke brain thereby reducing the negative effects associated with BBB disruption. In this review, we propose the combination of mannitol-induced BBB permeation and NF-kB decoy for enhancing the therapeutic benefits of cell therapy in stroke.
Keywords: Stem cells, transplantation, cerebral palsy, neurotrophic factor, blood brain barrier, mannitol, NF-kB
Introduction
Cell therapy continues to be an experimental therapy for neurodegenerative and hematologic disorders [1-3]. Many diseases, including those characterized by inflammatory, traumatic, degenerative and autoimmune alterations, have been examined as target indications for stem cell application.
Stroke is the third main cause of death and the first cause of chronic disability in the United States [4]. Unfortunately, treatment for stroke remains limited. To date, the only FDA-approved treatment for ischemic stroke is tissue plasminogen activator (tPA), which has a narrow time window of only approximately 4.5 hours after the onset of stroke symptoms. Approximately only 3-5% of all patients that suffer a stroke benefit from tPA, primarily due to the narrow therapeutic window [5]. The lack of available therapies and the devastating effects of these diseases compel researchers and clinicians to find more effective treatments.
Transplantation of stem cells has shown great potential as an alternative therapy for stroke, especially with its wider therapeutic window. Investigations into the mechanisms underlying stem cell-mediated functional recovery have focused on the transdifferentiation of stem cells, but a majority of these studies have failed to show that stem cells of hematologic origin are able to differentiate into a neuronal phenotype [6, 7]. Therefore, the therapeutic efficacy of these cells has been proposed to be achieved via the chaperone effect. Due to their logistical advantages (e.g., low risks to baby and mother, ample supply of cells, and very young donor age) and minimal ethical concerns, human umbilical cord blood (HUCB) cells seem as an ideal source for cell therapy [8].
The two major mechanisms implicated in the functional recovery exerted by cell therapy advance the concepts that transplanted cells: a) actually replace the damaged host cells and b) serve as transport vehicles to deliver neurorestorative substances such as neurotrophic, angiogenic/vasculogenic, and anti-inflammatory factors. In many studies utilizing stem cells for transplantation very few grafted cells survive, but neurological deficits are attenuated in both animal models and patients with central nervous system (CNS) disorders [9, 10]. Indeed, functional recovery in stroke ensues without graft cell entry into the CNS, granting that therapeutic molecules exuded by these cells crossed the BBB and reach the injury site or alternatively promote recovery via a peripheral mechanism such as an effect upon the spleen [11, 12].
The aim of this review is to analyze the preclinical evidence that shows transplantation of HUCB cells delivered in conjunction with mannitol via intravenous delivery presents as an effective and efficient method for delivery of neuroprotective trophic factors to the site of injury in the brain for the amelioration of stroke-related deficits [13]. Further, new research has shown promising results regarding the neuroprotective effect of the inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) which reduces deleterious inflammation at the injury site [14]. Because the permeation of the BBB with mannitol may allow systemic inflammation to exacerbate stroke, the combination of HUCB cell transplantation and NF-κB should be considered to realize the mannitol- facilitated CNS entry of stem cell-derived neurotrophic factors while suppressing the deleterious effects of peripheral inflammatory response.
Blood Brain Barrier: The Pivotal Barrier for CNS Homeostasis
The blood-brain barrier provides physical protection and regulates homeostasis within the brain [15]. The selective nature of this barrier is vital to protect the CNS and maintain its essential functions. The BBB is comprised of endothelial cells, which restrict the movement of molecules into the brain. The BBB consists of not only endothelial cells but also tight junctions and astrocytic endfeet, which support the selective permeability of this barrier.
Due to the fact that molecules larger than 400 daltons are unable to cross the BBB, strategies to disrupt the BBB have been developed to allow for the delivery of therapeutic agents to the CNS [16-17]. Intracerebral delivery of stem cells has been utilized to physically bypass the BBB, allowing cell deposits into specific regions of the injured brain. However, the invasiveness of intracerebral transplantation may pose trauma and surgical complications to the patient, suggesting that a minimally invasive transplant procedure may be more practical in the clinic [18]. The challenge for a minimally invasive peripheral administration route is the presence of the BBB. For the transplanted cells to optimally penetrate the BBB and enter the brain from the periphery, the administration of a BBB permeabilizer, such as mannitol, may be needed as an adjunct therapy. The infusion of hypertonic solutions can also be used for chemical disruption of the BBB [16]. These agents act by shrinking the endothelial cells that comprise the BBB and stretch the tight junctions, an integral part of the barrier [16]. BBB disruption can result from the delivery of agents such as mannitol, glucose, prodrugs, molecular Trojan horses, and polymeric nanoparticles [14, 19, 20].
Mannitol has been coupled with HUCB cells delivered intravenously to increase the permeability of the BBB to macromolecules [12, 14]. However, with the BBB already compromised after stroke and the secondary injury ensuing thereafter including systemic inflammation contributing to the disease progression [14], the treatment with mannitol may have an unintended consequence of further permeabilizing the BBB that can lead to additional CNS recruitment of inflammatory response from the periphery. Inflammation is an immediate response to injury due to the activation of transcription factors [21], thus disrupting the BBB requires regulation of the inflammatory response generated by the injury and the agent which disrupts the BBB.
