Abstract
Chaperones are the primary regulators of the proteostasis network and are known to facilitate protein folding, inhibit protein aggregation, and promote disaggregation and clearance of misfolded aggregates inside cells. We have tested the effects of five chaperones on the toxicity of misfolded oligomers preformed from three different proteins added extracellularly to cultured cells. All the chaperones were found to decrease oligomer toxicity significantly, even at very low chaperone/protein molar ratios, provided that they were added extracellularly rather than being overexpressed in the cytosol. Infrared spectroscopy and site-directed labeling experiments using pyrene ruled out structural reorganizations within the discrete oligomers. Rather, confocal microscopy, SDS-PAGE, and intrinsic fluorescence measurements indicated tight binding between oligomers and chaperones. Moreover, atomic force microscopy imaging indicated that larger assemblies of oligomers are formed in the presence of the chaperones. This suggests that the chaperones bind to the oligomers and promote their assembly into larger species, with consequent shielding of the reactive surfaces and a decrease in their diffusional mobility. Overall, the data indicate a generic ability of chaperones to neutralize extracellular misfolded oligomers efficiently and reveal that further assembly of protein oligomers into larger species can be an effective strategy to neutralize such extracellular species.
Keywords: protein homeostasis, protein misfolding, protein aggregates, amyloid, extracellular chaperones
The ability of living systems to maintain their peptides and proteins in soluble and functional states is the result of evolutionary selection of the physicochemical and conformational characteristics of these macromolecules (1) and is also attributable to an array of biological mechanisms that, together, act to ensure proteostasis (2). Failures of the protective and regulatory mechanisms can result in a wide variety of pathological conditions, including those associated with the accumulation of protein aggregates, both in the cytosol and in the extracellular space (3, 4). Molecular chaperones are proteins that play a central role in the avoidance of protein misfolding and aggregation (5–8). Most known chaperones are intracellular (7, 8), but some of them are secreted and are collectively referred to as extracellular chaperones (9). Chaperones are known to have a range of different functions, such as assisting in protein folding (7), inhibiting protein aggregation (10), causing the disaggregation of aberrant protein oligomers (6), and facilitating the degradation of misfolded proteins (11).
In this study, we have examined the effects of two intracellular and three extracellular chaperones on the toxicity of extracellularly added oligomers formed by three different peptides/proteins, namely, the 42-residue amyloid β peptide (Aβ42), the islet amyloid polypeptide (IAPP), and the N-terminal domain of the HypF protein from Escherichia coli (HypF-N). We focused our attention on small oligomers because such species are generally highly toxic to cultured cells and are thought to be the major deleterious species in a range of protein aggregation diseases (4). We show that the chaperones markedly decrease the toxicity of preformed oligomers, with significant effects being observed even at molar ratios of protein/chaperone as high as 500:1. In addition, we show that chaperones bind to the oligomers and promote their clustering into larger aggregates in the absence of any disaggregation or major structural reorganization. Overall, these results provide suggestions on additional mechanisms by which molecular chaperones are cytoprotective extracellularly and also reveal previously undescribed determinants of protein oligomer toxicity and strategies to convert protein aggregates that are toxic to cultured cells into innocuous species.
Results
Chaperones Reduce Oligomer Toxicity.
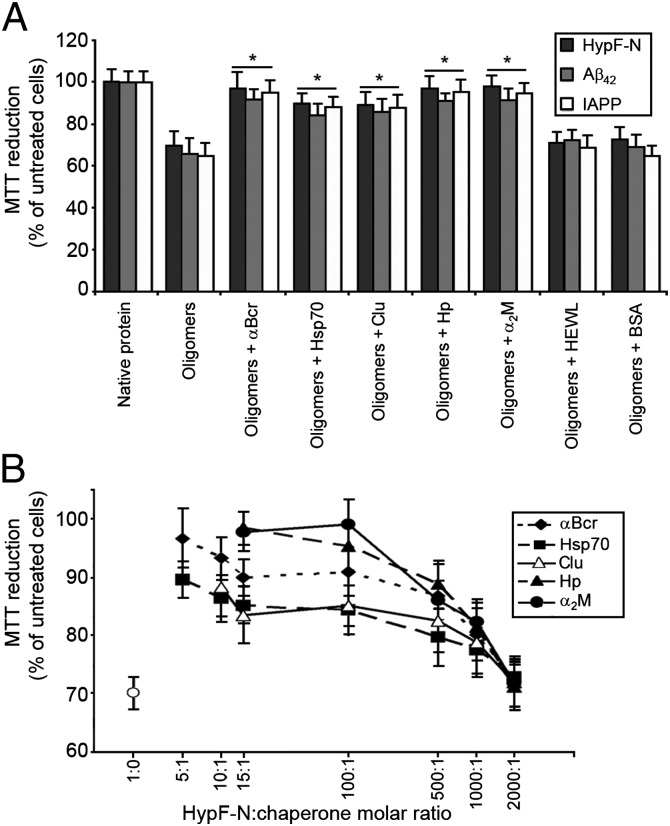
We generated toxic oligomers from Aβ42, IAPP, and HypF-N, as described previously (12–14). After 1 h of incubation in the cell culture medium, they were added to human SH-SY5Y neuroblastoma cultured cells at a corresponding monomer concentration of 12 μM. Under these conditions, all oligomers were found to decrease the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction of the cells by 30–40%, demonstrating their toxic nature (Fig. 1A). The oligomers were then subjected to the same procedure but incubated for 1 h in the cell culture medium containing either αB-crystallin (αBcr), heat shock protein 70 (Hsp70), clusterin (Clu), haptoglobin (Hp), or α2-macroglobulin (α2M) before their addition to SH-SY5Y cells. For these five chaperones and all our experiments, the protein/chaperone molar ratios were 5:1, 5:1, 10:1, 15:1, and 100:1, respectively, unless otherwise stated. In each case, the cells were found to reduce MTT to levels similar to untreated cells and to cells treated with the native proteins (Fig. 1A). Hsp70 was found to be equally effective with or without ATP (SI Appendix, Fig. S1).
