Abstract
Objective
To identify the role of triglyceride-rich lipoproteins (TGRLs) and apoE, a major apolipoprotein in TGRLs, in adipose tissue inflammation with high-fat diet (HFD)–induced obesity.
Methods
Male apoE−/− and C57BL/6J wild-type (WT) mice fed HFD for 12 weeks were assessed for metabolic and inflammatory parameters. ApoE−/− and WT mice were orally gavaged with [3H]palmitic acid to examine the role of apoE in fat delivery to adipose tissue. VLDL from obese apoE−/− mice were intravenously injected into lean WT or apoE−/− mice to test potential contribution of TGRLs-derived fat delivery to inflammation in adipose tissue and the role of apoE.
Results
ApoE−/− mice gained less body weight, and had less fat mass and lower triglyceride levels in skeletal muscle than WT. ApoE−/− mice on HFD had better insulin sensitivity than WT even when comparing body weight–matched mice. Compared to WT mice, apoE−/− mice on HFD had lower levels of inflammatory cytokines/chemokines and CD11c in adipose tissue, and lower levels of inflammatory markers in skeletal muscle. At 6 hours after oral gavage with [3H]palmitic acid, incorporation of [3H]palmitic acid into adipose tissue and skeletal muscle was lower in apoE−/− mice. After repeated daily injection for 3 days, VLDL from obese apoE−/− mice induced inflammation in adipose tissue of recipient WT but not apoE−/− mice.
Conclusion
In HFD-induced obesity, apoE plays an important role in inflammation in adipose tissue and skeletal muscle, likely by mediating TGRL-derived fat delivery to these tissues.
Keywords: adipose tissue, inflammation, insulin resistance, obesity, lipoproteins, apolipoprotein E
Obesity is associated with chronic inflammation in adipose tissue [1–3], which plays crucial roles in the development of insulin resistance and type 2 diabetes [3–6]. However, the mechanisms underlying adipose tissue inflammation in obesity remain poorly defined. Obesity is caused by excess caloric intake, frequently as fat, over energy expenditure. After ingestion, dietary fat (triglycerides [TG]) is hydrolyzed in the gut and the released fatty acids (FAs) reformed into TG by enterocytes, which release chylomicrons (CM), a type of TG-rich lipoproteins (TGRLs), into lymph, ultimately reaching the plasma compartment. The TG in CM is delivered to peripheral tissues such as adipose tissue (and skeletal muscle) where TG is lipolyzed and the released FAs are transferred to adipocytes and converted again to TG in adipocytes. Hepatocytes synthesize and secrete TG in very low density lipoproteins (VLDL), which are metabolized in a way similar to TG in CM. When a large amount of fat is consumed, the overall process mediated by TGRLs including CM and VLDL increases, resulting in enhanced FA influx into adipocytes causing excessive fat deposition (adipocyte hypertrophy) and adiposity [7], which may play critical roles in adipose tissue inflammation. Nevertheless, a potential role of TGRLs in adipose tissue inflammation has never been studied and the key molecules in TGRLs that mediate fat transport and potential induction of adipose tissue inflammation in obesity have not been identified.
Apolipoproteins are involved in lipoprotein and lipid metabolism through their transport of lipids, activation of cholesterol esterification and stimulation of lipolysis [8,9]. ApoE, a major apolipoprotein in TGRLs, is important for TGRL metabolism by binding to apoE-dependent receptors on cell surfaces. Based on the discussed roles of TGRLs and apolipoprotein, we hypothesize that in obesity, TGRLs play an important role in adipose tissue inflammation by mediating excess lipid delivery into adipose tissue and that apoE is essential for this TGRL-mediated effect, thereby contributing to obesity-linked insulin resistance. Indeed, previous studies have shown that apoE plays a role in high-fat diet (HFD)–induced weight gain and insulin resistance [10–12] and in lipid accumulation in cultured adipocytes [13]. However, the role of apoE in obesity-associated inflammation and fat transport into adipose tissue has not been well studied.
In the present study, we used apoE−/− and wild-type (WT) mice with HFD-induced obesity or with intravenous injection of VLDL to determine effects of TGRLs on adipose tissue inflammation and the role of apoE in inflammation and lipid delivery to adipose tissue and skeletal muscle, another important insulin-responsive tissue target.
MATERIALS AND METHODS
An expanded Methods section is available in the Supplemental Materials.
Animals and diets
Male apoE−/− mice, backcrossed to C57BL/6J mice for at least 10 generations, and C57BL/6J WT mice (Jackson Laboratories, Bar Harbor, ME) were used. After weaning, mice were fed a normal diet (ND) containing 4.5% (w/w) fat. At 6 weeks of age, they were either switched to an HFD consisting of 21% (w/w) fat (Dyets, Bethlehem, PA) or maintained on ND ad libitum for an additional 12 weeks. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Metabolic analyses
Blood was obtained from mice after a 6-hour fast. Plasma levels of glucose, insulin, cholesterol, TG and nonesterified FAs (NEFAs) were measured. Glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed in mice after a 6-hour fast.
Insulin-stimulated Ser473-phosphorylation of Akt was examined in quadriceps femoris and perigonadal adipose tissue of mice at 10 minutes after an intraperitoneal injection of human insulin (1.5 units/kg body weight).
Histology
Perigonadal adipose depots were fixed, embedded in paraffin and sectioned. Sections were stained with hematoxylin–eosin (H&E) and photographed. The diameter of adipocytes was calculated using Image J (1.43u) software.
Quantitative RT-PCR
Total RNA was isolated from tissues. Quantitative reverse-transcription polymerase chain reaction (qPCR) was performed to examine mRNA levels of selected molecules [2,5].
Flow cytometric analysis
Stromal/vascular cells (S/Vs) were isolated from adipose tissue [5], stained with appropriate fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated antibodies or isotype negative controls, and analyzed using a BD FACScan Flow Cytometer with CellQuest (BD Biosciences) or FlowJo software [2,5,14].