Acute inflammation accompanies the early stages of stroke which can result in detrimental side effects including contradicting the therapeutic benefits of stem cell therapy. Any treatment regimen must address the inflammatory response to injury, which may be exacerbated by mannitol-induced BBB permeation. Approaches focusing on the inhibition of signaling pathways involved in the inflammatory response may be a viable option [14]. We have subdivided this review paper into these two major topics, namely the use of mannitol for BBB permeation to aid the CNS entry of peripherally administered stem cells, and the use of NF-κB to abrogate the systemic inflammation as a result of BBB permeation.
The Challenge: BBB Permeation for Selective CNS Entry of Stem Cells While Blocking Inflammatory Cells
The restrictive permeability of the BBB makes delivery of stroke therapies to the brain from peripheral sites challenging [14]. The BBB can be temporarily disrupted via osmotic means [14]. Mannitol works by shrinking endothelial cells and stretching tight junctions thus allowing the penetration of molecules through the membrane of the BBB [14]. Although BBB permeation may facilitate stem cell transplantation via the peripheral route, such BBB damage may also stimulate systemic inflammation. Inflammation is a natural immune response, however it can overshadow therapeutic benefits and may even exacerbate stroke [14]. Therefore, strategies must be designed to combat inflammation by arresting the systemic inflammatory response while still allowing the entry of stem cells and therapeutic drugs into the brain [8].
The transcription factor NF-κB plays a key role in endothelial cell activation and the inflammatory response [14]. Endothelial cells are a main component of the BBB, while NF-κB activation leads to an inflammatory response in BBB. New therapeutic approaches work to inhibit NF-κB activation in the BBB and thus allow entry of molecules through the membrane without subsequent inflammation.
Ischemic Injury: Its Current Status and Potential Treatments with Stem Cells
Ischemic stroke occurs when oxygen and blood to the brain have been disrupted by obstruction, usually as a consequence of plaque or other fatty deposits. It is estimated that approximately two million brain cells die every minute that the tissue is deprived of oxygen and glucose [22]. Permanent damage and even death may result if blood flow is not restored to the penumbra in a timely manner. Due to the limited treatment options for stroke, different therapeutic modalities are being explored for stroke. Although tPA helps protect from ischemic damage, it unfortunately has a limited therapeutic window of approximately 4.5 hours post-ischemia.
As mentioned earlier, stroke is the third cause of death in the United States and is the main cause of disability [22]. Drug therapy has a dismal record for the treatment of stroke in the clinical setting. The narrow therapeutic window for therapeutic intervention in stroke is partially due to the fact that therapeutics cannot cross the BBB therefore they cannot enter the brain. Stem cells may have a wider therapeutic window than the current 3 hour therapeutic window of tPA, but are subject to the same logistical limitations of drug therapies in terms of crossing the BBB. Stem cells that are administered peripherally (i.e. intravenously) may not be able to cross the BBB and enter the brain. Strategies designed to achieve BBB permeation are needed for optimal therapeutic efficacy of stem cells for stroke.
Stem cell based therapy is currently being explored in many neurological diseases. Embryonic stem cell characteristics include high plasticity, trophic support, and proliferation [23, 24]. However, due to the high proliferative capacity of embryonic stem cells, they also carry the risk of tumor formation following transplantation. Unlike embryonic stem cells, adult stem cells display low risk of tumor formation, thus they may be a better stem cell source for transplantation. Emerging evidence suggests that adult stem cells have neurorestorative capacity, extending the time window of opportunity they offer for rehabilitation [25]. Neurorestoration is advantageous later on as treatment due to its stable baseline deficit [25]. Thus, the stroke patients that missed the narrow tPA therapeutic window may benefit from neurorestorative therapies.
Stem cell delivery in stroke by intravascular methods is less invasive than intracerebral transplantation. For patients that seek therapy during the acute stages of stroke may benefit from intravenous delivery since chemoattractant cues at this early stage of the disease are highly upregulated and may stem cells to migrate from the periphery to the brain [25]. Intracerebral delivery of stem cells has shown to be more advantageous in the chronic phase of stroke since the signaling pathways that attract the stem cells to the injured brain may have already waned [25].
Since the peripheral method of transplantation is preferred and is a more feasible approach in the clinical setting, overcoming the BBB restrictive features are vital to improving this therapeutic method. Hence, the ultimate goal is to get the stem cells across the BBB without the risk of inflammation or rejection so that they may initiate the repair process immediately after stroke onset. HUCB cells are explored in this review based on our long-standing interest in this easily accessible stem cell population, as well as minimal ethical concerns [12, 13, 19, 26].