Fig. 1.
Reduction of protein oligomer toxicity by chaperones. (A) Preformed oligomers of HypF-N, Aβ42, and IAPP were resuspended in the cell culture medium at a corresponding monomer concentration of 12 μM, incubated for 1 h without or with the indicated chaperones or control proteins, and then added to the cell culture medium of SH-SY5Y cells. The asterisks indicate P ≤ 0.01 relative to the experiment without chaperones. (B) Similarly treated HypF-N oligomers (12 μM monomer) were incubated for 1 h without (○) or with the indicated chaperones at the indicated HypF-N/chaperone molar ratios and then added to SH-SY5Y cells. The scale on the x axis is logarithmic.
When the oligomers were incubated in the cell culture medium with proteins that do not act as chaperones, either hen lysozyme (HEWL) or bovine serum albumin (BSA), they maintained their toxicity (Fig. 1A). Moreover, chaperones were not found to be protective against other forms of stress, such as H2O2-mediated oxidation (SI Appendix, Fig. S2). These results therefore indicate that all five chaperones markedly decrease the toxicity of oligomers formed by three different proteins, with these effects being specific to the chaperones (relative to other proteins) and to protein oligomer toxicity (relative to other forms of stress).
The experiments with HypF-N oligomers and chaperones were repeated by varying the concentrations of each of the five chaperones (from 2.4 μM to 6 nm), while maintaining constant that of HypF-N (12 μM). All chaperones had significant effects even at HypF-N/chaperone molar ratios of 500:1, becoming ineffective only at molar ratios of 2,000:1 (Fig. 1B). Thus, chaperones appear to decrease oligomer toxicity markedly at greatly substoichiometric concentrations. The experiments were repeated with HypF-N, Aβ42, and IAPP oligomers (12 μM) using varying concentrations of antibodies sequence-specific to the proteins (from 2.4 μM to 6 nm). Compared with the chaperones, higher concentrations of antibodies were required to reduce the toxicity of the respective oligomers (SI Appendix, Fig. S3).
Chaperones Prevent the Interaction of Oligomers with Cellular Membranes.
For the following experiments, we chose to focus on the oligomers from a single protein, HypF-N. Indeed, under different experimental conditions, HypF-N can aggregate into toxic (type A, the same used in the present work) or nontoxic (type B) oligomers; the two species are morphologically similar as determined by atomic force microscopy (AFM) and bind thioflavin T (ThT) to similar levels, making it possible to carry out valuable control experiments (14). In addition, differences in the structure of toxic and nontoxic oligomers have been detected using the fluorescent probe N-(1-pyrene)maleimide (PM) (14), providing a valuable method to probe structural changes experienced by the oligomers following their exposure to chaperones (see below).
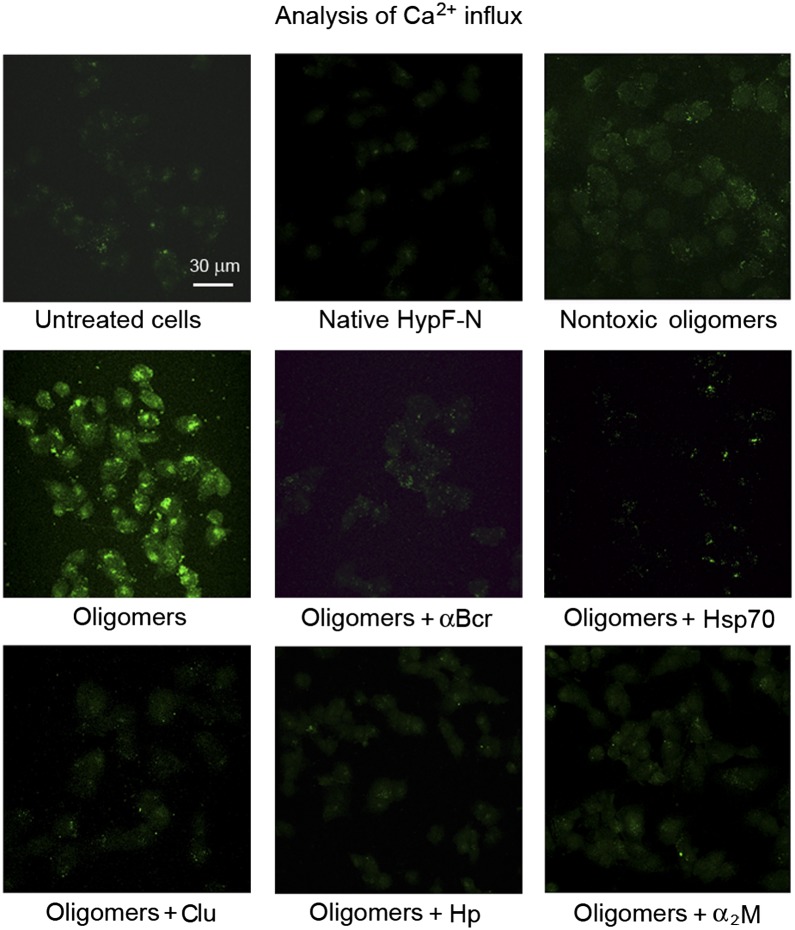
HypF-N oligomers have been shown to be toxic via aberrant interactions with the cell membrane, causing a rapid influx of Ca2+ ions from the cell culture medium to the cytosol (15). This event triggers a slower and complex cellular cascade manifested by increases in intracellular reactive oxygen species and caspase-3 activity, and as a release of intracellular calcein from cells, eventually leading to apoptosis (15). Preincubation of HypF-N oligomers (12 μM monomer) with each of the five chaperones in the cell culture medium for 1 h, before addition to the cells, was found to inhibit the increase of intracellular Ca2+ levels caused by the oligomers (Fig. 2), with the degree of inhibition increasing with preincubation time (SI Appendix, Fig. S4). All other events were also reduced markedly, with the extent of reduction again being dependent on the time of preincubation (SI Appendix, Figs. S5–S7). We conclude, therefore, that all five chaperones examined here can inhibit the initial biochemical events induced by toxic HypF-N oligomers, namely, the influx of Ca2+, thus eliminating the occurrence of later downstream effects. In addition, the observed dependence of the degree of protection on the preincubation time indicates that the chaperones generically reduce toxicity by interacting with the oligomers, rather than through a separate protective pathway mediated by interaction of the chaperones with the cells.
