Abstract
Macromolecules enter cells by endocytosis and are sorted to different cellular destinations in early/sorting endosomes. The mechanism and regulation of sorting are poorly understood, although transitions between vesicular and tubular endosomes are important. We found that the antihypertensive drug Prazosin inhibits endocytic sorting by an off-target perturbation of the G protein-coupled receptor dopamine receptor D3 (DRD3). Prazosin is also a potent cytokinesis inhibitor, likely as a consequence of its effects on endosomes. Prazosin stabilizes a normally transient interaction between DRD3 and the coatomer COPI, a complex involved in membrane transport, and shifts endosomal morphology entirely to tubules, disrupting cargo sorting. RNAi depletion of DRD3 alone also inhibits endocytic sorting, indicating a noncanonical role for a G protein-coupled receptor. Prazosin is a powerful tool for rapid and reversible perturbation of endocytic dynamics.
Keywords: small-molecule inhibitor, endocytic tubulation, unconventional G protein-coupled receptor function, drug off-target effects, membrane trafficking
G protein-coupled receptors (GPCRs) are the most important chemosensing receptors in animals and the targets of many therapeutic drugs. They have mostly been studied from the perspective of signal transduction and pharmacology, but there have been hints that GPCRs are important regulators of basic cellular processes (1, 2). The dopamine receptors (D1–5) are GPCRs with important functions in the brain, and they are targeted by numerous drugs used to treat neurological disorders ranging from Parkinson disease to schizophrenia. Although there is a large amount of literature about the regulation of GPCR signaling by endocytosis, much less is known about how or if GPCRs, in turn, regulate endocytosis (3–5). Our data show that the dopamine receptor D3 (DRD3) plays an unexpected role in endocytic sorting.
Many different proteins and complexes enter cells by endocytosis, and they must be rapidly sorted for transport to different locations in the cell. For example, some cargoes are recycled to the plasma membrane, whereas others are sent to lysosomes. Sorting occurs in specialized compartments called early or sorting endosomes. Highly dynamic trafficking occurs between these compartments and multiple other cellular compartments, driven by sorting, budding, fission, and fusion reactions (6). Although some individual steps in endocytic sorting have been elucidated, their coordination in cells remains mysterious. Dynamic transitions between vesicular and tubular endosomes seem to be key factors in determining the fate of endocytic cargoes; the coatomer complex COPI may play a role in these dynamics (7, 8), but precisely how these transitions are regulated is unclear. One reason that it has been difficult to elucidate sorting endosome dynamics in living cells has been the lack of small-molecule tools to rapidly and reversibly perturb them. Elucidation of Golgi dynamics benefitted greatly from use of Brefeldin (9), a small molecule that inhibits Arf guanine-nucleotide exchange factor (ArfGEF), perturbing the functions of COPI at the Golgi (10).
Prazosin, which we describe here as a tool for endocytosis research, is an important drug that has been used clinically for decades to treat hypertension, prostate hyperplasia, posttraumatic stress disorder, and scorpion stings (11). Its primary known mechanism is to antagonize α1-adrenergic receptors, a subfamily of GPCRs. GPCR receptor drugs often bind to GPCRs other than the primary target, and such off-target interactions can play important roles in therapy and toxicity. Here, we report an interesting off-target activity at DRD3.
Results
Prazosin Inhibits Late Stages During Cell Division.
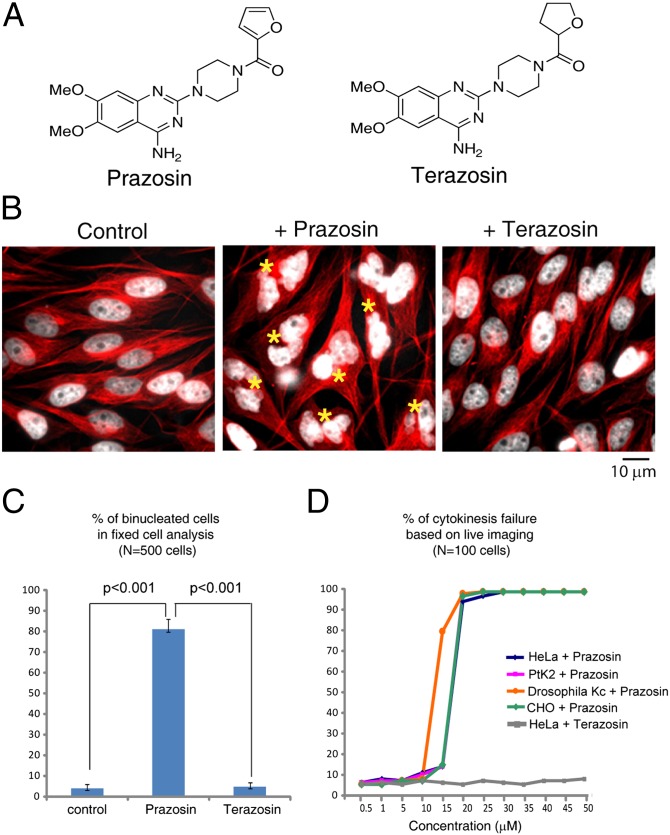
In a screen for small-molecule inhibitors of cytokinesis (12), the final step of cell division, Prazosin (Fig. 1A) unexpectedly scored as a strong hit, with over 80% of dividing HeLa cells becoming binucleated (Fig. 1 B and C). Our initial screen was in Drosophila Kc167 cells, but we found that the actions of Prazosin were similar in all mammalian cell lines tested (Fig. 1D), suggesting a fairly general mechanism. A chemically related compound, Terazosin (Fig. 1A), was inactive and is used as a control throughout this work. Because Terazosin antagonizes α1-adrenergic receptors as effectively as Prazosin, the effect of Prazosin on cytokinesis is likely to be an off-target interaction. Time-lapse imaging showed that Prazosin blocks cytokinesis at the abscission stage after furrow constriction (SI Appendix, SI Materials and Methods and Fig. S1). Abscission is thought to require complex plasma membrane dynamics, including secretion and endocytosis (13), which suggests that Prazosin might perturb these dynamics.