HUCB as An Efficacious Stem Cell Source for Transplant Therapy in Stroke
As noted above, two schools of discipline advance the concept of therapeutic benefits of cell therapy, namely cell replacement and bystander “neurotrophic factor” effects. In stroke, neuroprotection can be provided by systemic delivery of HUCB cells intravenously [12]. This neuroprotection is provided by the neurotrophic factors released by the grafted cells whether by direct effect at the injury site or peripherally [6]. HUCB cells do not cross the BBB when delivered peripherally therefore common evaluation techniques, such as visualization of the stem cells within the brain could not be utilized [12]. Instead, researchers use infarct volumes, behavioral testing, immunohistochemistry, ELISA, and blood concentration levels of neurotrophic factors such as GDNF, NGF, and BDNF to evaluate the effect of HUCB cells as a treatment for acute ischemic stroke [12]. Neurotrophic factors are considered neuroprotective and must also cross the BBB in order to reach injured brain tissue [23]. Intravenous delivery of HUCB cells was evaluated using four treatment protocols: HUCB cells (200,000 cells) with mannitol; HUCB cells with phosphate buffered saline (PBS); PBS alone (vehicle); and mannitol alone. HUCB cells were delivered by injection into the jugular vein over 10 minutes [12]. PBS was also delivered through the jugular vein for 10 minutes. Immediately following the initial injection, mannitol or PBS was delivered over 5 minutes to the HUCB group and the PBS group [12]. This intravenous delivery in animals was carried out immediately during the sixty minute occlusion [12]. This type of protocol may not be feasible in the clinic; however, other studies have tested HUCB delivery at 24 and 72 hours post stroke [12, 14].
The HUCB grafted cells improved motor and cognitive performance [12]. Behavioral testing was done at day 3 after stroke because it was determined that at this time point the infarct volume was at its maximum [12]. These tests measured motor asymmetry by using the elevated body swing test (EBST) and passive avoidance test to measure cognitive performance by acquisition time, and retention time of tasks. Cognitive and motor deficits were reduced in animals treated with HUCB/mannitol [12]. Reduced cerebral infarct volumes were also seen in this experiment although mannitol was not shown to reduce edema [12].
Furthermore, there was an absence of detectable HUCB grafts in the stroke brain. GFP-labeled HUCB cells were not found in the brain of the stroke animals [12]. Immunohistochemistry was also employed to detect the human-specific N-CAM in the brain. This test also revealed that no HUCB cells resided in the brain of the subject animals. Thus the grafted cells did not cross the BBB. This was true for both HUCB treatment groups [12].
Although HUCB cells were not detected in the brain they were found in three organs. Human N-CAM positive cells were discovered in these peripheral tissues at day 3 [12]. This was found in both HUCB groups. HUCB cells show sufficient mobility and growth in tissue. Thus, HUCB cells can travel to the brain; however they cannot infiltrate the BBB to access the ischemic site.
HUCB Graft Neuroprotection by Trophic Factor Mediation
In the pioneering stroke study using stem cell therapy and mannitol [12], HUCB cells did not penetrate the BBB, however cognitive improvements were shown. These results suggest that HUCB cells either produced a direct trophic effect on the damaged tissue by secreting neurotrophic factors in to the blood stream or the HUCB cells elevated the endogenous levels of trophic factors by inducing existing cells to produce neurotrophic factors. Elevated brain levels of glial cell line-derived neurotrophic factor (GDNF) were found at day 3 in HUCB/mannitol treated rats [27]. GDNF plays a key role in neuroprotection and its presence in the brain has been correlated with reduced cognitive and motor deficits post stroke. This was tested by exposing HUCB cells to antibodies against GDNF, nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF) before transplantation [27]. The HUCB/mannitol treatment was given as before and the benefits of this treatment were ameliorated by the antibodies, thus blocking the protective effects [12]. This experiment lends evidence to a direct link between the neurotrophic factors and neuroprotection.
The next step was to determine the source of the trophic factors. In order to show that HUCB cells are an effective treatment option even in when administered peripherally, the trophic factors must be traced back to the HUCB cells. This was determined by the blood concentration of each trophic factor measured in circulating blood. The concentrations of control animals to HUCB treated animals were compared to determine if endogenous cells or the grafted cells are the source of the trophic factors. Enzyme-linked immunosorbent assay (ELISA) found low, yet detectable levels in comparison to the control group [12]. This concentration was only found in the [13] HUCB/mannitol treatment group, no detectable levels were found in the other three groups [12]. The peripheral organs that tested positive for HUCB graft cells were tested for the presence of trophic factors as well. Both HUCB treatment groups showed significant elevations of trophic factors above the control group. The vehicle and mannitol only groups showed a low detection above the control groups [12].
Stem Cell Therapies in Animal Models of Neonatal Hypoxic-Ischemic Injury
Neonatal hypoxia-ischemia (HI) brain injury defines in neonatal encephalopathy, neonatal stroke cerebral palsy, and is a major cause of disability in neonates [29]. HI brain injuries are considered acute ischemic insults [29]. Neonatal hypoxia-ischemia brain injury in rat models was developed by Vannucci [29-31]. This modal was developed to experimentally mimic neonatal brain injury [29]. One of the treatments in neonatal encephalopathy includes mild systemic hypothermia [32-34]. Acute ischemic injuries to the brain involve cell therapy that is preferably administered via intravenous injection [35]. While acute injuries focus on neurorestorative elements, HUCB cells administered peripherally may also provide neuroprotection [35,36].
As in adult stroke animal models, the age of the animal plays a key role in brain plasticity [29]. The brain plasticity of young animals influences the outcome of HI injuries [29] and may affect the therapeutic outcome of stem cell treatment [29]. Gender of the neonate is also an important factor in the outcome of HI injury recovery [29]. Even without treatment, young female rats show smaller infarct volumes compared to their male counterpart males [29]. Unlike adult stroke injury, spontaneous cognitive recovery during the early stages of insult can occur in neonatal brain injuries [35]. This spontaneous recovery must be accounted for in behavioral testing by long-term monitoring and measured evaluation [35].