Tissue uptake of dietary [3H]palmitic acid
After fasting overnight, mice were gavaged with 10 μCi [3H]palmitic acid (PA; MP Biomedicals, Inc. Solon, OH) in triolein. Blood, adipose tissue, quadriceps femoris, diaphragm and liver were harvested. 3H radioactivity was measured in plasma and lipid extract from tissues.
Effect of intravenously injected VLDL on adipose tissue inflammation
VLDL isolated from (donor) mouse plasma by density-gradient ultracentrifugation was intravenously injected into recipient mice once daily for 3 days. Inflammatory markers in adipose tissue of recipient mice were then determined.
Lipid peroxidation assay
Lipid peroxidation was measured in adipose tissue homogenate using Thiobarbituric acid-reactive substances (TBARS) Assay kit (Cayman Chemical, Ann Arbor, MI).
TG content in tissues
Total lipids were extracted from tissues with hexane/isopropanol. TG content was determined by an enzymatic assay kit (Wako Diagnostics, Richmond, VA).
Statistical analysis
Data are expressed as mean±SEM. Unpaired two-tailed Student’s t tests (for comparison between 2 groups) or one-way ANOVA followed by Tukey post hoc pairwise tests (for comparison of ≥3 groups) were performed for statistical analysis using GraphPad Prism 5 (San Diego, CA). Differences were considered significant when P<0.05.
EXPERIMENTAL RESULTS
Less fat mass and improved glucose tolerance and insulin sensitivity in apoE−/− mice on HFD independent of weight change
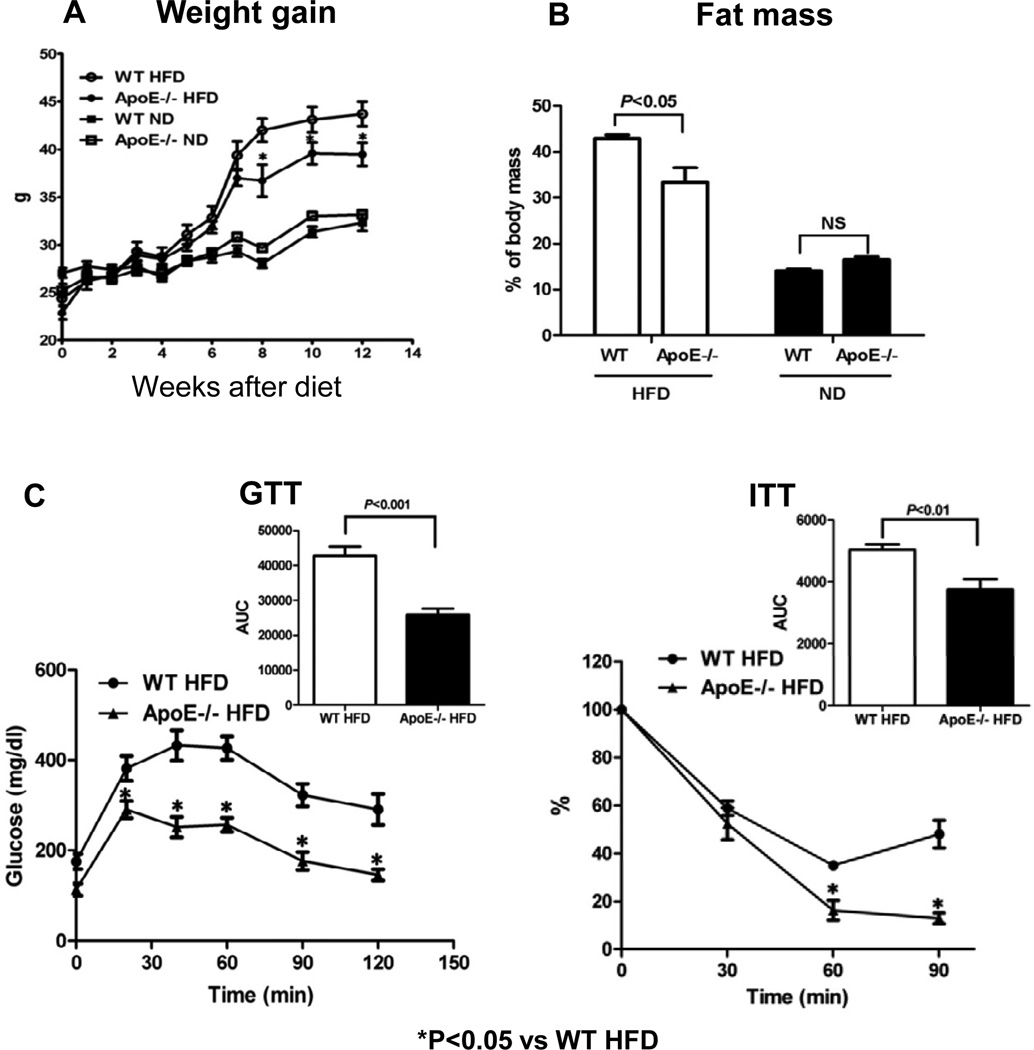
After HFD, apoE−/− and WT mice had similar weight gains for the first 7 weeks. However, after 7 weeks, weight gain in apoE−/− mice was less than that in WT mice (Figure 1A). Consistent with previous studies [11–13], body weight, perigonadal fat and liver weights and total fat mass were all lower in apoE−/− mice than in WT mice (Supplemental Figure I) at 12 weeks after HFD. Because differences in body weights may affect fat mass, we also compared apoE−/− and WT mice with similar body weights (Supplemental Figure II) and found that apoE−/− mice on HFD had less fat mass than body weight–matched WT mice (Figure 1B), indicating a body weight–independent effect of apoE on total fat mass in mice.