Fig. 2.
Reduction of HypF-N oligomer-induced intracellular free Ca2+ influx by chaperones. Confocal microscope images of SH-SY5Y cells show intracellular free Ca2+ following exposure to HypF-N oligomers (at a corresponding monomer concentration of 12 μM) preincubated with or without the indicated chaperones. The green fluorescence arises from the intracellular Fluo3 probe bound to Ca2+.
Extracellular chaperones can interact with misfolded proteins and are thought to facilitate their clearance via endocytosis mediated by cell surface receptors, such as the lipoprotein receptor-related protein 1 (LRP-1) and 2 (LRP-2) or scavenger receptors (9). This possibility was, however, excluded in our system as shown by confocal microscopy and anti–HypF-N antibodies (SI Appendix, Figs. S8 and S9). In fact, HypF-N oligomers, unlike the native protein and nontoxic oligomers, were found to be internalized following preincubation for 1 h in the absence of chaperones. Analysis of the confocal images at median planes parallel to the coverslip revealed that HypF-N appeared inside the cells only on treatment with the toxic oligomers (SI Appendix, Fig. S8B). Preincubation of the oligomers with each chaperone led to little or no HypF-N entry (SI Appendix, Figs. S8 and S9). The oligomers were predominantly detected outside or attached to the cells but not within them, as confirmed by analyzing the confocal images at median planes. These observations thus show that the chaperones inhibit oligomer internalization, at least under the conditions used here, rather than stimulating their intracellular degradation following endocytosis.
We next took advantage of HEK293 cell lines overexpressing either human Hsp70 (HSPA1A) or Hsp22 (HSPB8) in the cytosol (16, 17); the latter is a small heat shock protein containing a α-crystallin domain. Both cell lines were found to be resistant to intracellular protein aggregation-associated cell death (16, 17) (SI Appendix, Fig. S10). As observed with the SH-SY5Y cells, treatment of wild-type HEK293 cells with HypF-N oligomers caused a decreased MTT reduction by 41 ± 8%, whereas preincubation of the oligomers with either Hsp70 or αBcr prevented such an effect (SI Appendix, Fig. S10). Importantly, the HypF-N oligomers were also toxic to HEK293 cells overexpressing Hsp70 or Hsp22, but preincubation of the oligomers with Hsp70 or αBcr prevented their toxicity in the corresponding cell lines (SI Appendix, Fig. S10).
Overall, therefore, the deleterious effects of HypF-N oligomers can be abolished by chaperones, provided that the chaperone-oligomer interactions occur before the oligomers are able to interact with the cell membranes and initiate the Ca2+ influx. In agreement with this conclusion, chaperones overexpressed intracellularly are not protective against extracellular oligomers.
Chaperones Bind to and Assemble the Oligomers into Larger Species.
Three possible nonexclusive molecular mechanisms can be hypothesized to explain how chaperones reduce oligomer toxicity: (i) the chaperones disassemble the oligomers, (ii) they bind to oligomers and promote their assembly into larger and innocuous aggregates, or (iii) they catalyze a structural reorganization into nontoxic forms. The ability of HypF-N oligomers to bind ThT was maintained following preincubation with each of the chaperones, whereas neither native HypF-N nor free chaperones had such ability (SI Appendix, Fig. S11). A process of chaperone-mediated oligomer disassembly can thus be excluded.