Similar to the concept of combined stem cell therapy and mannitol in adult stroke, this experimental treatment has been tested in neonatal HI [35]. Intravenous administration of HUCB cells 24-72 hours after injury with a dose as low as 200,000 cells can produce significant behavioral improvements [13]. Further work in HUCB cell therapy in neurological disorders is warranted.
When transplanted with HUCB cells, mannitol was demonstrated to improve behavioral recovery of HI damaged animals via a series of motor tests conducted 7 and 14 days after transplantation [13]. However, neither mannitol nor vehicle alone showed any cognitive improvement. This study shows strong evidence that mannitol combined with HUCB cells enhances the recovery of neurologically injured animals. Furthermore, ANOVA analysis demonstrated that in transplanted HI injured animals, CNS growth factors were upregulated by mannitol combined with HUCB [13]. This combination of HUCB with mannitol increased GDNF, NGF, BDNF growth factors levels in the CNS and other trophic factors, which may have a role in the observed functional improvement as previously mentioned [13]. However, no trophic factors were detected in the peripheral blood nor were HUCB survival increased with mannitol treatment. Both of these findings support the hypothesis that the therapeutic outcome detected is not based on graft survival per se but through the mechanism of entry of neuroprotective and neurorestorative cells into the CNS and that the functional recovery seen was mediated by the increased of trophic factors after injection of HUCB with mannitol in HI injured animals.
Functional improvement was observed in HUCB cell transplantation with mannitol were greater than the improvements observed in IV administered HUCB cells alone. The EBST and Rotarod tests showed amelioration of behavioral deficits by 40-50% through improvements observed in motor asymmetry and motor coordination 7 and 14 days after transplantation of HUCB with mannitol [13]. Extended examination of motor tasks should be explored further and be used to determine the optimal therapeutic conditions for HUCB and mannitol transplantation in HI. Also, investigations in cognitive abilities by testing learning and memory should also be explored in HI models with HUCB and mannitol treatment.
In both adult stroke and neonatal HI, mannitol significantly reduced the therapeutic effective dose of HUCB. For the neonatal HI, the cell dose is 15,000 [13] which is lower compered to the cell dose of 200,000 we used in the previous study [12]. When these doses are combined with mannitol they mirror the effects that were reported with higher doses (>500,000) without mannitol [28, 36]. The possible explanation could be the high capability of plasticity of the neonatal brain as compared to the adult brain. Whereas the initial protocol was for the donor cells to be injected shortly after ischemic injury, it was identified that cerebral palsy does not occur until a few day or weeks after birth. Therefore, the time of transplantation was adjusted to strengthen the clinical application of this potential treatment cerebral palsy. We demonstrate that even one week after injury, mannitol allowed for the therapeutic effects of HUCB to occur [12], suggesting that BBB passage in neonates is key for the functional outcome via peripheral administration.
As noted above, our previous study in adult stroke indicated that the BBB opening produced by the insult was not enough to allow trophic factors to secrete into the brain, thus exogenous manipulation was needed [13]. BBB may not completely be developed in the neonatal rats but is still capable of blocking factors from entering as proved in the neonatal animals that received HUCB cells alone without mannitol. Conversely, mannitol with HUCB cells when transplanted into neonatal rats showed an increase in CNS levels of growth factors, inferring that even an incomplete BBB needs to be bypassed to allow entry of growth factors. A BBB disturbed by mannitol aids in mobilization of graft derived trophic factors to be in the brain for their desired outcomes. Experimentation shows strong evidence that HUCB grafts were the source of growth factor levels in the brain based on the lack of those growth factors in HI animals that received vehicle or mannitol solely.
The HI neonatal rat model [22, 30-31] used in our study [13] has mimicked the damage and malformations seen in cerebral palsy newborns. HI injury leads to hippocampal cell loss and allows for potential therapeutic for cerebral palsy [37, 38]. This neurologic disorder typically occurs during childbirth but can manifest within the first two years of life and may be caused by a number of factors, including intrapartum asphyxia, genetic syndromes, brain infections, head injury, accidents, abuse, or neglect [38-44]. Currently there is no cure for cerebral palsy, but treatments such as drugs or rehabilitaation therapy have proven effective in reducing the symptoms [43]. Accordingly, these neonatal HI results [13], combined with the adult stroke study [12], suggest that BBB permeation by mannitol advances the therapeutic potential of intravenous transplantation of HUCB cells for cerebral palsy. Indeed, clinical trials of HUCB transplantation have been initiated in cerebral palsy patients, and it may be envisioned that mannitol as an adjunct therapy may further improve the clinical outcome.
Despite the many advantages of HUCB cells for both adult stroke and neonatal HI, we are cognizant of the inflammatory responses from the systemic circulation that may be exacerbated by strategies designed to manipulate the BBB permeability. To this end, we propose the use of an anti-inflammatory approach that may be used as an adjunct therapy to HUCB transplantation and mannitol treatment.