Fig. 1.
Prazosin inhibits cytokinesis. (A) Chemical structures of Prazosin and its inactive analog Terazosin. (B) Fixed cell analysis shows that Prazosin induces cytokinesis failure and binucleated cell formation, whereas Terazosin does not. HeLa cells were incubated with DMSO, 20 μM Prazosin, or Terazosin for 36 h before being fixed and stained for microtubules (red) and DNA (white). Binucleated cells are indicated by an asterisk. (Scale bar: 10 μm.) (C) Quantification of B. (D) Quantification of cytokinesis failure in different cell lines after Prazosin treatment shows that Prazosin-induced cytokinesis inhibition is conserved. Terazosin-treated HeLa cells are also included (SI Appendix, SI Materials and Methods).
Prazosin Induces Endosomal Tubules and Inhibits Endosomal Sorting.
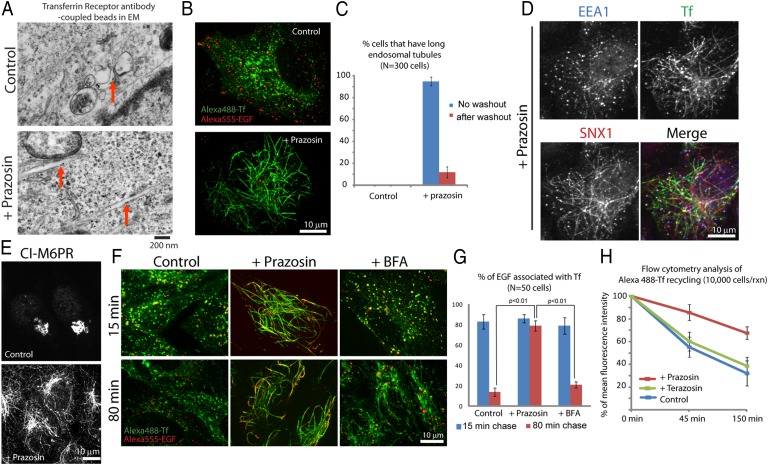
EM analysis revealed that Prazosin treatment induced striking membrane tubules within the cytoplasm up to 20 μm in length and ∼100 nm in diameter (Fig. 2A and SI Appendix, SI Materials and Methods). These tubules morphologically resembled one reported form of early endosomes (7). Prazosin-induced tubules were strongly labeled by fluorescent transferrin, a marker of endocytic trafficking (Fig. 2B), and gold nanoparticles coupled to transferrin receptor antibodies localized to tubules (Fig. 2A). Robust transferrin-stained tubules formed within 10 min of 20 μM Prazosin treatment and were present in nearly 100% of cells, indicating a lack of cell cycle dependence. The effect was reversible; tubules disappeared within minutes after drug washout (Fig. 2C and SI Appendix, Fig. S2A).
Fig. 2.