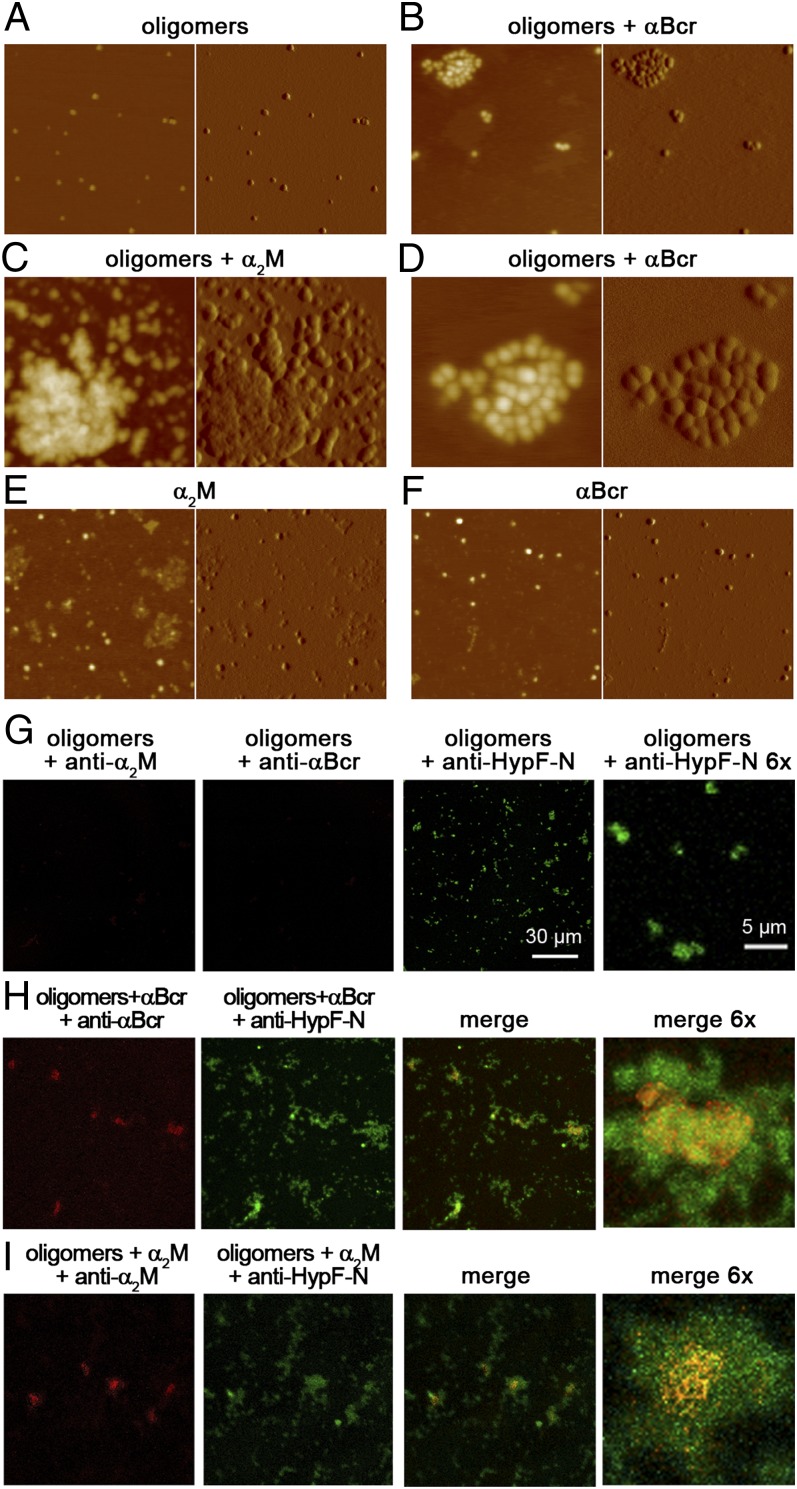
To investigate whether chaperones bind to the oligomers and promote their further assembly, we first used AFM. Discrete oligomers with a height of 2–6 nm were observed by AFM in the absence of chaperones (Fig. 3A), but significantly larger aggregates are evident with αBcr (Fig. 3B) or α2M (Fig. 3C), used here as representative chaperones. On higher magnification of these images, the aggregates appear as clusters of oligomers (Fig. 3D). In some cases, more complex structures are observed, consisting of very large aggregates of irregular shape with typical heights of a few tens of nanometers, often surrounded by clusters of more distinct oligomers. Large assemblies are not observed in samples containing only chaperones (Fig. 3 E and F).
Fig. 3.
Assembly of HypF-N oligomers induced by chaperones. (A–F) AFM images (Left, height data; Right, amplitude data) show oligomers and chaperones preincubated in phosphate buffer alone or in combination, as indicated; the scan size is 630 nm. (D) Enlargement of a 250-nm × 250-nm portion of B. Z range: 10 nm (A), 13 nm (B and D), 25 nm (C), 6 nm (E), and 10 nm (F). (G) Representative confocal microscope images show HypF-N oligomers without chaperones and treated with anti-α2M (red), anti-αBcr (red), or anti-HypF-N (green) antibodies, as indicated. The absence of red fluorescence indicates the absence of cross-reaction. (H, I) Images show HypF-N oligomers incubated with αBcr (H) or α2M (I) and treated with the same three antibodies, as indicated. The colocalization of oligomers and chaperones is shown in the merged images (yellow).
The AFM data show that chaperones promote the assembly of the oligomers into larger species but do not provide information on whether or not the chaperones remain bound to them. The oligomers can also be observed with confocal microscopy because, unlike free chaperones, they readily adhere to the glass coverslips (Fig. 3G). Images obtained using oligomers preincubated with αBcr or α2M show larger aggregates and colocalization of the chaperones with the large oligomer clusters (Fig. 3 H and I). Although this technique has a resolution that enables only visualization of clusters of oligomers or areas enriched with oligomers (not individual oligomers), it shows that the chaperones are bound to the large clusters of oligomers.
