Abstract
The inability to acquire protective immunity against Plasmodia is the chief obstacle to malaria control, and inadequate T-cell responses may facilitate persistent blood-stage infection. Malaria is characterized by a highly inflammatory cytokine milieu, and the lack of effective protection against infection suggests that memory T cells are not adequately formed or maintained. Using a genetically targeted strain of Plasmodium berghei, we observed that the Plasmodium ortholog of macrophage migration inhibitory factor enhanced inflammatory cytokine production and also induced antigen-experienced CD4 T cells to develop into short-lived effector cells rather than memory precursor cells. The short-lived effector CD4 T cells were more susceptible to Bcl-2–associated apoptosis, resulting in decreased CD4 T-cell recall responses against challenge infections. These findings indicate that Plasmodia actively interfere with the development of immunological memory and may account for the evolutionary conservation of parasite macrophage migration inhibitory factor orthologs.
Keywords: vaccine, immune evasion
Vector-borne parasites such as the Plasmodium spp. that are responsible for malaria rely on an inefficient mode of infection but nevertheless elude eradication. People living in malaria-endemic regions can sustain a low-level parasitemia and eventually may achieve tolerance to symptoms; however, these individuals are protected only partially from disease manifestations. The partial protection wanes quickly in the absence of reinfection, and sterilizing immunity is not established against natural Plasmodium infections (1). The inability of the host to clear Plasmodia completely allows the parasites to mature and survive during the low-transmission season.
Early studies identified the importance of cell-mediated immune pathways in the adaptive response against malaria (2). Selective depletion of different immune cell populations indicated that control of blood-stage infection is dependent on CD4 T cells, which can reduce parasitemia and promote host survival (3–7). The ability of Plasmodium-specific memory CD4 T cells to develop and be maintained in the host appears to be altered during malaria (8, 9), and this phenomenon likely contributes to the lack of a long-term, sterilizing immunity. CD4 T cells are activated initially by antigen and inflammatory signals from antigen-presenting cells (APCs). Following activation, CD4 T cells proliferate rapidly and acquire critical effector functions; these cells then undergo a dramatic contraction phase after the peak of infection (10), and the cells that remain after the contraction phase become established as memory T cells. Responding terminal effector T cells do not survive the contraction period (11, 12) and thus do not confer protection to reinfection.
The fate of antigen-primed T cells is dependent on both the host cytokine milieu and the persistence of antigen. Although inflammatory cytokines such as TNF-α and IFN-γ act to control the malaria parasite burden (13, 14), high levels of inflammation also promote the development of terminally differentiated effector cells. In viral infections, elevated expression of IL-12 favors the development of responding T cells into short-lived, terminal effector cells rather than memory precursor effector cells (11, 15); however less is known about the effects of inflammatory cytokines on the development of memory T cells during Plasmodium infections. There is evidence that during blood-stage Plasmodium infection IFN-γ is detrimental to the survival of Plasmodium-specific CD4 T cells by regulating its contraction phase (12, 16). The observation that concurrent infection with Plasmodium falciparum impairs the development of vaccine-induced Plasmodium antigen-specific memory CD4 T cells (17) further suggests that the formation of T-cell–mediated immunological memory is impaired during malaria.
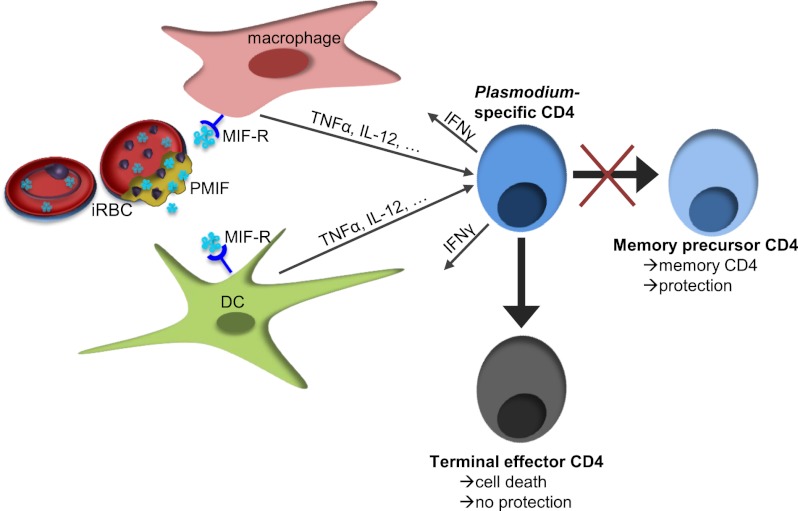
We describe herein a central role for the Plasmodium ortholog of the cytokine macrophage migration inhibitory factor (PMIF) in regulating the host inflammatory response to malaria and its subsequent effect on the development of CD4 T-cell–mediated immune protection. Challenge infections showed that CD4 T cells activated in the presence of PMIF do not produce a robust recall response to homologous parasites. These studies provide evidence for an active mechanism by which Plasmodia interfere with the generation of Plasmodium-specific memory CD4 T cells, thereby facilitating parasite persistence and transmission.