Prazosin induces endosomal tubulation and inhibits sorting. (A) Electron micrographs of Prazosin-induced transferrin receptor positive tubules. Transferrin receptor antibody-coupled gold beads were added to HeLa cells for 10 min before control DMSO, or 30 μM Prazosin was added for 1 additional h. Red arrows show gold beads in vesicles or tubules. (Scale bar: 200 nm.) (B) Prazosin-treated HeLa cells exhibit robust transferrin receptor-positive endosomal tubules, whereas lysosomes are not affected. Alexa488-Transferrin and Alexa555-EGF were added to cells for 45 min. Then, cells were chased in the presence of DMSO or 30 μM Prazosin for 30 min in marker-free medium to allow EGF to reach the lysosomes and clear from earlier pathways. (C) Quantification of the endosomal tubules in Prazosin-treated cells and washout cells using fixed cell analysis. Cells were treated with DMSO or 30 μM Prazosin for 30 min followed by washing out with drug-free medium for 30 min. Endosomal tubules longer than 5 μm are classified as long endosomal tubules. (D) Prazosin induces an array of endosomal tubules that contain transferrin (and transferrin receptor and Rab11) (SI Appendix, Fig. S2B), SNX1 (and SNX2), and EEA1 (and Rab5) (SI Appendix, Fig. S2B). Representative micrographs show transferrin, SNX1, and EEA1 localization in a Prazosin-treated HeLa cell. Alexa 488-Transferrin (green) was added to HeLa cells for 10 min before 30 μM Prazosin was added for an additional 1 h. Cells were washed and fixed before staining with anti-SNX1 (red) and anti-EEA1 (blue) antibodies. (E) Prazosin-treated HeLa cells form tubules that contain CI-M6PR. (F) Endosomal sorting is disrupted in endosomal tubules induced by Prazosin but not Brefeldin A. Pulse and chase experiments in HeLa cells show that both transferrin and EGF are trapped in endosomal tubules during sorting. HeLa cells were treated with DMSO control, 30 μM Prazosin, or 10 μg/mL BFA for 1 h. Then, Alexa 488-Transferrin and Alexa 555-EGF were added for 10 min. Images were taken at indicated times after washing out with marker- and phenol red-free medium. (G) Quantification of results in F. (H) Flow cytometry analysis of transferrin recycling in control, 30 μM Prazosin, or Terazosin-treated HeLa cells.
Prazosin perturbed a specific step in the endocytic pathway. Initial vesicle internalization from the plasma membrane was not perturbed by Prazosin (SI Appendix, Fig. S3A). Localization of clathrin (SI Appendix, Fig. S3B) was not perturbed, and lysosome-associated membrane protein (LAMP1) (a marker of lysosomes) (SI Appendix, Fig. S3B) or fluorescently labeled EGF resident in lysosomes after pulse-chase experiments were not perturbed (Fig. 2B). Endoplasmic reticulum or Golgi resident proteins were also not affected (SI Appendix, Fig. S3B), and endosomal pH was not affected. Recycling endosome markers (transferrin, transferrin receptor, and Rab11) showed robust staining along the tubules along with sorting nexin 1 (SNX1) (Fig. 2D and SI Appendix, Fig. S2B) and proteins that traffic between endosomes and the Golgi, such as cation-independent mannose 6 phosphate receptor (CI-M6PR) (Fig. 2E). Prazosin-induced tubules contain patches of early endosomal markers, a portion of which resided stably in tubules while the rest existed as vesicles (Fig. 2D and SI Appendix, Fig. S2B).
To test if Prazosin inhibits endosomal sorting, we added two differentially labeled cargoes, EGF and transferrin, to live cells. In untreated cells, both were trafficked through the endocytic pathway; however, EGF then moved to lysosomes, and transferrin moved to recycling endosomes and eventually, the plasma membrane as expected (14). We showed, in Fig. 2B, that EGF already resident in lysosomes is not affected by Prazosin. However, EGF localized to and remained in tubules if it was added to cells pretreated with Prazosin; transferrin did the same, and recycling was inhibited by Prazosin (Fig. 2 F–H and SI Appendix, SI Materials and Methods). Unlike Brefeldin, which induces transferrin-positive endosomal tubules without blocking sorting (Fig. 2 F and G), Prazosin seems to be an inhibitor of endosomal sorting.
Some endosomal pathways use microtubules as highways to transport vesicles to cellular locations as required. Early steps of sorting are thought to be independent of microtubules (15–18), but the morphology of the membrane tubules suggested a possible cytoskeleton involvement. We found that Prazosin-induced tubules are not much affected by microtubule depolymerization (SI Appendix, Fig. S4 A and B). Prazosin-induced tubules were also independent of actin (SI Appendix, Fig. S4C). To our knowledge, Prazosin treatment is the only type of cellular perturbation that can cause complete but reversible tubulation of sorting endosomes, providing an opportunity to study the factors that regulate the formation and turnover of these important trafficking platforms.
Effects of Prazosin on Endocytic Sorting Are Independent of Its Clinical Targets, the Adrenergic Receptors.
To determine if the adrenergic receptors are involved in Prazosin-induced endosome tubulation, we used RNAi to deplete α1-adrenergic receptors in HeLa cells, which did not inhibit their response to Prazosin (SI Appendix, Fig. S5). Other small-molecule adrenergic antagonists, either closely related to Prazosin (e.g., Terazosin) (Fig. 1A) or chemically unrelated (e.g., Corynanthine), did not cause the same phenotypes as Prazosin, even at high concentrations (SI Appendix, Fig. S5).
Effects of Prazosin on Endocytic Sorting Are Mediated by DRD3.