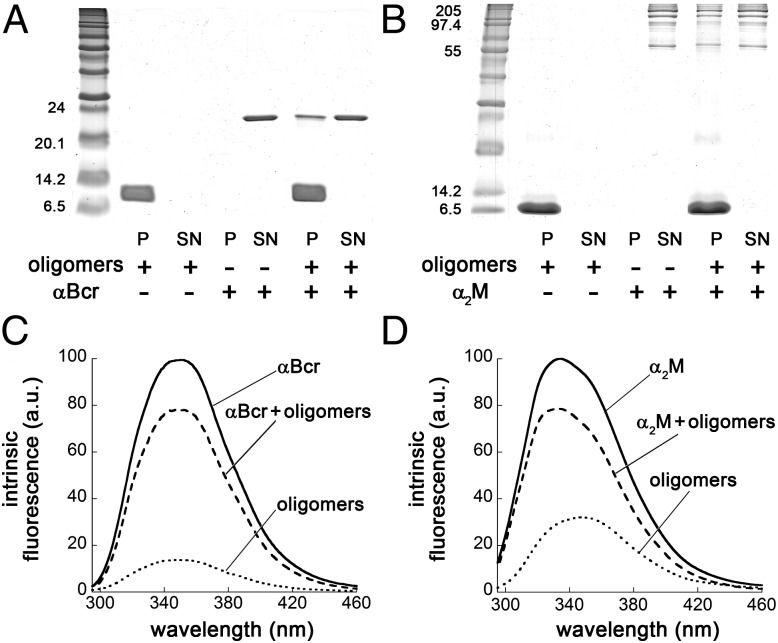
In another experiment, samples of HypF-N oligomers incubated with each chaperone were centrifuged to separate the soluble [supernatant (SN)] and insoluble [pellet (P)] fractions, which were then analyzed by SDS-PAGE. In the control samples containing oligomers or αBcr alone, the HypF-N monomer (molecular mass ∼10.5 kDa) and the αBcr monomer (molecular mass ∼20 kDa) bands were found only in the P and SN fractions, respectively (Fig. 4A). In the sample containing both species, the HypF-N band was found only in the P fraction, whereas αBcr was found to partition between the P and SN fractions (Fig. 4A). This finding indicates that a fraction of αBcr is bound to the oligomers. Moreover, the αBcr found in the P fraction remains tightly associated with the oligomers after resuspension of the P and further incubation (SI Appendix, Fig. S15). Similar results were obtained using α2M (Fig. 4B), Hsp70 (SI Appendix, Fig. S12A), Clu (SI Appendix, Fig. S13A) and Hp (SI Appendix, Fig. S14A). The results thus confirm that binding occurs between the oligomers and all five chaperones studied here. Intrinsic fluorescence spectra of the SN fractions collected in each experiment were also acquired (Fig. 4 C and D and SI Appendix, Figs. S12B, S13B, and S14B). The SNs obtained from the samples where both oligomers and chaperones were present yielded fluorescence spectra that were less intense than the corresponding samples in which only the chaperone was present, confirming that a fraction of the chaperone population had been separated through centrifugation.
Fig. 4.
Binding of chaperones to HypF-N oligomers. (A) SDS-PAGE analysis of the P and SN fractions of samples containing HypF-N oligomers (lanes 2 and 3), αBcr (lanes 4 and 5), and oligomers with αBcr (lanes 6 and 7). The bands at ∼10 kDa and 20 kDa indicate monomeric HypF-N and αBcr, respectively. The HypF-N concentration was 48 μM. (B) SDS-PAGE analysis for α2M; conditions and lanes are as in A. The α2M bands range from ∼60 kDa to ∼160 kDa. (C) Intrinsic fluorescence spectra of the SN fractions of samples containing HypF-N oligomers (dotted line), αBcr (solid line), and oligomers with αBcr (dashed line). The spectrum of HypF-N oligomers has been subtracted from that of chaperone plus oligomers to clear the contribution of the former. All spectra are the means of three experiments. (D) Intrinsic fluorescence analysis of α2M. Conditions and spectra are as in C. a.u., arbitrary units.
Overall, the AFM data indicate that chaperones promote the assembly of the oligomers into larger species, whereas the other methods of analysis indicate that the chaperones are bound to the assembled oligomers. The finding that a small fraction of chaperones is bound to the oligomers, prompted by SDS-PAGE and intrinsic fluorescence measurements, suggests that chaperones act as nucleation sites for the assembly of oligomers into larger species and explains why they are effective in reducing markedly oligomer toxicity even at highly substoichiometric concentrations.
Molecular Structure of the Oligomers Is Preserved After Chaperone Interaction.
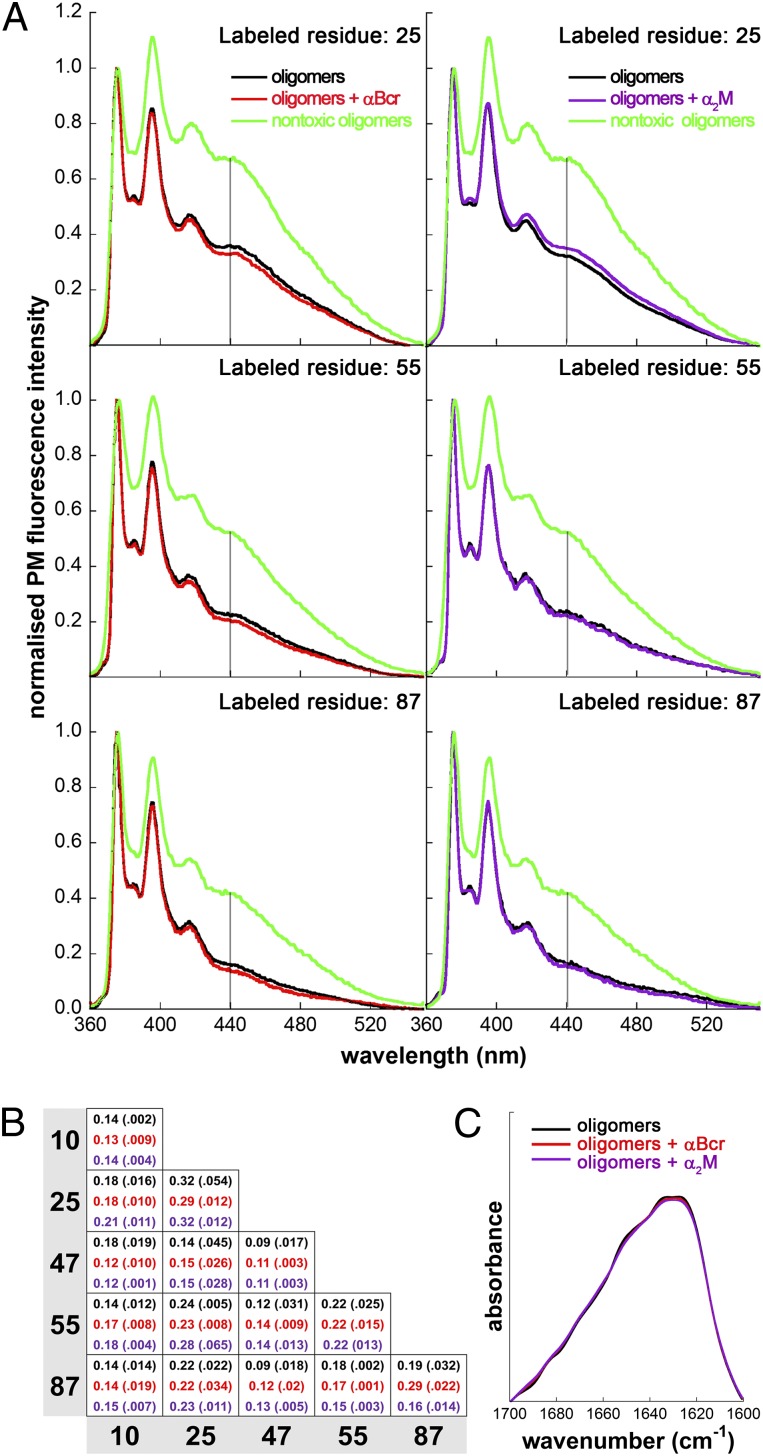
As a next step, we set out to assess if chaperones promote a structural reorganization of the oligomers, in addition to inducing their assembly. We took advantage of the ability to discriminate between the toxic HypF-N oligomers used in this work (type A) and nontoxic ones (type B) through the measurement of the fluorescence properties of oligomers labeled with PM at different positions in the sequence (14). This probe monitors the presence of a short distance (≤10 Å) between labeled positions in the oligomers, which becomes evident as an excimer band at 440 nm (14). In particular, the fluorescence spectra of nontoxic oligomers formed by HypF-N labeled with PM at position 25, 55, or 87 show an excimer band at 440 nm that is very weak in the corresponding spectra obtained with PM-labeled toxic oligomers (14) (Fig. 5A). The fluorescence spectra obtained for the toxic oligomers labeled at each position after incubation with or without αBcr or α2M are very similar, and all show the absence of an excimer band (Fig. 5A), excluding a structural conversion. Similar data were obtained with Hsp70, Clu, and Hp (SI Appendix, Figs. S12C, S13C, and S14C).