Results
IL-12 and IFN-γ Interfere with the Development of T-Cell–Mediated Protection Against Malaria.
We studied the Plasmodium berghei ANKA (PbA) blood-stage infection model (18) to investigate the effect of inflammatory cytokines on Plasmodium-specific CD4 T cells. Upon infection, the cytokines IL-12 and IFN-γ promote the differentiation of naive CD4 T cells into Th1 cells, which are known to mediate antibody-independent protection against Plasmodium infection (3). IL-12 and IFN-γ can further influence CD4 T-cell fate (19, 20), and malaria-induced IFN-γ has been implicated in regulating the contraction of Plasmodium-specific CD4 T cells (12, 16). Although the effects of IL-12 and IFN-γ on T-cell development and survival are well characterized in many infection models (11, 15, 21, 22), they have not been investigated thoroughly during malaria. Because IL-12 regulates IFN-γ production, we were interested in studying the effects of elevated IFN-γ and IL-12 levels on Plasmodium-specific CD4 T-cell expansion and function during blood-stage malaria.
The effects of IL-12 and IFN-γ on CD4 T cells during blood-stage malaria were examined by administering neutralizing antibodies directed against IFN-γ and IL-12 on days −1, 1, 3, and 5 post PbA infection. In the BALB/c PbA blood-stage infection model, immunoneutralization of IFN-γ and IL-12 did not significantly affect parasitemia during the acute phase of infection (Fig. S1A), a finding that is consistent with reports from other murine Plasmodium blood-stage infection models (23, 24). The peak of the inflammatory response to PbA infection of BALB/c mice occurs around days 4 and 5 post infection (25), and this acute phase of the response is followed by contraction of responding CD4 T cells starting around day 7 post infection (16).
No significant differences in parasitemia were observed in the groups treated with IgG (control) or anti–IFN-γ/IL-12 at day 7 post infection, indicating that the two groups were exposed to comparable levels of Plasmodium antigens. We then examined the effects of these cytokines on CD4 T-cell development during blood-stage malaria. The lack of defined CD4 T-cell epitopes has hindered efforts to characterize CD4 T-cell function during malaria, and we therefore used cell proliferation as a surrogate for identifying CD4 T cells that respond to PbA infection (26). In these experiments, T-cell proliferation was detected by expression of the nuclear protein Ki67. We observed no significant difference in the number of PbA-responsive CD4 T cells in control IgG- and anti–IFN-γ/IL-12–treated animals at day 7 post infection (Fig. S1B), indicating that IL-12 and IFN-γ do not contribute significantly to the initiation of the anti-Plasmodium CD4 T-cell response.
Studies in lymphocytic choriomeningitis virus have shown that increased inflammatory responses can promote the development of a terminally differentiated, short-lived effector cell phenotype rather than a memory precursor phenotype in responding T-cell populations (11, 27). We hypothesized that IL-12 and IFN-γ may have similar effects on Plasmodium-responsive CD4 T cells. T-bet is a transcription factor that is regulated by IFN-γ and IL-12 signaling and that is elevated in terminally differentiated T cells (11, 28). We observed that the increase in IL-12 and IFN-γ levels during blood-stage PbA infection promotes the up-regulation of T-bet at day 7 post infection. Comparison of PbA-responsive CD4 T cells from control IgG- and anti–IFN-γ/IL-12–treated mice revealed elevated levels of T-bet in the presence of IFN-γ and IL-12 (Fig. 1A), suggesting that these cytokines influence the differentiation of responding CD4 T cells during blood-stage malaria.