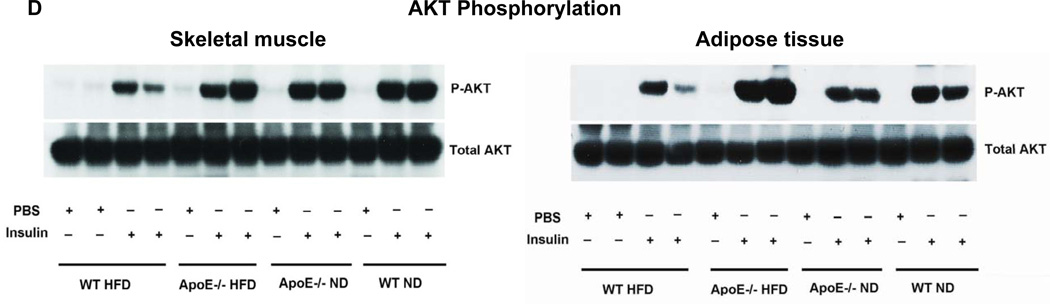
Figure 1. ApoE−/− mice on HFD had less fat mass and improved glucose tolerance and insulin sensitivity independent of altered weight gain.
(A) Weight gain in all examined mice fed high-fat diet (HFD) or normal diet (ND) for 12 weeks starting at 6 weeks of age. (B) Total fat mass, (C) glucose tolerance test (GTT) and insulin tolerance test (ITT) for mice with matched body weight (body weight shown in Supplemental Figure II). (D) Insulin-stimulated Ser473 phosphorylation of Akt in skeletal muscle and adipose tissue determined by western blot. AUC: area under the curve. n=5–12 mice/group. NS: not significant.
Consistent with previous studies [11–13], we also found improved metabolic dysfunctions in apoE−/− mice compared with WT mice on HFD (Supplemental Figure III). As different body weights could substantially influence metabolic parameters, we examined glucose tolerance (using GTT) and insulin sensitivity (using ITT) in body weight–matched apoE−/− and WT mice on HFD, and found that glucose tolerance and insulin sensitivity remained improved in apoE−/− mice (Figure 1C), suggesting a body weight–independent role of apoE in insulin resistance induced by HFD.
Insulin-stimulated phosphorylation of the insulin-signaling protein, Akt, was examined in adipose tissue and skeletal muscle. Compared to WT mice on ND, WT on HFD showed blunted Akt phosphorylation in both adipose tissue and skeletal muscle, indicating insulin resistance in both tissues in obese WT mice. In contrast, insulin-stimulated Akt phosphorylation was not reduced in either adipose tissue or skeletal muscle in obese apoE−/− mice compared with lean apoE−/− mice, and was higher in obese apoE−/− mice than in obese WT mice (Figure 1D), indicating better insulin sensitivity in adipose tissue and skeletal muscle of obese apoE−/− mice compared with obese WT mice. Plasma cholesterol and TG levels were higher in apoE−/− than WT mice (Supplemental Figure IV).