Potential Therapeutic Benefits Of Mannitol And NF-KB
Mannitol is an obligate extracellular solute that is used as a delivery system for drugs or therapeutic agents directly into the brain [45]. Mannitol works by shrinking endothelial cells hyperosmotically, and this in turn concurrently stretches the tight junctions allowing for the passage of molecules through the membrane [45]. The optimal time for drug or cell delivery with the use of mannitol is about 30 minutes [45]. As discussed above, mannitol has assisted peripheral stem cell delivery to the adult and neonatal injured brain as a hypertonic agent. However, to counteract the unintended con sequence of mannitol administration, inhibition of signaling pathways associated with inflammation have been studied to enhance the therapeutic potential of combined mannitol and stem cell therapy in ischemic models. During ischemic brain injury, specific genes that induce inflammation are transcribed. Following hypoxic or ischemic brain insult, endothelial cells are a major target of CNS injury. When the endothelial cells are damaged by stroke, cerebral blood flow may be halted leading to a compromised BBB resulting in leakage and hence increased permeability and edema. Post ischemic insults to the endothelial cells may also increase leukocyte adhesion and consequently activate an inflammatory response [21]. Pro-inflammatory transcription factors are activated post stroke and include NF-kB which code for inflammatory genes and cytokines. Secondary injury is targeted for post-stroke treatment and a way to manage this is by inhibiting the inflammatory response associated with stroke [21].
Swelling and inflammation occurs briefly after stroke in ischemic areas [46]. This inflammatory result is characterized by microglial activation, edema, and leukocyte infiltration, [44, 47, 48] and is enhanced by cell migration from the blood which coalesce with release of cytokines, free radicals, and microglial and astrocytic activation. With all of these responses working together, the neurotoxicity is amplified and negates the healing effects that inflammation is supposed to have, and has been suggested that it promotes nervous tissue secondary damage [49].
A novel approach to suppress this inflammatory response is to inhibit NF-kB activation so that these inflammatory factors are not encoded for initiation of inflammation. ICAM-1 is the target gene that is explored for this inhibition. ICAM-1 expression by endothelial cells results in enhanced leukocyte adhesion and permeation of BBB [21]. An oligonucleotide decoy, which bears a DNA binding sequence for NF-kB, has been shown to compete with NF-kB for binding. Transcriptional decline of NF-kB was achieved by this decoy and consequently ICAM-1 transactivation was also diminished. This study used mannitol to assist the delivery of the decoy into the endothelial cells, since it is known to disrupt the BBB and so that inhibition could occur. It can be envisioned that once inflammation has subsided, injection of HUCB can be employed to enhance the repair of functionality in the damaged regions of the brain Fig. (1). This innovative strategy advances the notion that stem cell therapy with mannitol and NF-kB decoy may allow a highly regulated entry of stem cells across the BBB, while reducing the stroke-induced inflammatory and therefore the secondary damage associated with the disease evolution.
Fig. (1).
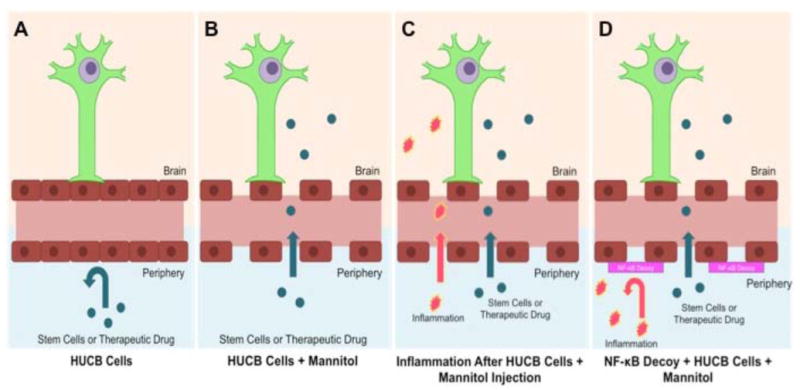
The blood brain barrier under normal condition limits access of many stem cells and therapeutic drugs from the periphery to the brain (A). Following stroke, BBB breakdown ensues, allowing entry of stem cells into the brain which is enhanced by mannitol injection (B). However, stroke-induced BBB leakage may also allow entry of pro-inflammatory molecules thereby exacerbating stroke pathology (C). The combination of mannitol-aided stem cell transplantation with NF-kB decoy may lead to an improved regulation the BBB by allowing stem cells to penetrate the brain, while reducing the stroke-induced inflammatory response.
Strategies to inhibit the molecular mechanisms that cause inflammation after stroke have been studied [14]. Specific transcription factors were employed to inhibit the expression of trophic factors that are known to produce inflammation. In vitro culture of two cell lines under cytokine-induced and hypoxia-reoxygenation (H/R) induced inflammation were used to measure the protein expression and effect of inhibition. These inflammations are caused by the expression of ICAM-1. Researchers attempted to create a targeted genetic disruption of NF-κB which leads to the activation in human brain microvascular endothelial (HBME) cells and IT-1 cell lines. NF-κB plays a key role in endothelial cell activation. Endothelial cell activation is part of a normal inflammatory response. Reducing the inflammatory response after stroke can produce neuroprotective effects [14]. NF-kB activation through cytokine or H/R induced inflammation results in the upregulation of ICAM-1 [14]. The ICAM-1 molecule is involved in the immune response by stabilizing cell signaling which induces inflammation [14]. TNF-α is a cytokine which induces the activation of NF-κB. Three types of NF-κB decoys (TFD) were employed to determine which would best manipulate ICAM-1 transcription and thus reduce inflammation [14].