Fig. 5.
Lack of structural reorganization of HypF-N oligomers following treatment with chaperones. (A) Fluorescence emission spectra of HypF-N oligomers labeled with PM at positions 25 (Top), 55 (Middle), and 87 (Bottom). The HypF-N concentration was 12 μM. The spectra refer to oligomers incubated in 20 mM phosphate buffer, pH 7.0, without (black) and with αBcr (red) or α2M (purple). For comparison, the corresponding spectra of nontoxic oligomers are reported in each graph (green). The spectra are normalized to the intensity of the peak centered at 375 nm. The vertical lines at 440 nm indicate the position of the excimer band. (B) Ratios between the PM fluorescence intensities measured at 440 nm (excimer peak) and 375 nm (PM monomer peak) for HypF-N oligomers prepared with 1:1 mixtures of HypF-N chains PM-labeled at positions 10, 25, 47, 55, and 87. Colors are as in A. The total HypF-N concentration was 12 μM. The SDs are reported in brackets. (C) FTIR amide I spectra of HypF-N oligomers after incubation without (black) and with αBcr (red) or α2M (purple).
We extended our analysis to oligomers formed from 1:1 mixtures of HypF-N monomers labeled with PM at five different positions (residues 10, 25, 47, 55, and 87), thereby obtaining excimer ratio values for a total of 15 differently labeled HypF-N oligomers. The patterns of 15 excimer ratio values obtained in the presence of αBcr or α2M, used as representative chaperones, are essentially identical to the pattern obtained in their absence (Fig. 5B), indicating that the spatial distribution of residues in the oligomers is preserved following interaction with the chaperones.
Furthermore, examination with Fourier transform infrared (FTIR) spectroscopy indicated that no changes in secondary structure appeared to have occurred following the incubation of the oligomers with either αBcr or α2M (Fig. 5C).
Discussion
Our data show that chaperones inhibit the cellular toxicity of extracellular protein oligomers under the conditions studied here. This behavior appears to result from the ability of the chaperones to bind to oligomers and promote their further assembly into larger species, in the absence of any significant reorganization of their internal molecular structure. This mechanism is extremely effective because it allows the chaperones to reduce oligomer toxicity at highly substoichiometric levels.
Such ability of molecular chaperones adds to their well-established functions in facilitating protein folding, inhibiting protein aggregation, and promoting the disaggregation and clearance of protein aggregates (6, 7, 10, 11). In all our experiments, the oligomers are formed before adding the chaperones, showing that the protective action of the latter also includes neutralization of toxic oligomers after they have formed. This protective mechanism is not entirely undescribed, but evidence has been very sparse in the past, with reports often being contradictory and with undefined mechanisms (9, 18–22).
These data also suggest that the size of extracellular protein aggregates is an inverse correlate of their toxicity. The chaperone-induced oligomer clusters are characterized by a reduction in their exposed hydrophobic surface and diffusional mobility, both of which are expected to reduce their toxicity to cells. It is therefore clear that chaperones can be valuable tools for understanding the factors governing oligomer toxicity.
Importantly, the results shown here suggest that the structure, function, and mechanism of action of molecular chaperones may serve to guide the design of therapeutic interventions against diseases originating from extracellular protein aggregation. Indeed, the finding that natural molecular chaperones can inhibit the toxicity of aberrant protein aggregates after they are formed, with broad specificity and at very low concentrations, suggests that therapeutics based on the same type of intervention could be effective against such diseases.
Materials and Methods
Proteins.
HypF-N was purified as previously described (14). Aβ42 and IAPP were purchased from Sigma–Aldrich. Human Hsp70 and αBcr were purified as previously described (23, 24). Human Clu, α2M, and Hp were purified as previously reported (25–27). BSA and HEWL were obtained from Sigma–Aldrich.