Reduced inflammation in adipose tissue of apoE−/− mice on HFD
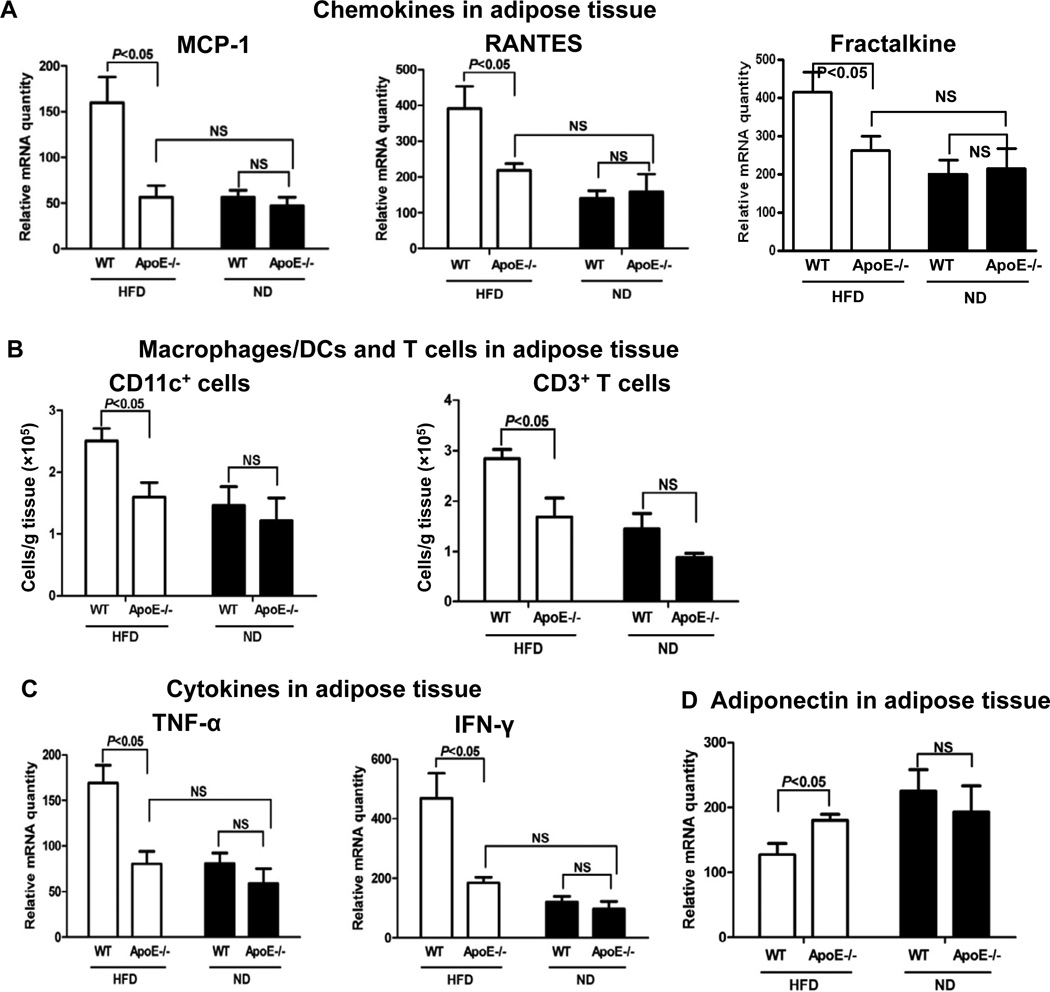
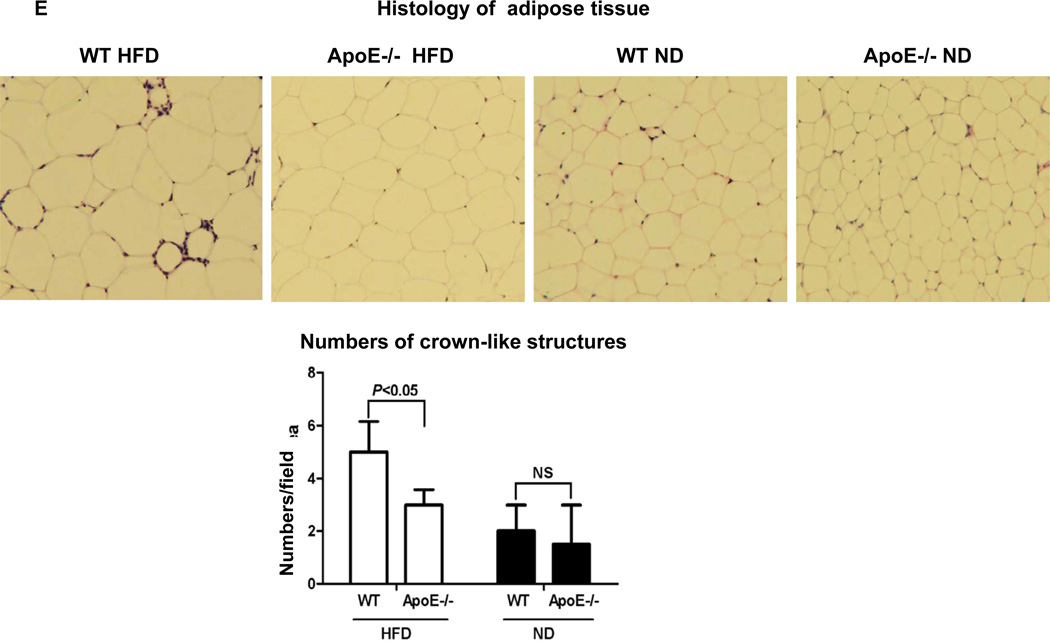
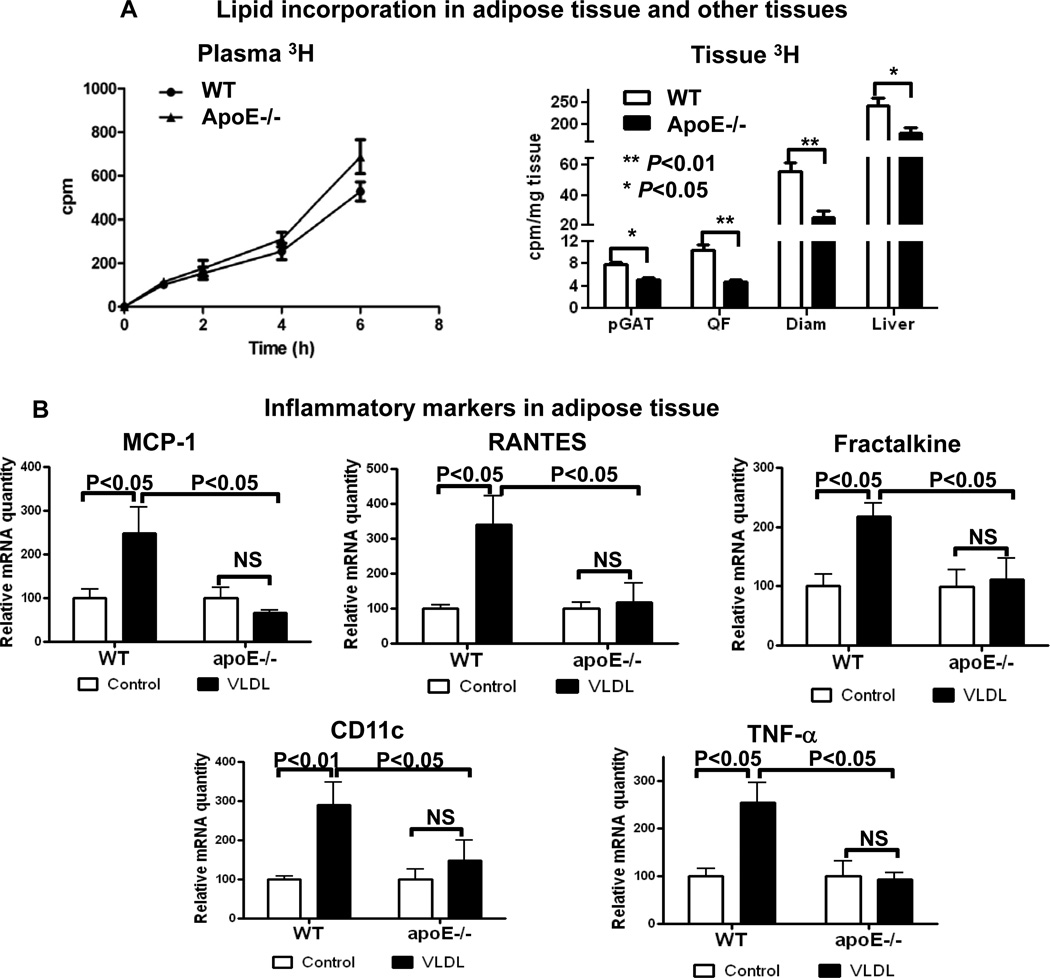
We next examined adipose tissue inflammation, a process implicated in the development of insulin resistance in obesity. Due to the difficulties in matching the body weights of apoE−/− and WT mice on HFD for all the studies, we used HFD-fed apoE−/− mice with slightly lower body weight than WT on HFD, but significantly greater body weight than WT and apoE−/− mice on ND (Supplemental Figure V), for the following studies. Consistent with previous reports [1–3,5,15,16], obese WT mice had increased inflammation in adipose tissue, indicated by higher levels of chemokines including monocyte chemotactic protein–1 (MCP-1), regulated upon activation, normal T-cell expressed, and secreted (RANTES) and fractalkine; higher numbers of F4/80+ macrophages, CD11c+ cells and T cells; higher levels of macrophage/DC and T cell activation markers including tumor necrosis factoR α (TNF-α) and interferon-γ (IFN-γ); and lower adiponectin levels in adipose tissue compared with lean WT mice (Figures 2A, 2B, 2C, 2D; Supplemental Figure VI). In contrast, obesity in apoE−/− mice did not significantly change levels of these inflammatory markers in adipose tissue. The adipose tissue levels of adiponectin were higher and of other markers were lower in obese apoE−/− mice than in obese WT mice (Figures 2A, 2C, 2D; Supplemental Figure VI). Obese apoE−/− mice had fewer F4/80+ macrophages, CD11c+ cells and CD3+ T cells in adipose tissue than obese WT mice as examined by flow cytometry (Figure 2B; Supplemental Figure VI) and confirmed by histology showing lower numbers of crown-like structures, which consist of macrophages and T cells [1,17], in adipose tissue of obese apoE−/− mice compared with obese WT mice (Figure 2E). Adipocytes from apoE−/− mice were also smaller than those from WT mice (Figure 2E; Supplemental Figure VI).