ICAM-1 is one of the inflammatory endothelial genes in the human genome. The transcription of these genes leads to the activation of endothelial cells. Once endothelial cells are activated, they upregulate the surface expression of cell adhesion molecules and secretes cytokines. Cytokines act by sequestering neutrophils in the ischemic zone [14]. Since many pathways are involved in the gene expression of inflammatory endothelial cells, NF-kB was identified as a common pathway involved in the upregulation of multiple genes because it binds to a number of genes involved in the inflammatory response. In the resting state, NF-kB is bound by an inhibitor, IkB, in the cytoplasm. After phosphorylation by IKK, IkB is degraded by the proteasome [10]. This degradation of IkB releases NF-kB, which travels to the nucleus where it binds to ciselements in the promoters of genes involved in the inflammatory cascade [14]. Transcriptions factor “decoys” (TFD) are double-stranded oligodeoxyribonucleotides that compete with endogenous proteins for the binding at the regulatory regions of gene promoters for the binding of transcription factors [14].
The IT-1 and cultured HBME cell lines were exposed to three treatment protocols to determine specificity of TFD to the host DNA: NF-kB TFD, a mutated NF-kB TFD, and a scrambled TFD. The mutated NF-kB TFD contained a mutated sequence with a change in 3 bases in the NF-kB binding site and the scrambled TFD contained a scrambled genetic sequence at the binding site. The sequences reflect specific binding of NF-kB, rather than the nonspecific effects of the full oligonucleotide. Both cell lines were subjected to hypertonic loading with mannitol, which allowed the TFD proteins to pass through the plasma membrane. Mannitol was able to transfect the TFD into the endothelial cell nuclei [14]. After a recovery period, cells were stimulated with TNF-α or placed in the hypoxia chamber [14]. Although the TFD was able to permeate into the nuclei of all cell lines, only the NF-kB TFD was able to competitively bind to the host DNA and inhibit gene expression. The inhibition of NF-kB blocks the upregulation of ICAM-1. This was shown through detecting ICAM-1 mRNA levels through Northern Blot analysis and the specificity of NF-kB TFD binding and surface expression of ICAM-1 was determined through electrophoretic mobility shift assay (EMSA).
Targeting inflammation by interrupting the signal cascade is an approach that is commonly used for various therapies. Inhibiting NF-kB, with the use of an oligonucleotide decoy, reduces the levels of ICAM-1 in the nucleus and on the surface of endothelial cells. Loss of this protein reduces the harmful inflammatory response usually activated after a stroke. Since the source of inflammation may not affect the inhibitory action of NF-kB decoy, this treatment can likely attenuate stroke-induced central and systemic inflammation.
Conclusions
Cell therapies are a revolutionary treatment for neurodegenerative diseases. Similar to traditional drug therapies, experimentation must determine many factors for the safety and efficacy of stem cell treatments such as the correct dose, delivery route, and optimal time of intervention [50]. Stem cell lines must be validated through multiple laboratories with different animal models before clinical trials can be authorized [50]. This year, the National Institute of Health is studying the safety and efficacy of stem cells used as treatment for stroke in clinical trials at various stages of recruitment and completion [51]. Eight stem cell products are being evaluated in these trials [50]. Laboratory experimentation is crucial to confirm the mechanisms by which particular stem cells, like HUCB cells, provide their neuroprotective as well as neuroregenerative effects in stroke treatment [52-56]. The key to maintaining parallel and thus confirmatory experimentation requires the use of standard protocols for stem cell therapies. The experimental design paradigms created by Stroke Therapy Academic Industry Round Table (STAIR) and Stem cell Therapeutics as an Emerging Paradigm in Stroke (STEPS) have developed guidelines imperative to successful translation of stem cell treatments from the lab to the clinic [52-56]. Future work should focus on HUCB cell treatment which includes mannitol and NF-κB decoys in small and large animal models. These animal models must be fully tested to determine possible side effects of the inhibition of NF-κB. Experiments must isolate the effects of mannitol alone and the NF-κB decoy while monitoring the system in vivo. Other transcription factor decoys should also be identified and tested for use with stem cell therapy. Signaling pathways are complex communication systems that require careful navigation when inhibition is triggered upstream as in the case of NF-κB. Guidelines put forth by STAIR and STEPs must be carefully considered throughout the lab-to-clinic translational process.
Experimentation with mannitol has shown significant improvement in cell therapy. Mannitol is an effective BBB permeabilizer that has been shown to be effective at lower concentrations and may allow for the earlier entry of stem cells into the injured brain. HUCB in conjunction with mannitol and NF-κB decoys may serve as a valuable method of neuroprotection and neurorestoration. Additional laboratory studies are warranted to examine the safety and efficacy of the combination of HUCB transplantation with mannitol and NF-κB decoys, including optimization of the optimal dosage, route, and timing of delivery of the combination therapy after stroke.
Although this review focuses on stem cells in combination with mannitol and NF-κB as a potential therapeutic for ischemic stroke, the same method of BBB permeabilization can similarly be applied to facilitate the entry of therapeutic drugs from the periphery to the diseased brain, while suppressing systemic inflammation. In addition, whereas this review focuses on stroke, other CNS disorders characterized by BBB damage may benefit from the peripheral administration of stem cells with NF-κB decoy treatment.