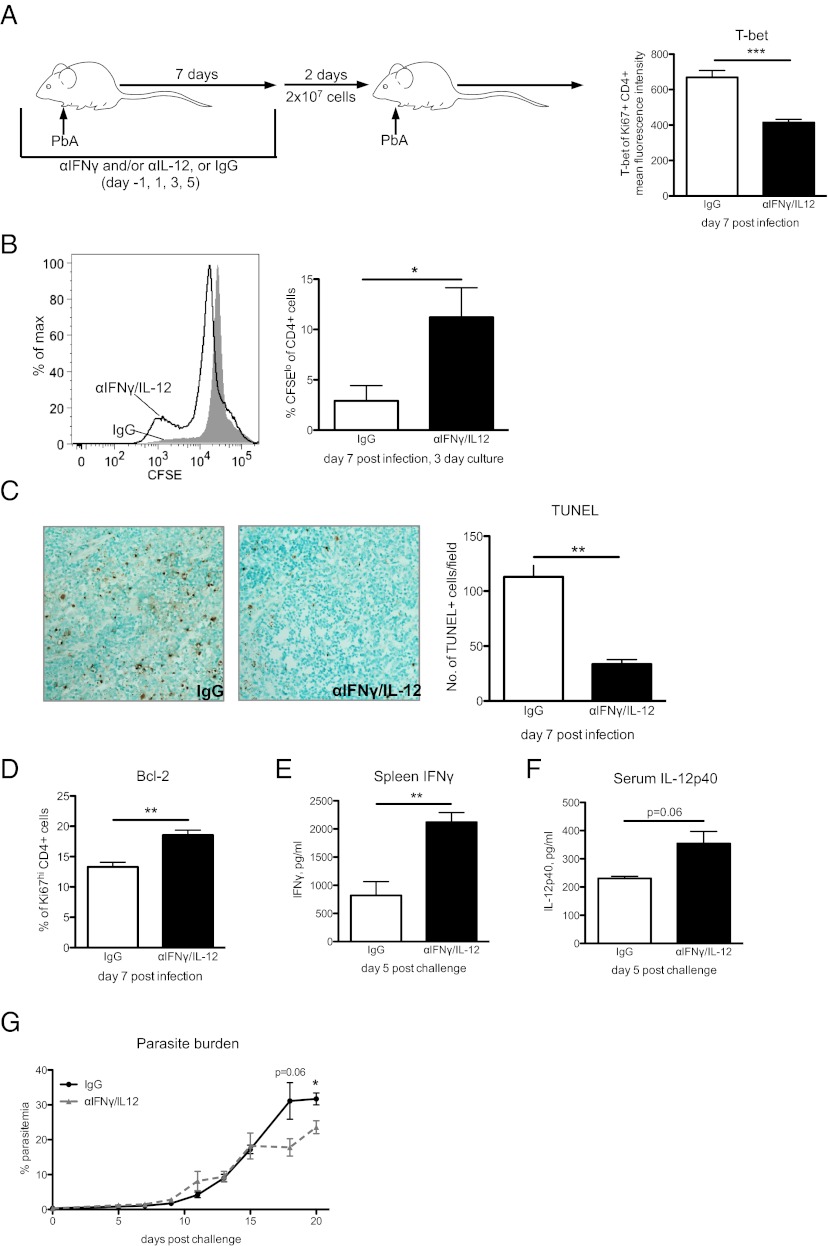
Fig. 1.
IL-12 and IFN-γ suppress CD4 T-cell proliferation and diminish CD4 T-cell recall responses. (A) BALB/c mice were infected by i.p. injection of 106 PbA-infected RBCs and were treated with control IgG or neutralizing antibodies against IFN-γ and IL-12 on indicated days (see schematic). T-bet expression in PbA-responsive CD4 T cells (Ki67hi, CD4+) at day 7 post infection was detected by intranuclear staining and analyzed by flow cytometry. (B) On day 7 post infection, 5 × 106 splenocytes were isolated, labeled with CFSE, and cultured for 3 d ex vivo without additional stimulation. Then proliferation was detected by CFSE dilution in CD4 T cells. (C) TUNEL staining of spleen histologic sections at day 7 post infection. Three fields were counted per spleen section to determine the number of TUNEL+ cells. (Magnification: 20×.) n = 4 per group. (D) Bcl-2 was detected in the Ki67hi, CD4+ T-cell population at day 7 post infection. (E–G) Splenocytes (2 × 107) from anti–IFN-γ/IL-12– or IgG-treated mice were labeled with CFSE and incubated with 10 μM chloroquine for 2 h and then were adoptively transferred i.v. into naive BALB/c mice. Recipients were challenged with PbA on day 2 post transfer. One group of recipients was killed at day 5 post challenge, and a second group was monitored for parasitemia. In mice killed at day 5 post challenge, IL-12 was measured in the serum, and IFN-γ secreted by 5 × 106 splenocytes after 18 h in culture was measured in the supernatant. Parasitemias of recipient mice were determined by quantitative PCR detection of P. berghei 18S rRNA copies/μL blood. One representative experiment of two independent experiments with n = 5 mice per group is shown; data are shown as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.005 by two-tailed t test.