Figure 2. ApoE−/− mice on HFD had reduced inflammatory markers in adipose tissue.
(A) mRNA levels of MCP-1, RANTES and fractalkine in adipose tissue. (B) Numbers of CD11c+ cells and T cells in adipose tissue examined by flow cytometry. (C) mRNA levels of TNF-α and IFN-γ in adipose tissue. (D) mRNA levels of adiponectin in adipose tissue. (E) Representative histological images of adipose tissue with numbers of crown-like structures per field. Magnification, ×100. n=5–10 mice/group.
Reduced delivery of oral [3H]PA to adipose tissue in apoE−/− mice
Enhanced lipid delivery from diet by CM and from liver by VLDL to adipose tissue with excessive TG accumulation in adipocytes as observed in obesity may be a crucial contributor to adipose tissue inflammation. Therefore, we examined dietary lipid delivery into adipose tissue and the role of apoE. After oral administration of [3H]PA in mice, 3H radioactivity appeared in blood (Figure 3A), indicating intestinal [3H]PA absorption and incorporation into blood lipoproteins (CM/VLDL). At 6 hours post [3H]PA administration, 3H radioactivity appeared in adipose tissue. As indicated by similar 3H radioactivity in blood of WT and apoE−/− mice, particularly at earlier timepoints (Figure 3A), deficiency of apoE did not affect dietary PA absorption into blood. However, compared with WT mice, 3H radioactivity in adipose tissue was lower in apoE−/− mice (Figure 3A), indicating that the absence of apoE impaired dietary PA deposition into adipose tissue. Compared to WT mice, apoE−/− mice also had decreased dietary [3H]PA deposition in skeletal muscle and liver as indicated by lower 3H radioactivity in quadriceps femoris, diaphragm and liver (Figure 3A). These data support an essential role of apoE in lipid delivery to adipose tissue, skeletal muscle and liver.
Figure 3. ApoE−/− mice had impaired delivery of dietary [3H]palmitic acid ([3H]PA) into adipose tissue and reduced inflammatory response in adipose tissue to VLDL administration.
(A) ApoE−/− and WT mice were gavaged with [3H]PA. 3H radioactivity was determined in blood at timepoints indicated and in perigonadal adipose tissue (pGAT), quadriceps femoris (QF), diaphram (Diam) and liver at 6 hours after gavage. n=4–5 mice/group. (B) WT and apoE−/− mice received daily intravenous injection of VLDL from apoE−/− mice on HFD or PBS (control). After 3 injections, mRNA levels of inflammatory markers were examined in adipose tissue. n=4–6 mice/group.
VLDL induction of adipose tissue inflammation in WT mice and reduced inflammatory response to apoE-deficient VLDL in adipose tissue of apoE−/− mice
To test whether obesity-associated TGRL-derived lipid transfer induces inflammation in adipose tissue, we intravenously injected VLDL from obese apoE−/− mice into lean WT and apoE−/− mice, and examined inflammatory markers in adipose tissue of the recipient mice. For the first time, we observed that repeated daily injection (3 times) of VLDL from obese mice into lean WT mice significantly increased adipose tissue levels of inflammatory markers, including MCP-1, RANTES, fractalkine, CD11c and TNF-α (Figure 3B), implying that lipid transfer from the injected “obese” VLDL (which would acquire apoE in WT mice) into “lean” adipose tissue induced adipose tissue inflammation. In contrast, injection of the apoE-deficient VLDL did not induce significant inflammatory response in adipose tissue of apoE−/− mice (Figure 3B), suggesting an essential role of apoE in VLDL-induced adipose tissue inflammation.
Oxidative stress in adipose tissue
To test whether oxidative stress contributes to the inflammatory changes, malondialdehyde (MDA) was examined in adipose tissue. Unexpectedly, adipose tissue MDA levels tended to be lower in obese WT than in lean WT mice. Compared to obese WT, obese apoE−/− mice did not show significant difference in adipose tissue MDA levels (Supplemental Figure VII).