Acknowledgments
DCH is funded by NIN NS055728
Footnotes
Conflict Of Interests: PRS and CVB are Founder and Consultant, respectively, for Saneron CCEL Therapeutics, Inc. Both are inventors on patent applications related to stem cell research. CVB is supported by NIH NINDS R01NS071956-01, James and Esther King Foundation for Biomedical Research Program, SanBio Inc., Celgene Cellular Therapeutics, KMPHC and NeuralStem Inc.
References
- 1.Blau H, Brazelton T, Keshet G, Rossi F. Something in the eye of the beholder. Science. 2002;298:361–2. doi: 10.1126/science.298.5592.361c. [DOI] [PubMed] [Google Scholar]
- 2.Mezey E, Nagy A, Szalayova I, et al. Comment on “Failure of bone marrow cells to transdifferentiate into neural cells in vivo”. Science. 2003;299:1184. doi: 10.1126/science.1079318. [DOI] [PubMed] [Google Scholar]
- 3.Theise ND, Krause DS, Sharkis S. Comment on “little evidence for developmental plasticity of adult hematopoietic stem cells”. Science. 2003;299:1317. doi: 10.1126/science.1078412. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morbidity and Mortality Weekly Report. 2001;50:120–5. [PubMed] [Google Scholar]
- 5.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 6.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 7.Weimann JM, Charlton CA, Brazelton TR, Hackman RC, Blau HM. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc Natl Acad Sci U S A. 2003;100:2088–93. doi: 10.1073/pnas.0337659100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess D, Borlongan C. Stem cells and neurological diseases. Cell Prolif. 2008;41:94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VMY, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149:310–21. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- 10.Lindvall O, Brundin P, Widner H, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science. 1990;247:574–7. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- 11.Vendrame M, Gemma C, Pennypacker KR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199:191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–9. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 13.Yasuhara T, Hara K, Maki M, Xu L, Yu G, Ali M, et al. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14:914–21. doi: 10.1111/j.1582-4934.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess DC, Howard E, Cheng C, Carroll J, Hill WD, Hsu CY. Hypertonic mannitol loading of NF-kappaB transcription factor decoys in human brain microvascular endothelial cells blocks upregulation of ICAM-1. Stroke. 2000;31:1179–86. doi: 10.1161/01.str.31.5.1179. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.11.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Blanchette M, Fortin D. Blood-brain barrier disruption in the treatment of brain tumors. Methods Mol Biol. 2011;686:447–63. doi: 10.1007/978-1-60761-938-3_23. [DOI] [PubMed] [Google Scholar]
- 17.Ferber D. Bridging the blood-brain barrier: New methods improve the odds of getting drugs to the brain cells that need them. PLoS Biol. 2007;5:e169. doi: 10.1371/journal.pbio.0050169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borlongan CV, Sanberg PR, Freeman TB. Neural transplantation for neurodegenerative disorders. Lancet. 1999;353(1):S129–30. doi: 10.1016/s0140-6736(99)90229-5. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Banderas L, Holgado MA, Venero JL, Alvarez-Fuentes J, Fernandez-Arevalo M. Nanostructures for Drug Delivery to the Brain. Curr Med Chem. 2011;18:5303–21. doi: 10.2174/092986711798184262. [DOI] [PubMed] [Google Scholar]
- 20.Stonestreet BS, Sadowska GB, Hanumara RC, Petrache M, Petersson KH, Patlak CS. Comparative effects of glucose-and mannitol-induced osmolar stress on blood-brain barrier function in ovine fetuses and lambs. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.114. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park DH, Eve DJ, Musso J, III, et al. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev. 2009;18:693–702. doi: 10.1089/scd.2009.0008. [DOI] [PubMed] [Google Scholar]
- 22.Rice JE, III, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 23.Okragly AJ, Haak-Frendscho M. An acid-treatment method for the enhanced detection of GDNF in biological samples. Exp Neurol. 1997;145:592–6. doi: 10.1006/exnr.1997.6500. [DOI] [PubMed] [Google Scholar]
- 24.National Stroke Association [homepage on the Internet] Centennial. CO: National Stroke Association; [cited 2011 December 31]. Availible from: http://stroke.org. [Google Scholar]
- 25.Daar A, Bhatt A, Singer P. Stem cell research and transplantation: Science leading ethics. Transplant Proc. 2004;36:2504–6. doi: 10.1016/j.transproceed.2004.08.129. [DOI] [PubMed] [Google Scholar]
- 26.Takagi Y, Nishimura M, Morizane A, et al. Survival and differentiation of neural progenitor cells derived from embryonic stem cells and transplanted into ischemic brain. J Neurosurg. 2005;103:304–10. doi: 10.3171/jns.2005.103.2.0304. [DOI] [PubMed] [Google Scholar]
- 27.Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8:1193–201. doi: 10.1586/14737175.8.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman MB, Davis CD, Borlongan CV, Emerich D, Sanberg PR. Transplantation of human umbilical cord blood cells in the repair of CNS diseases. Expert Opin Biol Ther. 2004;4:121–30. doi: 10.1517/14712598.4.2.121. [DOI] [PubMed] [Google Scholar]