The effect of IL-12 and IFN-γ on the expression of T-bet in PbA-responsive CD4 T cells indicated that these cytokines may contribute to CD4 T-cell contraction after the peak of the response. To examine the effects of malaria-induced IFN-γ and IL-12 on CD4 T-cell contraction, splenocytes were isolated from control IgG- or anti–IFN-γ/IL-12–treated mice at day 7 post infection and were labeled with carboxyfluorescein succinimidyl ester (CFSE). The cells then were cultured without additional stimulation, and the ability of CD4 T cells to maintain proliferation was detected by assessing CFSE dilution in CD4 T cells 3 d later. We observed that although CD4 T cells from IFN-γ/IL-12–neutralized mice continued to proliferate ex vivo, CD4 T cells from control IgG-treated mice did not sustain the ability to divide in culture (Fig. 1B). These data suggest that the IL-12 and IFN-γ responses to blood-stage malaria are involved in promoting CD4 T-cell contraction and support a recent report that CD4 T-cell contraction is reduced in P. berghei-infected IFN-γ−/− mice (16).
Terminally differentiated effector T cells are less protected from apoptotic cell death during the contraction phase than their memory precursor effector T-cell counterparts (10). Consistent with the elevated levels of T-bet and shortened proliferation, TUNEL staining of spleen histologic samples indeed revealed more apoptotic cell death in the presence of IFN-γ and IL-12 (Fig. 1C). We further observed lower Bcl-2 expression in CD4 T cells that express high levels of T-bet (Fig. S2), and the loss of Bcl-2 is known to increase susceptibility to apoptosis (10). We detected higher levels of Bcl-2 in PbA-responsive CD4 T cells from anti–IFN-γ/IL-12–treated mice than in control IgG-treated mice by flow cytometric analysis (Fig. 1D), suggesting that IL-12 and IFN-γ may increase apoptosis via a Bcl-2–dependent mechanism during the contraction phase of the T-cell response to blood-stage malaria.
We next investigated whether regulation of the CD4 T-cell response by IFN-γ and IL-12 influenced the recall response against a PbA challenge infection. Because BALB/c mice are unable to clear PbA infections, we adoptively transferred 2 × 107 splenocytes from control IgG- or anti–IFN-γ/IL-12–treated mice into naive recipients and challenged the recipients with PbA. Mice that received splenocytes from anti–IFN-γ/IL-12–treated donors showed a stronger anti-malaria cytokine response and better parasite control than mice that received splenocytes from control IgG-treated donors (Fig. 1 E–G). These data indicate that the inflammatory response produced during acute PbA infection promotes CD4 T-cell contraction, and increased splenocyte apoptosis is likely a major factor in the diminished anti-Plasmodium recall responses observed during challenge infection.
Circulating PMIF Levels Are Associated with Inflammation in Malaria Patients.
The discovery that orthologs of the human cytokine, macrophage migration inhibitory factor (MIF), are expressed by evolutionarily distant parasites (29) prompted us to consider that such orthologs may function in pathways of immune evasion. Human MIF is an upstream mediator that promotes inflammatory cytokine production (30), and the structural similarities between human MIF and PMIF (31) led us to hypothesize that PMIF likewise may promote inflammation in the host.