Reduced inflammation in skeletal muscle of apoE−/− mice on HFD
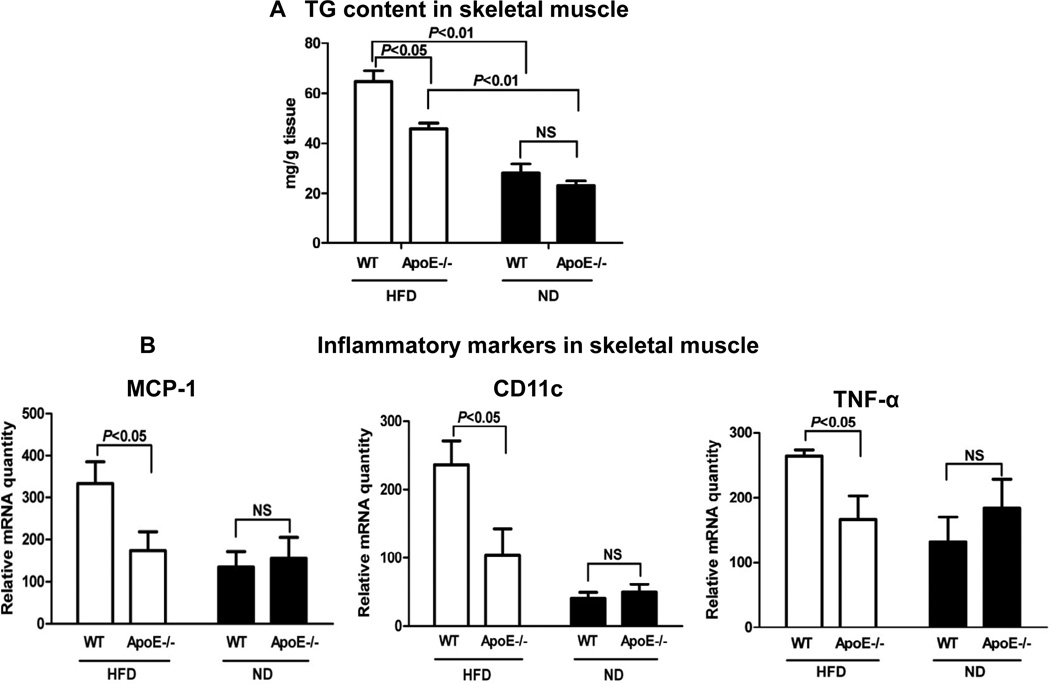
Next, we examined lipid content and inflammatory markers in skeletal muscle. Along with impaired insulin sensitivity in skeletal muscle (Figure 1D), obese WT mice had increased TG content and elevated levels of inflammatory markers, including MCP-1, CD11c and TNF-α, in skeletal muscle compared with lean WT mice (Figures 4A, 4B). Consistent with the data indicating a role of apoE in lipid delivery into skeletal muscle, TG content was significantly lower in skeletal muscle of obese apoE−/− mice than in that of obese WT mice (Figure 4A). Along with these differences, obese apoE−/− mice had lower levels of MCP-1, CD11c and TNF-α in skeletal muscle compared with obese WT mice (Figure 4B). Lean apoE−/− mice did not differ significantly from lean WT mice in TG content (Figure 4A) and levels of inflammatory markers in skeletal muscle (Figure 4B).
Figure 4. ApoE−/− mice on HFD had reduced TG content and inflammatory markers in skeletal muscle.
(A) TG content in skeletal muscle. (B) mRNA levels of inflammatory markers in skeletal muscle. n=8–10 mice/group.
DISCUSSION
In the current study, we first report that deficiency of apoE in obese mice decreased inflammation in adipose tissue and skeletal muscle. We also made the novel observations that VLDL from obese mice increased inflammation in adipose tissue of lean mice and that deficiency of apoE on VLDL reduced this effect. Further studies revealed that decreased lipoprotein-mediated lipid delivery into adipose tissue (and skeletal muscle) in the absence of apoE may underlie the reduced inflammation in these tissues of apoE−/− mice on HFD. Therefore, our study indicates that in obesity induced by HFD, TGRLs play an important role in inflammation in adipose tissue (and possibly skeletal muscle), likely by delivering excess fat into the tissue, and that apoE is essential for the TGRL-mediated effects and is thereby involved in obesity-related inflammation in adipose tissue and skeletal muscle, which may contribute to insulin resistance in these tissues.
ApoE−/− mice with hyperlipidemia are a commonly used mouse model for studying atherosclerosis, and display severe inflammation and atherosclerosis in blood vessels [14]. Because obesity and hyperlipidemia may both contribute to inflammation [18], we had expected that the combination of obesity and hyperlipidemia in apoE−/− mice with HFD would enhance inflammation in adipose tissue. However, on the contrary, apoE−/− mice on HFD had decreased inflammation in adipose tissue compared to WT mice.
The mechanisms for adipose tissue inflammation in obesity remain poorly defined. Although increased levels of chemokines such as MCP-1 and RANTES may play important roles in adipose tissue inflammation by recruiting and activating leukocytes [2,19], it remains unknown how chemokines are upregulated in adipose tissue in obesity. Obesity is associated with enlarged adipocytes with increased lipid accumulation (adipocyte hypertrophy), which is associated with increased adipocyte inflammation [20]. Circulating TGRLs deliver dietary and liver-derived fat to adipocytes, thereby playing crucial roles in the development of adipocyte hypertrophy in obesity. Our in vivo mouse study showing that VLDL from obese mice induced adipose tissue inflammation in lean WT mice, provides the first evidence for an important role of TGRLs in adipose tissue inflammation. From our study, we speculate that in HFD-induced obesity, enhanced TGRL-derived fat delivery to adipocytes, with an attendant increase in fat (mainly in the form of FAs) influx and greater fat accumulation in adipocytes, plays a critical role in adipocyte inflammation, particularly expression of chemokines, which contributes to initiation and maintenance of adipose tissue inflammation by recruiting inflammatory leukocytes. In support of this, FAs, which are released from TG in TGRLs by lipoprotein lipase, induce inflammation, with increased expression of chemokines in adipocytes, possibly through interacting with toll-like receptor 4 on adipocytes [21].