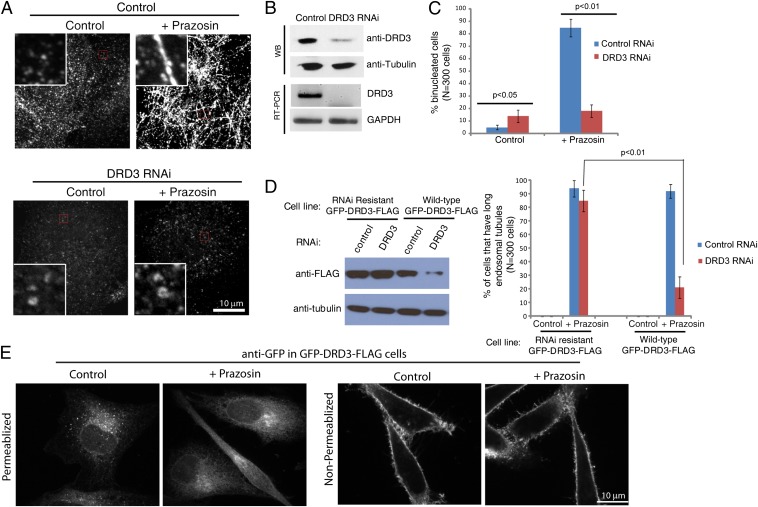
Drugs that target GPCRs can be promiscuous in binding activity between related GPCRs. We profiled the activity of Prazosin across a GPCR panel using functional readouts and found that, in addition to adrenergic receptors, it robustly antagonized 5 of 158 GPCRs tested (SI Appendix, Table S1A), including dopamine receptors D1 and D2. RNAi knockdown of these receptors in HeLa did not significantly affect the Prazosin response. Unexpectedly, however, RNAi of the related DRD3, which was not in the GPCR panel, completely blocked the endosome tubulation activity of Prazosin (Fig. 3 A and B) as well as its cytokinesis-inhibiting activity (Fig. 3C). Unlike other dopamine receptors that are significantly enriched in the brain, DRD3 seems to be expressed at low levels in most tissues (www.genevestigator.com) as well as HeLa cells (Fig. 3B and SI Appendix, SI Materials and Methods) (19) and other cancer cells (20), suggesting that it might play a broader role. To confirm that our observations were not caused by off-target effects of the siRNAs targeting this receptor, we constructed a cell line that expressed an RNAi-resistant GFP-DRD3 allele (SI Appendix, SI Materials and Methods). As expected for a specific knockdown, DRD3 RNAi in the RNAi-resistant cell line did not inhibit the effects of Prazosin on tubulation and cytokinesis (Fig. 3D).
Fig. 3.
DRD3 is involved in endocytic sorting and mediates the effects of Prazosin. (A) DRD3 RNAi prevents Prazosin-induced endosomal tubule formation. Transferrin receptor staining is shown. HeLa cells were treated with control or DRD3 siRNA for 3 d before DMSO or 30 μM Prazosin was added for 1 h. (B) Western blot and semiquantitative RT-PCR show the expression and knockdown of DRD3 in HeLa cells. (C) DRD3 RNAi causes an increase in binucleated cells and prevents strong cytokinesis inhibition induced by Prazosin. (D) Western blots show the specificity and efficiency of DRD3 RNAi knockdown. Cells with long endosomal tubules (longer than 5 μm) were counted in three independent experiments. (E) The localization of DRD3. GFP-DRD3-FLAG-HeLa cells were treated with control DMSO or 30 μM Prazosin for 1 h before being fixed in PBS + formaldehyde. Cells were processed with anti-GFP antibody staining using two different conditions. Left shows cells that were permeabilized using 0.1% Triton X-100, and Right shows unpermeablized cells. Live GFP-DRD3-FLAG-HeLa cells show similar localizations to the anti-GFP staining in permeablized cells, indicating that the membrane population of DRD3 is relatively low compared with its intracellular population, which is similar to transferrin receptor. The commercially available anti-DRD3 antibody shows highly unspecific staining and is not suitable for immunofluorescence. (Scale bar: 10 μm.)
Using a standard downstream signaling (cAMP level) assay and a β-Arrestin Recruitment Tango Assay (21) (SI Appendix, SI Materials and Methods), we tested if Prazosin acts on DRD3 in a classical manner. We found that it did not behave as a classical GPCR agonist, antagonist, or allosteric modulator (SI Appendix, Fig. S6 and Table S1B). This finding is consistent with our observation that treatment with other DRD3 agonists/antagonists, including 7-OH-DPAT and SB-27701-A, does not give rise to the same tubulation, inhibition of transferrin recycling, or cytokinesis block phenotypes as Prazosin (SI Appendix, Fig. S7). This finding suggests that Prazosin does not act at traditional small-molecule binding sites on DRD3.
Knockdown of DRD3 Affects Endocytic Sorting Independently of Prazosin.