Formation of Protein Oligomers.
HypF-N was converted into toxic (type A) or nontoxic (type B) aggregates as previously reported (14). Oligomers were centrifuged at 16,100 × g for 10 min, dried under N2, and resuspended in the cell culture medium without cells (for cell biology tests) or in 20 mM phosphate buffer, pH 7.0 (for biophysical/biochemical analysis). No significant dissolution of the oligomers or change in morphology/structure could be detected after this procedure, as previously reported (14). Toxicity was measured in all cases under physiological conditions (pH 7.4, 37 °C, no organic solvents). Aβ42 and IAPP oligomers were prepared as previously described (12, 13) and resuspended in the cell culture medium to 12 μM. Native proteins were diluted to 12 μM into the same medium. All oligomers were incubated in the appropriate media for 1 h at 37 °C while shaking, without or with chaperones, and then added to cultured cells or subjected to biophysical/biochemical analysis. The HypF-N(Aβ42/IAPP)/chaperone molar ratio was 5:1 (αBcr), 5:1 (Hsp70), 10:1 (Clu), 15:1 (Hp), and 100:1 (α2M), unless stated otherwise (all proteins are considered as monomers, except Clu and Hp as αβ dimers and α2M as a tetramer).
MTT Reduction Assay.
Oligomers of HypF-N, Aβ42, and IAPP (at a corresponding monomer concentration of 12 μM) were incubated for 1 h without or with αBcr, Hsp70, Clu, Hp, α2M, HEWL, or BSA (HypF-N/chaperone molar ratios as described above, HypF-N/HEWL and HypF-N/BSA molar ratios were 5:1) and then added to SH-SY5Y cells. The MTT reduction assay was performed as previously described (14). Further details are given in SI Appendix, Materials and Methods.
Measurement of Intracellular Ca2+.
HypF-N oligomers (at a corresponding monomer concentration of 12 μM) were incubated for 1 h in the cell culture medium without or with αBcr, Hsp70, Clu, Hp, or α2M and then added to SH-SY5Y cells seeded on glass coverslips for 60 min at 37 °C. Cells were then loaded with 10 μM fluo3-AM (Molecular Probes), as previously described (14, 15). Cells were also treated with nontoxic HypF-N oligomers or the native protein (12 μM monomer). Cell fluorescence was analyzed by confocal scanning microscopy, as previously described (14, 15).
AFM.
Samples prepared as detailed above were diluted from 5- to 100-fold, as required, and 10-μL aliquots were deposited on freshly cleaved mica and dried under a mild vacuum. Tapping mode AFM images were acquired in air using a Multimode SPM, equipped with a “E” scanning head (maximum scan size of 10 μm) and driven by a Nanoscope IV controller, and a Dimension 3100 SPM, equipped with a “G” scanning head (maximum scan size of 100 μm) and driven by a Nanoscope IIIa controller (Digital Instruments, Bruker AXS GmbH). Single-beam uncoated silicon cantilevers (type OMCL-AC160TS; Olympus) were used. The drive frequency was 290–310 kHz, and the scan rate was 0.4–0.8 Hz. Aggregate sizes were measured from the height in cross-section of the topographic AFM images. The reported heights result from the obtained values multiplied by a shrinking factor of 2.2, which was evaluated comparing the heights of native HypF-N under liquid and after drying.
Confocal Microscopy Analysis for Chaperone-Oligomer Binding.
HypF-N oligomers (at a corresponding monomer concentration of 48 μM) were incubated for 1 h without or with αBcr or α2M and then centrifuged to obtain Ps that were resuspended and incubated subsequently for 30 min at 37 °C in solutions containing: (i) 1:4,000 rabbit polyclonal anti–HypF-N antibodies (Primm) and goat polyclonal anti-αBcr antibodies or goat polyclonal anti-α2M antibodies (Santa Cruz Biotechnology), (ii) 1:1,000 Alexa Fluor 488-conjugated anti-rabbit secondary antibodies (Molecular Probes), and (iii) 1:4,000 Texas red-conjugated anti-goat secondary antibodies (Santa Cruz Biotechnology). After every incubation, samples were centrifuged for 10 min at 16,100 × g, washed in PBS, and centrifuged again. Finally, the P was resuspended in 20 μL PBS and spotted on glass coverslips. The cross-reactivity of oligomers was tested by subsequent incubations with primary and secondary anti-αBcr or anti-α2M antibodies. Confocal microscope images were acquired as previously described (14).
SDS-PAGE.
HypF-N oligomers, αBcr, and α2M were incubated for 1 h in 20 mM phosphate buffer at pH 7.0 in isolation and in combination (48 μM HypF-N). Samples were then centrifuged for 10 min at 16,100 × g. Aliquots of the P and SN fractions were subjected to SDS-PAGE using 15% (wt/vol) polyacrylamide gels.
Intrinsic Fluorescence.
Intrinsic fluorescence spectra of the SN fractions collected for SDS-PAGE were acquired at 37 °C using a Perkin–Elmer LS 55 spectrofluorometer and a 2-mm × 10-mm quartz cell, with an excitation wavelength of 280 nm. The spectrum of HypF-N oligomers was subtracted from that of the chaperone in the presence of HypF-N oligomers, and all the spectra were normalized to the maximum fluorescence intensity of the chaperone spectrum.
Pyrene Fluorescence.
HypF-N variants carrying a single cysteine residue were labeled with PM as previously described (14), converted into toxic and nontoxic oligomers in homogeneous or 1:1 mixtures, and then diluted fourfold into 20 mM phosphate buffer at pH 7.0. Fluorescence emission spectra of these samples were acquired after 1 h without or with chaperones and analyzed as previously described (14). The HypF-N concentration was 12 μM.