ApoE is a major apolipoprotein in TGRLs and plays an important role in hepatic clearance of TGRLs by binding to apoE receptors on cell surfaces. Previous studies have also indicated a role of apoE in lipid accumulation within cultured adiocytes and potentially in fat delivery into adipose tissue [10,13]. Our observation that apoE−/− mice had reduced fat delivery into adipose tissue provides the first direct evidence for an essential role of apoE in fat delivery to adipose tissue in vivo. Given that apoE−/− mice had normal dietary fat absorption, apoE likely mediates fat delivery by a role in lipid transfer from blood TGRLs into the tissue. Based on the discussed role of TGRL-derived fat delivery in adipose tissue inflammation, reduced fat delivery, with less fat influx into adipocytes, is expected to result in decreased secretion of chemokines such as MCP-1 and RANTES by adipose tissue, which was observed in apoE−/− mice and may underlie the reduction in macrophage/DC and T cell accumulation and activation, as also observed in adipose tissue of apoE−/− mice on HFD. As additional support, intravenous injection of apoE-deficient VLDL, which, with acquisition of apoE from WT mice, induced inflammation in adipose tissue of lean WT mice, did not increase inflammation in adipose tissue of apoE−/− mice.
Oxidative stress may occur in obesity and contribute to inflammation [4]. Unexpectedly, we observed lower levels of MDA, a lipid peroxidation product and a marker for oxidative stress, in adipose tissue of obese WT compared with lean WT mice. Consistent with our findings, Sohet, et al reported reduced MDA levels in adipose tissue and liver of mice on HFD [22]. Higher vitamin E content in HFD (61 IU/kg in ND versus 120 IU/kg in HFD) may underlie the reduced MDA levels [22]. These data suggest that lipid peroxidation may not play a crucial role in adipose tissue inflammation in our mouse models.
In addition to excess fat deposition in adipose tissue, obesity is commonly associated with ectopic lipid deposition in skeletal muscle, which may result in inflammation in skeletal muscle and contribute to insulin resistance [23]. Indeed, we found higher TG content, increased inflammatory markers and enhanced insulin resistance in skeletal muscle of obese WT mice. Importantly, we demonstrated that deficiency of apoE in obese mice also decreased dietary lipid delivery into skeletal muscle, with lower TG content in skeletal muscle. To our knowledge, this is the first report on an essential role of apoE in lipid deposition in skeletal muscle associated with obesity. Skeletal muscle expresses apoE receptors such as VLDL receptor, which has been shown to play a role in mediating TGRL delivery to skeletal muscle [24]. The reduced fat delivery, with less fat content, in skeletal muscle may also underlie the decreased inflammation and improved insulin sensitivity in this tissue of apoE−/− mice on HFD.
Studies from Mazzone’s group showed an essential role of adipocyte-expressed apoE in adipocyte functions [25]. Our current study does not exclude a potential role of adipocyte-expressed apoE in fat uptake and inflammation in adipose tissue. However, given the similar effects of apoE deficiency on fat delivery and inflammation in adipose tissue and skeletal muscle, we postulate that apoE on TGRLs exerts the primary effects as we present in the current study. Additional support for this is that apoE-containing VLDL, but not apoE-deficient VLDL, induced adipogenesis with increased lipid accumulation in apoE-deficient bone marrow stromal cells [13].
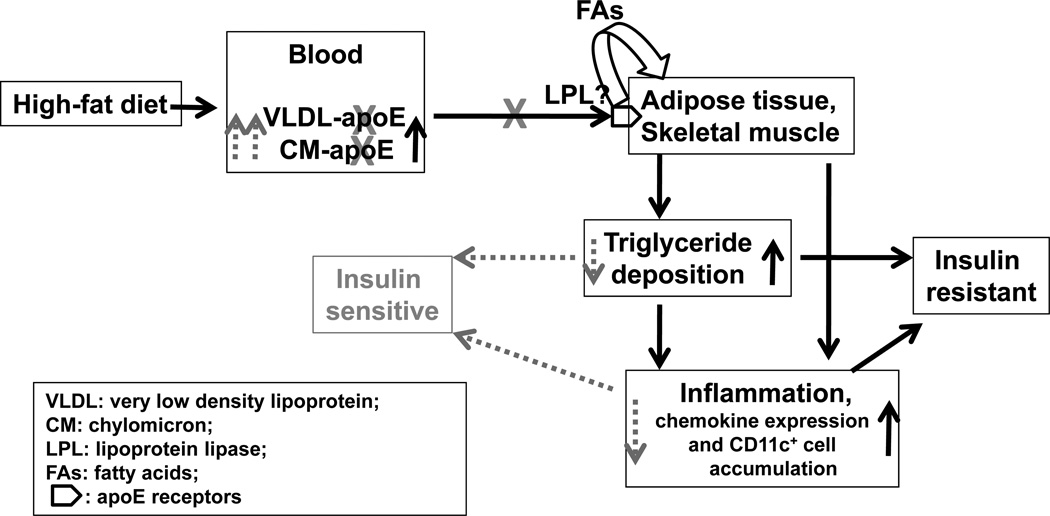
In summary, using an apoE-deficient mouse model, we demonstrated an essential role of apoE in inflammation in adipose tissue and skeletal muscle associated with HFD-induced obesity. Based on our data, we propose a working model (Figure 5) for the role of apoE (apoE-containing TGRLs) in adipose tissue and skeletal muscle inflammation in HFD-induced obesity. Continuous intake of HFD leads to increased production of apoE-containing TGRLs, including intestinally-derived CM and hepatocyte-synthesized VLDL, with resultant increases in plasma levels of TG (TGRLs). ApoE on TGRLs delivers a surfeit of fat (TGRL-TG) into adipose tissue and skeletal muscle via binding to apoE receptors on adipocytes and skeletal muscle, a step that may also involve lipoprotein lipase with release and transport of FAs, and results in excess fat deposition in these tissues. This continuous apoE-dependent TGRL-derived fat delivery may play critical roles in the development of inflammation and insulin resistance in adipose tissue and skeletal muscle. Deficiency of apoE (shown in gray in Figure 5) in TGRLs reduces fat delivery into adipose tissue and skeletal muscle, thereby leading to decreased fat influx and less fat accumulation and subsequently to amelioration of inflammation and improvement of insulin resistance in these tissues.