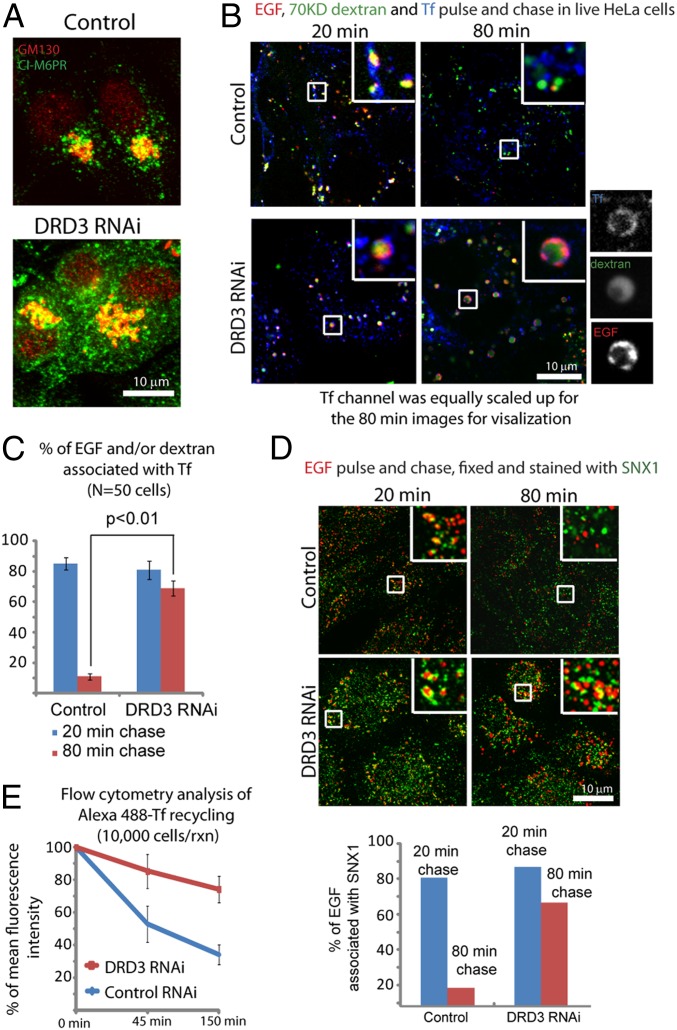
To test if DRD3 regulates endocytic sorting independent of Prazosin action, we evaluated the effect of DRD3 RNAi and found that DRD3 knockdown alone shares some of the effects of Prazosin on cells. Like Prazosin, DRD3 knockdown did not affect initial internalization of fluorescent transferrin or EGF (SI Appendix, Figs. S3A and S8A) or localization of clathrin or dynamin (SI Appendix, Figs. S3B and S8B). However, both perturbed transferrin trafficking, and DRD3 knockdown caused a modest increase in binucleated cells (two- to threefold) (Fig. 3C and SI Appendix, Fig. S8C). Trafficking between endosomes and the Golgi, as visualized by CI-M6PR staining, was also perturbed by both treatments (Figs. 2E and 4A). DRD3 knockdown caused an overall reduction of transferrin staining, and the remaining vesicles were enlarged (Figs. 3A and 4B), which can be a consequence of endocytic sorting defects. To test if endocytic sorting was affected by the absence of DRD3, we treated cells with a pulse of fluorescently labeled markers (transferrin, EGF, and dextran) and observed their progress through the endocytic transport network after chasing with unlabeled medium as described above for Prazosin. In control and DRD3 RNAi cells, most vesicles contained all three markers 20 min after treatment (Fig. 4 B and C), suggesting that they had reached sorting endosomes and were being prepared for transport to their respective pathways; 80 min after chasing in control cells, the markers had separated almost completely, and each marker had arrived at its appropriate destination (Fig. 4 B and C). In contrast and similar to Prazosin treatment, the fluorescent markers continued to be highly colocalized at 80 min in DRD3-depleted cells (Fig. 4 B and C), and many of these endosomes were positive for a sorting endosome marker, SNX1 (Fig. 4D). Also similar to Prazosin treatment, transferrin recycling, measured by FACS analysis, was inhibited in DRD3 knockdown cells (Fig. 4E). Thus, loss of dopamine receptor DRD3 seems to slow down endocytic sorting.
Fig. 4.
DRD3 is involved in endocytic sorting. (A) DRD3 RNAi causes increased CI-M6PR localization to vesicles in addition to the Golgi. Control or DRD3 RNAi-treated cells were stained for CI-M6PR (green) and a resident Golgi marker GM130 (red). (B) Pulse and chase experiments show sorting defects in DRD3 RNAi cells. HeLa cells were treated with control or DRD3 RNAi for 3 d before Alexa 555-EGF, FITC-70 kD-dextran, and Alexa 647-Transferrin were added for 10 min followed by washing out with marker- and phenol red-free medium. Pictures were taken at indicated time points in live cells. (C) Quantification of EGF and/or dextran that colocalize or partially colocalize with transferrin; (D) Endocytic cargoes are trapped in SNX1-positive endosomes after DRD3 RNAi. HeLa cells were treated with control or DRD3 RNAi for 3 d before Alexa 555-EGF (red) was added for 10 min followed by washing out with marker-free medium for indicated time points. Cells were than washed, fixed, and stained with anti-SNX1 (green). EGF that colocalizes or partially colocalizes with SNX1 was quantified. For each condition, around 50 cells were counted. Mean values from two independent experiments are shown. (Scale bar: 10 μm.) (E) Flow cytometry analysis of transferrin recycling in control or DRD3 RNAi knockdown HeLa cells.