- 29.Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci. 2005;27:81–6. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- 30.Vannucci RC, Vannucci SJ. Cerebral carbohydrate metabolism during hypoglycemia and anoxia in newborn rats. Ann Neurol. 1978;4:73–9. doi: 10.1002/ana.410040114. [DOI] [PubMed] [Google Scholar]
- 31.Vannucci S, Vannucci R. Glycogen metabolism in neonatal rat brain during anoxia and recovery. J Neurochem. 1980;34:1100–5. doi: 10.1111/j.1471-4159.1980.tb09946.x. [DOI] [PubMed] [Google Scholar]
- 32.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalo-pathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 33.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 34.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 35.Carroll JE, Borlongan CV. Adult stem cell therapy for acute brain injury in children. CNS Neurol Disord Drug Targets. 2008;7:361–9. doi: 10.2174/187152708786441812. [DOI] [PubMed] [Google Scholar]
- 36.Park DH, Lee JH, Borlongan CV, Sanberg PR, Chung YG, Cho TH. Transplantation of umbilical cord blood stem cells for treating spinal cord injury. Stem Cell Rev. 2011;7:181–94. doi: 10.1007/s12015-010-9163-0. [DOI] [PubMed] [Google Scholar]
- 37.Dabney KW, Lipton GE, Miller F. Cerebral palsy. Curr Opin Pediatr. 1997;9:81–8. doi: 10.1097/00008480-199702000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Lequin MH, Barkovich AJ. Current concepts of cerebral malformation syndromes. Curr Opin Pediatr. 1999;11:492–6. doi: 10.1097/00008480-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Berger R, Garnier Y. Pathophysiology of perinatal brain damage. Brain Res Brain Res Rev. 1999;30:107–34. doi: 10.1016/s0165-0173(99)00009-0. [DOI] [PubMed] [Google Scholar]
- 40.Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther. 2006;86:1534–40. doi: 10.2522/ptj.20050397. [DOI] [PubMed] [Google Scholar]
- 41.Hermansen MC, Hermansen MG. Perinatal infections and cerebral palsy. Clin Perinatol. 2006:315–33. doi: 10.1016/j.clp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Lipson A, Gillerot Y, Tannenberg A, Giurgea S. Two cases of maternal antenatal splenic rupture and hypotension associated with Moebius syndrome and cerebral palsy in offspring. Further evidence for a utero placental vascular aetiology for the Moebius syndrome and some cases of cerebral palsy. Eur J Pediatr. 1996;155:800–4. doi: 10.1007/BF02002911. [DOI] [PubMed] [Google Scholar]
- 43.Spencer N, Devereux E, Wallace A, et al. Disabling conditions and registration for child abuse and neglect: a population-based study. Pediatrics. 2005;116:609–13. doi: 10.1542/peds.2004-1882. [DOI] [PubMed] [Google Scholar]
- 44.Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, Deans RJ, Hess DC, Carroll JE, Borlongan CV. Behavioral and histological characterization of intrahippocampal grafts of human bone marrow-derived multipotent progenitor cells in neonatal rats with hypoxic-ischemic injury. Cell Transplant. 2006;15:231–8. doi: 10.3727/000000006783982034. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda M, Bhattacharjee AK, Kondoh T, Nagashima T, Tamaki N. Synergistic effect of cold mannitol and Na(+)/Ca(2+) exchange blocker on blood-brain barrier opening. Biochem Biophys Res Commun. 2002;291:669–74. doi: 10.1006/bbrc.2002.6495. [DOI] [PubMed] [Google Scholar]
- 46.Kriz J. Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol. 2006;18:145–57. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 47.Emsley HC, Smith CJ, Tyrrell PJ, Hopkins SJ. Inflammation in acute ischemic stroke and its relevance to stroke critical care. Neurocrit Care. 2008;9:125–38. doi: 10.1007/s12028-007-9035-x. [DOI] [PubMed] [Google Scholar]
- 48.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–69. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takano T, Oberheim NA, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40:S8–S12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borlongan CV, Weiss MD. Baby STEPS: a giant leap for cell therapy in neonatal brain injury. Pediatr Res. 2011;70:3–9. doi: 10.1203/PDR.0b013e31821d0d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clinical Trials [homepage on the internet] Washington, D.C.: ClinicalTrials.gov; [cited 2011 December 15]. Available from: http://clinicaltrials.gov. [Google Scholar]
- 52.Borlongan CV, Chopp M, Steinberg GK, et al. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med. 2008;3:249–50. doi: 10.2217/17460751.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borlongan CV. Cell therapy for stroke. Stroke. 2009;40:s146–8. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chopp M, Steinberg GK, Kondziolka D, et al. Who's in favor of translational cell therapy for stroke: STEPS Forward Please? Cell Transplant. 2009;18:691–3. doi: 10.3727/096368909X470883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parolini O, Alviano F, Bergwerf I, et al. Toward cell therapy using placenta-derived cells: disease mechanisms, cell biology, preclini-cal studies, and regulatory aspects at the round table. Stem Cells Dev. 2010;19:143–54. doi: 10.1089/scd.2009.0404. [DOI] [PubMed] [Google Scholar]
- 56.Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:51. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]