Figure 5. A working model for the role of apoE (apoE-containing TGRLs) in inflammation in adipose tissue and skeletal muscle and its contribution to insulin resistance in HFD-induced obesity.
Continuous apoE-mediated (TGRL-derived) fat delivery with increased fat influx leads to excess fat deposition and inflammation in adipose tissue and skeletal muscle, which contributes to insulin resistance in these tissues. Deficiency of apoE (gray) in TGRLs reduces lipid delivery into adipose tissue and skeletal muscle, resulting in less lipid accumulation and decreased inflammation, with subsequent amelioration of insulin resistance.
Our studies may have clinical implications for experimental therapy in humans. In obesity, increased production with elevated plasma levels of TGRLs leads to increased fat influx into adipose tissue, skeletal muscle and liver, resulting in adipocyte hypertrophy, ectopic fat deposition in skeletal muscle and fatty liver, all of which are thought to contribute to the development of insulin resistance and diabetes. Based on our working model, blockade of fat transfer from TGRLs into adipose tissue and skeletal muscle, with prevention of inflammation in these tissues, would be beneficial in treating obesity-linked insulin resistance and diabetes. However, blockade of TGRL-TG transfer to adipose tissue and skeletal muscle as in apoE−/− mice also slows lipid clearance by these tissues and raises lipid levels in blood, leading to hyperlipidemia (Fig. 5), which is a major risk factor for cardiovascular disease. Therefore, restriction of fat intake and/or inhibition of TGRL production with reduced secretion of TGRLs into blood and subsequently decreased fat influx into adipose tissue and skeletal muscle would be more beneficial in treating obesity-linked insulin resistance. Indeed, inhibition of TGRL production by an inhibitor of microsomal triglyceride transfer protein (MTP) reduced plasma TG levels and improved insulin sensitivity in obesity [26]. However, the MTP inhibitor may also cause hepatic fat accumulation [27]. The data on MTP inhibition suggest that fatty liver may be dissociated from insulin resistance, as has also been reported in other studies [28–30]. In combination with these studies [26–30], our studies emphasize a crucial role of TGRL-derived fat delivery into adipose tissue and skeletal muscle in the development of inflammation and insulin resistance in these tissues.
Supplementary Material
Acknowledgments
We thank Kerrie Jara for editorial assistance.
Sources of Funding
This work was supported by research grants from NIH (R01HL098839 to H.W., R01DK078847 to C.M.B.) and USDA/ARS (6250-51000-046 to C.W.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 3.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H, Perrard XD, Wang Q, et al. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2010;30:186–192. doi: 10.1161/ATVBAHA.109.198044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teusink B, Voshol PJ, Dahlmans VE, et al. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes. 2003;52:614–620. doi: 10.2337/diabetes.52.3.614. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi S, Suzuki J, Kohno M, et al. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J Biol Chem. 1995;270:15747–15754. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- 9.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann SM, Perez-Tilve D, Greer TM, et al. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes. 2008;57:5–12. doi: 10.2337/db07-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J, Katagiri H, Ishigaki Y, et al. Involvement of apolipoprotein E in excess fat accumulation and insulin resistance. Diabetes. 2007;56:24–33. doi: 10.2337/db06-0144. [DOI] [PubMed] [Google Scholar]
- 12.Karagiannides I, Abdou R, Tzortzopoulou A, Voshol PJ, Kypreos KE. Apolipoprotein E predisposes to obesity and related metabolic dysfunctions in mice. FEBS J. 2008;275:4796–4809. doi: 10.1111/j.1742-4658.2008.06619.x. [DOI] [PubMed] [Google Scholar]
- 13.Chiba T, Nakazawa T, Yui K, Kaneko E, Shimokado K. VLDL induces adipocyte differentiation in ApoE-dependent manner. Arterioscler Thromb Vasc Biol. 2003;23:1423–1429. doi: 10.1161/01.ATV.0000085040.58340.36. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Gower RM, Wang H, et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian S, Han CY, Chiba T, et al. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 18.Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 19.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao P, Chen Q, Shah S, et al. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes. 2009;58:104–115. doi: 10.2337/db07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohet FM, Neyrinck AM, Dewulf EM, et al. Lipid peroxidation is not a prerequisite for the development of obesity and diabetes in high-fat-fed mice. Br. J Nutr. 2009;102:462–469. doi: 10.1017/S0007114508191243. [DOI] [PubMed] [Google Scholar]
- 23.Hegarty BD, Cooney GJ, Kraegen EW, Furler SM. Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes. 2002;51:1477–1484. doi: 10.2337/diabetes.51.5.1477. [DOI] [PubMed] [Google Scholar]
- 24.Goudriaan JR, Espirito Santo SM, Voshol PJ, et al. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J Lipid Res. 2004;45:1475–1481. doi: 10.1194/jlr.M400009-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Huang ZH, Reardon CA, Mazzone T. Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 2006;55:3394–3402. doi: 10.2337/db06-0354. [DOI] [PubMed] [Google Scholar]
- 26.Dhote V, Joharapurkar A, Kshirsagar S, et al. Inhibition of microsomal triglyceride transfer protein improves insulin sensitivity and reduces atherogenic risk in Zucker fatty rats. Clin Exp Pharmacol Physiol. 2011;38:338–344. doi: 10.1111/j.1440-1681.2011.05513.x. [DOI] [PubMed] [Google Scholar]
- 27.Cuchel M, Bloedon LT, Szapary PO, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Perrard XD, Perrard JL, et al. Differential effect of weight loss with low-fat diet or high-fat diet restriction on inflammation in the liver and adipose tissue of mice with diet-induced obesity. Atherosclerosis. 2011;219:100–108. doi: 10.1016/j.atherosclerosis.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monetti M, Levin MC, Watt MJ, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Moon YA, Liang G, Xie X, et al. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.