Prazosin Stabilizes an Interaction Between DRD3 and the Coatomer Complex COPI.
Our data suggest that either Prazosin binds directly to DRD3 in a nonclassical way or DRD3 is required to manifest the effects of Prazosin binding to another target. We assayed the effects of Prazosin on interactions of other proteins with DRD3 using immunoprecipitation from cell lines stably expressing FLAG-tagged GFP-DRD3 (22) (SI Appendix, SI Materials and Methods). We focused on interaction partners likely to mediate membrane dynamics. Although the most obvious candidates, including dynamin and sorting nexins 1 and 2, showed not effect, Prazosin substantially increased the interaction between DRD3 and subunits of the COPI coatomer complex. We tested COPI, because it is involved in membrane trafficking and was previously associated with cytokinesis failure (12). All complex members for which we could obtain antibodies were strongly enriched in the DRD3 pull-down assay but only in the presence of Prazosin (Fig. 5A).
Fig. 5.
The COPI complex interacts with DRD3 in the presence of Prazosin and is involved in its effects on endocytosis. (A) Pull-down experiments using anti-FLAG antibody in GFP-DRD3-FLAG-HeLa cells treated with DMSO, Prazosin, or Terazosin show that the interaction of DRD3 with COPI subunits COPB, COPC, and COPG is increased after Prazosin treatment. (B) COPB, COPD, and COPG localizations are disrupted by Prazosin treatment. HeLa cells were treated with DMSO or 30 μM Prazosin for 1 h before fixing and staining with COP antibodies. (C) COPB1 or COPB2 RNAi prevents endosomal tubule formation in Prazosin-treated HeLa cells. Transferrin receptor staining is shown. HeLa cells were treated with siRNAs for 3 d before DMSO or 30 μM Prazosin was added for 1 h. (D) Pulse and chase experiments show sorting defects in COPB RNAi cells. HeLa cells were treated with control or COPB1 + COPB2 RNAi for 3 d before Alexa 488-Tf and FITC-70 kD-dextran were added for 10 min followed by washing out with marker- and phenol red-free medium. Individual COPB1 or COPB2 RNAi resulted in similar phenotypes. Pictures were taken at indicated time points without fixing the cells. (Scale bar: 10 μm.) (E) Flow cytometry analysis of transferrin recycling in control or COPB1 or COPB2 RNAi knockdown HeLa cells.
The COPI complex is important in retrograde transport from the Golgi to the endoplasmic reticulum (23, 24), and a role in the budding of vesicles from early endosomes has also been proposed (25–30). In untreated cells, COPI subunits localized mostly to the Golgi, and a small fraction localized to the cytoplasm or vesicles distributed throughout the cytoplasm. Prazosin treatment caused an increased fraction of COPI subunits to dissociate from the Golgi and localize to cytoplasmic non-Golgi regions (Fig. 5B). We previously reported that COPI RNAi results in cytokinesis failure, but generally, RNAi of most COPI subunits is cytotoxic, making it difficult to assess their functions (12). Although knockdown of either isoform of β-COP (COPB1 and COPB2) is also toxic to an extent, the remaining live cells show similar phenotypes to DRD3 RNAi and prevent the formation of Prazosin-induced endosomal tubules (Fig. 5C). This finding indicates that COPI may be required for the effect of Prazosin on endosomes and cytokinesis. β-COP knockdown in the absence of Prazosin also inhibited endosomal sorting and transferrin recycling in a manner similar to DRD3 knockdown (Fig. 5 D and E).
GPCRs can bind effector proteins through their cytoplasmic loops, and the third loop has been implicated in dopamine receptors’ interactions with multiple binding partners (31, 32). We constructed a cell line expressing DRD3-RNAi–resistant GFP-DRD3Δl-FLAG, where the third cytoplasmic loop was replaced with the shorter second cytoplasmic loop (SI Appendix, SI Materials and Methods). Prazosin-induced tubule formation was prevented in cells where the loop mutant replaced endogenous DRD3 (SI Appendix, Fig. S9A). Furthermore, COPI binding to DRD3 in the presence of Prazosin was reduced in cells expressing GFP-DRD3Δl-FLAG (SI Appendix, Fig. S9B). Although these data suggest that the third cytoplasmic loop of DRD3 is involved in the effects of Prazosin, we cannot exclude the possibility that the mutant is incorrectly folded and therefore, unable to rescue the WT response to Prazosin. Understanding how interactions between DRD3 and COPI contribute to endosome conformational changes and sorting defects is a high priority in the future.
Discussion
Our experiments implicate DRD3 and COPI in endocytic sorting. Individual removal of either DRD3 or COPI proteins leads to sorting defects (Figs. 4 B–D and 5D). However, neither of these treatments by themselves result in the formation of tubules, and both treatments prevent tubule formation in response to Prazosin. These data support a model in which DRD3 and COPI both function in sorting and the addition of Prazosin stabilizes a nonproductive complex between them and probably, other proteins (Fig. 5A). The concept of a small-molecule stabilizing interactions between proteins is familiar from chemical inducers of dimerization (33), suggesting that Prazosin may act similarly.