FTIR Spectroscopy.
HypF-N oligomers were incubated with or without αBcr or α2M (48 μM HypF-N) and then centrifuged and resuspended in D2O twice to achieve a final protein concentration of ∼1.4 mM. The sample was deposited on a potassium bromide window in a semipermanent liquid cell, and the FTIR spectra were recorded at room temperature using an FT/IR 4200 spectrophotometer (Jasco). The system was constantly purged with N2. The resulting spectra were baseline-corrected and smoothed.
Statistical Analysis.
All data were expressed as means ± SD. Comparisons were performed using ANOVA, followed by Bonferroni’s postcomparison test.
Supplementary Material
Acknowledgments
We thank Daniela Nichino for help in the AFM measurements. We thank the Italian Ministero dell’Istruzione, dell’Università e della Ricerca (F.C., B.M., C.C., R.C., S.C., A.R., and A.P.), the Royal Society for a Dorothy Hodgkin Fellowship (to S.M.), and the Spanish Ministry of Health according to the “Plan Nacional de I+D+I 2008-2011” through the Instituto de Salud Carlos III with cofunding by Fondo Europeo de Desarrollo Regional (to C.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117799109/-/DCSupplemental.
References
- 1.Monsellier E, Chiti F. Prevention of amyloid-like aggregation as a driving force of protein evolution. EMBO Rep. 2007;8:737–742. doi: 10.1038/sj.embor.7401034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 4.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weibezahn J, Schlieker C, Tessarz P, Mogk A, Bukau B. Novel insights into the mechanism of chaperone-assisted protein disaggregation. Biol Chem. 2005;386:739–744. doi: 10.1515/BC.2005.086. [DOI] [PubMed] [Google Scholar]
- 7.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 8.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Wilson MR, Yerbury JJ, Poon S. Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol Biosyst. 2008;4:42–52. doi: 10.1039/b712728f. [DOI] [PubMed] [Google Scholar]
- 10.Broadley SA, Hartl FU. The role of molecular chaperones in human misfolding diseases. FEBS Lett. 2009;583:2647–2653. doi: 10.1016/j.febslet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Pickart CM, Cohen RE. Proteasomes and their kin: Proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 12.Lambert MP, et al. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J Neurochem. 2001;79:595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- 13.Cecchi C, et al. Replicating neuroblastoma cells in different cell cycle phases display different vulnerability to amyloid toxicity. J Mol Med (Berl) 2008;86:197–209. doi: 10.1007/s00109-007-0265-3. [DOI] [PubMed] [Google Scholar]
- 14.Campioni S, et al. A causative link between the structure of aberrant protein oligomers and their toxicity. Nat Chem Biol. 2010;6:140–147. doi: 10.1038/nchembio.283. [DOI] [PubMed] [Google Scholar]
- 15.Zampagni M, et al. A comparison of the biochemical modifications caused by toxic and non-toxic protein oligomers in cells. J Cell Mol Med. 2011;15:2106–2116. doi: 10.1111/j.1582-4934.2010.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carra S, et al. Identification of the Drosophila ortholog of HSPB8: Implication of HSPB8 loss of function in protein folding diseases. J Biol Chem. 2010;285:37811–37822. doi: 10.1074/jbc.M110.127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabrizi C, Businaro R, Lauro GM, Fumagalli L. Role of α2-macroglobulin in regulating amyloid β-protein neurotoxicity: Protective or detrimental factor? J Neurochem. 2001;78:406–412. doi: 10.1046/j.1471-4159.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 19.Oda T, et al. Clusterin (apoJ) alters the aggregation of amyloid β-peptide (A β 1-42) and forms slowly sedimenting A β complexes that cause oxidative stress. Exp Neurol. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 20.Boggs LN, et al. Clusterin (Apo J) protects against in vitro amyloid-β (1-40) neurotoxicity. J Neurochem. 1996;67:1324–1327. doi: 10.1046/j.1471-4159.1996.67031324.x. [DOI] [PubMed] [Google Scholar]
- 21.Du Y, et al. α2-macroglobulin attenuates β-amyloid peptide 1-40 fibril formation and associated neurotoxicity of cultured fetal rat cortical neurons. J Neurochem. 1998;70:1182–1188. doi: 10.1046/j.1471-4159.1998.70031182.x. [DOI] [PubMed] [Google Scholar]
- 22.Yerbury JJ, et al. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007;21:2312–2322. doi: 10.1096/fj.06-7986com. [DOI] [PubMed] [Google Scholar]
- 23.Roodveldt C, et al. Chaperone proteostasis in Parkinson’s disease: Stabilization of the Hsp70/α-synuclein complex by Hip. EMBO J. 2009;28:3758–3770. doi: 10.1038/emboj.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waudby CA, et al. The interaction of alphaB-crystallin with mature α-synuclein amyloid fibrils inhibits their elongation. Biophys J. 2010;98:843–851. doi: 10.1016/j.bpj.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson MR, Easterbrook-Smith SB. Clusterin binds by a multivalent mechanism to the Fc and Fab regions of IgG. Biochim Biophys Acta. 1992;1159:319–326. doi: 10.1016/0167-4838(92)90062-i. [DOI] [PubMed] [Google Scholar]
- 26.French K, Yerbury JJ, Wilson MR. Protease activation of α2-macroglobulin modulates a chaperone-like action with broad specificity. Biochemistry. 2008;47:1176–1185. doi: 10.1021/bi701976f. [DOI] [PubMed] [Google Scholar]
- 27.Yerbury JJ, Rybchyn MS, Easterbrook-Smith SB, Henriques C, Wilson MR. The acute phase protein haptoglobin is a mammalian extracellular chaperone with an action similar to clusterin. Biochemistry. 2005;44:10914–10925. doi: 10.1021/bi050764x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.