Although our data suggest that the third cytoplasmic loop of D3 might be involved, exactly where Prazosin binds in this complex is not yet clear. The fact that it antagonizes DRD1 and -2 suggests that it can bind to closely related GPCRs and may, therefore, bind to DRD3 directly. Perhaps it binds to DRD3 in a nonstandard manner that is not competitive with traditional ligands, or perhaps it induces a conformational change, possibly perturbing receptor oligomerization, which is increasingly recognized as a key step in GPCR function (34). The very sharp dose–response curve observed for Prazosin (Fig. 1D) could support an oligomerization model, where a signaling cascade is triggered when a critical mass has been reached.
Although their ultimate effects on cells are different (e.g., Brefeldin perturbs Golgi, but Prazosin does not; morphology and stability of Prazosin- and Brefeldin-induced endosome tubules are different) (Fig. 2F and SI Appendix, Fig. S4), the mechanisms of action of Prazosin and Brefeldin involve the same protein complex, the coatomer COPI. Brefeldin has been used successfully to study Golgi dynamics (10). COPI localizes not only to the Golgi but also to small vesicles and the cytoplasm. Similarly, DRD3 is also found in different cellular pools (Fig. 3E). Prazosin seems to target specific subpools of both proteins. It triggers an increased association between DRD3 and COPI and their absence from the newly formed endosomal tubules. Taken together, these data suggest that a transient but important regulatory interaction between DRD3 and COPI, stabilized by Prazosin, is key in regulating the equilibrium between vesicular and tubular endosomes and therefore, endosomal sorting.
The effect of Prazosin on endosome dynamics is interesting clinically, both because endosomal trafficking is involved in numerous disease-related processes and because the action of Prazosin implicates a GPCR from an important family in a basic and general biological regulatory process. Given that many clinically approved drugs targets GPCRs, a better understanding of these receptors’ biological functions will be critical in developing more efficacious therapeutics. Prazosin is the only clinically approved drug that inhibits a specific step during endocytosis, and we know from its long history that it is relatively nontoxic. Targeting endocytic processes clinically would require higher Prazosin doses than those doses currently used in antihypertension therapy, because concentrations in patients’ blood are about 100-fold less than we reported here; however, Prazosin can be a starting point in additional development. Our findings highlight the increasingly accepted concept that even the most widely used and safe drugs often have unexpected targets and mechanisms. Off-target effects of compounds that have been thoroughly vetted in humans are always of great interest to both understand their efficacy and toxicity in their current indications and spark ideas for new indications.
Materials and Methods
Cell Culture and Immunofluorescence.
HeLa cells were grown in monolayers in DMEM supplemented with 10% (vol/vol) heat-inactivated FBS (Invitrogen) and 1% (vol/vol) penicillin/streptomycin (P/S; Cellgro). Prazosin (P7791) and Terazosin (T4680) are from Sigma.
For all immunofluorescence experiments, cells were fixed in either 3.7% (vol/vol) formaldehyde in PBS for 20 min or −20 °C methanol for 5 min, permeabilized with 0.1% Triton X-100 in TBS, blocked in AbDil (0.1% Triton X-100 in TBS + 2% (wt/vol) BSA + 0.1% NaN3), and probed with primary and secondary antibodies diluted in AbDil. Cells were stained with DAPI and mounted in Prolong gold mounting medium (Invitrogen).
The following antibodies and markers were used in immunofluorescence and Western blots: anti-GFP, Transferrin receptor, LAMP1, Clathrin, CI-M6PR, GM130, Giantin, COPB and COPD antibodies (Abcam), EEA1, SNX1 and SNX2 antibodies (BD Bioscience), TRAPα antibody (a gift from Tom Rapoport, Harvard Medical School, Boston, MA), DRD3 antibody (Calbiochem), fluorescently labeled transferrin and EGF (Invitrogen), FITC-70 kD dextran and mouse anti-FLAG antibody (Sigma), and COPG antibody (Santa Cruz).
Statistics.
For quantifications in the manuscript, mean values are shown in the figures, and SDs are shown as error bars. All images shown in figures are representative. 500 (Fig. 1C), 300 (Figs. 2C, 3 C and D, and 5C), or 50 (Figs. 2G and 4C) cells were counted for each condition in three independent experiments. Comparisons between treatments were analyzed by a two-tailed Student t test. P values are labeled in the figures for where data were compared. In Figs 2H, 4E, and 5E, 10,000 cells each were collected in three independent experiments. Mean fluorescence intensities normalized to 0 min are shown.
Supplementary Material
Acknowledgments
We thank the Nikon Imaging Center at Harvard Medical School for assistance with microscopy, the Flow Cytometry facility at the Dana-Farber Cancer Institute for assistance with flow cytometry, and Qingsong Liu and members of the U.S.E. laboratory for helpful discussions. We also thank Maria F. Sassano and Bryan L. Roth at the University of North Carolina and the National Institute of Mental Health Psychoactive Drug Screening Program for conducting the experiments shown in SI Appendix, Fig. S6. M.L.C. and T.J.M. were supported by National Institutes of Health Grant R01 GM023928. This project was funded by National Institutes of Health Grant R01 GM082834 (to U.S.E.) and the Dana-Farber Cancer Institute.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207821109/-/DCSupplemental.
References
- 1.Lappano R, Maggiolini M. G protein-coupled receptors: Novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol. 2011;23:393–403. doi: 10.1016/j.ceb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 5.Cavalli V, Corti M, Gruenberg J. Endocytosis and signaling cascades: A close encounter. FEBS Lett. 2001;498:190–196. doi: 10.1016/s0014-5793(01)02484-x. [DOI] [PubMed] [Google Scholar]
- 6.Gould GW, Lippincott-Schwartz J. New roles for endosomes: From vesicular carriers to multi-purpose platforms. Nat Rev Mol Cell Biol. 2009;10:287–292. doi: 10.1038/nrm2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruenberg J. The endocytic pathway: A mosaic of domains. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- 8.van Weering JR, Verkade P, Cullen PJ. SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin Cell Dev Biol. 2010;21:371–380. doi: 10.1016/j.semcdb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippincott-Schwartz J, et al. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 10.Deng Y, Golinelli-Cohen MP, Smirnova E, Jackson CL. A COPI coat subunit interacts directly with an early-Golgi localized Arf exchange factor. EMBO Rep. 2009;10:58–64. doi: 10.1038/embor.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Available at http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000625/. Accessed June 30, 2012.
- 12.Eggert US, et al. Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2004;2:e379. doi: 10.1371/journal.pbio.0020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guizetti J, Gerlich DW. Cytokinetic abscission in animal cells. Semin Cell Dev Biol. 2010;21:909–916. doi: 10.1016/j.semcdb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Collinet C, et al. Systems survey of endocytosis by multiparametric image analysis. Nature. 2010;464:243–249. doi: 10.1038/nature08779. [DOI] [PubMed] [Google Scholar]
- 15.Hunziker W, Mâle P, Mellman I. Differential microtubule requirements for transcytosis in MDCK cells. EMBO J. 1990;9:3515–3525. doi: 10.1002/j.1460-2075.1990.tb07560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kok JW, Hoekstra K, Eskelinen S, Hoekstra D. Recycling pathways of glucosylceramide in BHK cells: Distinct involvement of early and late endosomes. J Cell Sci. 1992;103:1139–1152. doi: 10.1242/jcs.103.4.1139. [DOI] [PubMed] [Google Scholar]
- 17.Matter K, Bucher K, Hauri HP. Microtubule perturbation retards both the direct and the indirect apical pathway but does not affect sorting of plasma membrane proteins in intestinal epithelial cells (Caco-2) EMBO J. 1990;9:3163–3170. doi: 10.1002/j.1460-2075.1990.tb07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elphick GF, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–1383. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- 20.Sachlos E, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–1297. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 21.Allen JA, et al. Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA. 2011;108:18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeanneteau F, Diaz J, Sokoloff P, Griffon N. Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol Biol Cell. 2004;15:696–705. doi: 10.1091/mbc.E03-05-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: Different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Beck R, Rawet M, Wieland FT, Cassel D. The COPI system: Molecular mechanisms and function. FEBS Lett. 2009;583:2701–2709. doi: 10.1016/j.febslet.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Gu F, Aniento F, Parton RG, Gruenberg J. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol. 1997;139:1183–1195. doi: 10.1083/jcb.139.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razi M, Chan EY, Tooze SA. Early endosomes and endosomal coatomer are required for autophagy. J Cell Biol. 2009;185:305–321. doi: 10.1083/jcb.200810098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu F, Gruenberg J. ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J Biol Chem. 2000;275:8154–8160. doi: 10.1074/jbc.275.11.8154. [DOI] [PubMed] [Google Scholar]
- 29.Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 30.Popoff V, Adolf F, Brügger B, Wieland F. COPI budding within the Golgi stack. Cold Spring Harb Perspect Biol. 2011;3:a005231. doi: 10.1101/cshperspect.a005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilani T, et al. Coupling of dopamine receptors to G proteins: Studies with chimeric D2/D3 dopamine receptors. Cell Mol Neurobiol. 2002;22:47–56. doi: 10.1023/a:1015341712166. [DOI] [PubMed] [Google Scholar]
- 32.Neve KA. The Dopamine Receptors. 2nd Ed. New York: Springer; 2010. [Google Scholar]
- 33.Fegan A, White B, Carlson JC, Wagner CR. Chemically controlled protein assembly: Techniques and applications. Chem Rev. 2010;110:3315–3336. doi: 10.1021/cr8002888. [DOI] [PubMed] [Google Scholar]
- 34.Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: Uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.