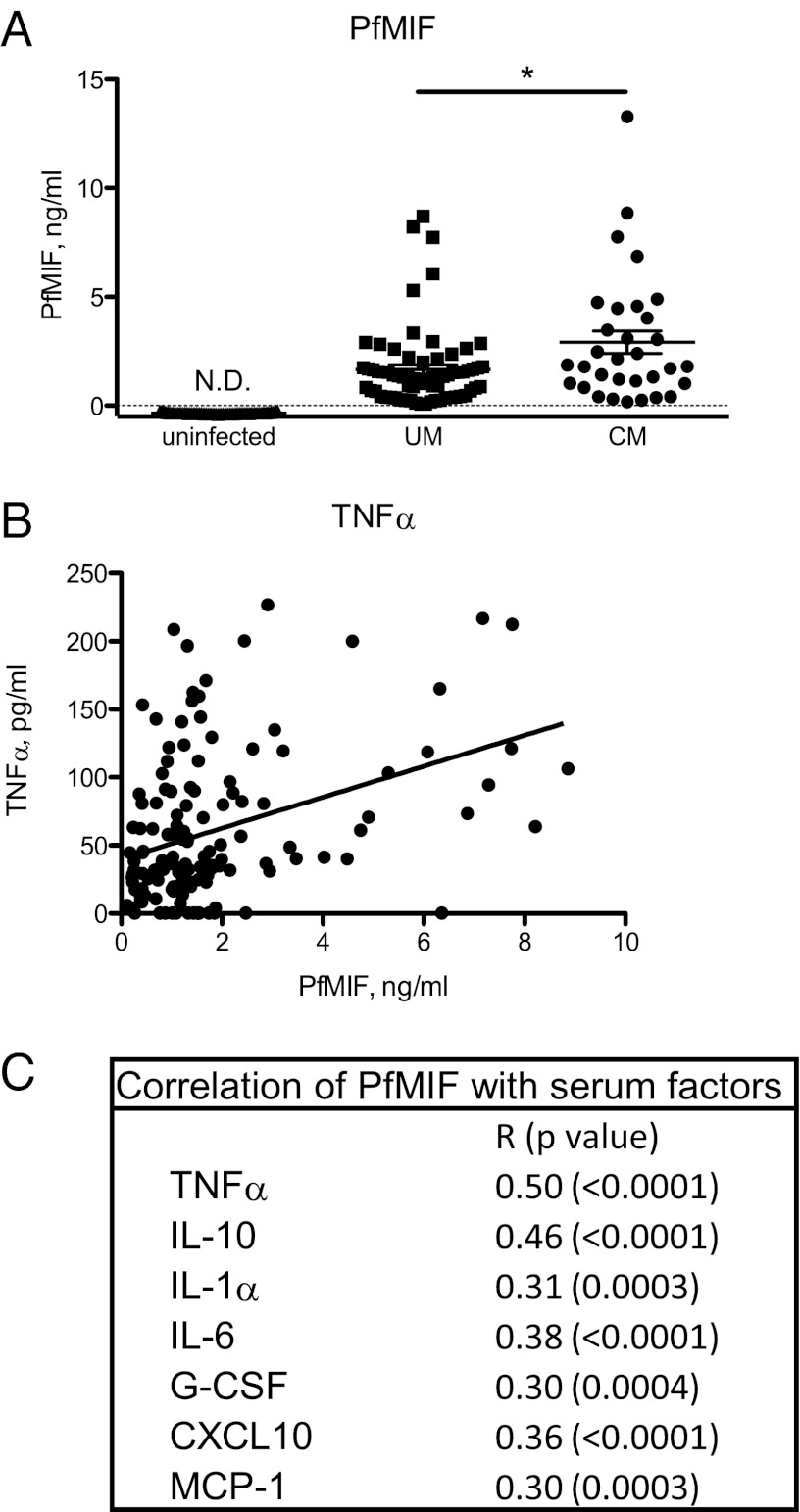
We developed a P. falciparum MIF (PfMIF)-specific ELISA and observed higher PfMIF levels in patients with cerebral malaria than in patients with uncomplicated malaria (Fig. 2A). Of note, cerebral malaria is a severe inflammatory manifestation of acute infection that is not correlated with parasitemia (32, 33). Positive associations between plasma concentrations of PfMIF and the inflammatory mediators TNF-α, Fas ligand, CCL2, and CXCL10 are likely contributing factors for the increased incidence of cerebral malaria (Fig. 2 B and C) (34). These data from malaria patients support the idea that parasite production of PMIF is associated with a greater proinflammatory state in the infected host.
Fig. 2.
Association of PfMIF with increased inflammatory cytokine levels in malaria patients. (A) Serum PfMIF levels in uninfected controls (n = 72), in patients with uncomplicated malaria (UM, n = 69), and in patients with cerebral malaria (CM, n = 32). P < 0.0001 between uninfected and UM and between uninfected and CM groups by Mann–Whitney test; *P < 0.05; N.D., not detected. (B) Circulating serum PfMIF levels correlated with serum TNF-α levels in malaria patients (n = 141, r = 0.35, P < 0.0001 by Pearson’s correlation). (C) Circulating inflammatory mediators were detected as previously described (33), and correlations of the listed mediators with PfMIF levels were calculated by Pearson’s correlation analysis for n = 126–140 patients.
PMIF Binds the Host MIF Receptor and Enhances TNF-α and IL-12 Production by APCs.
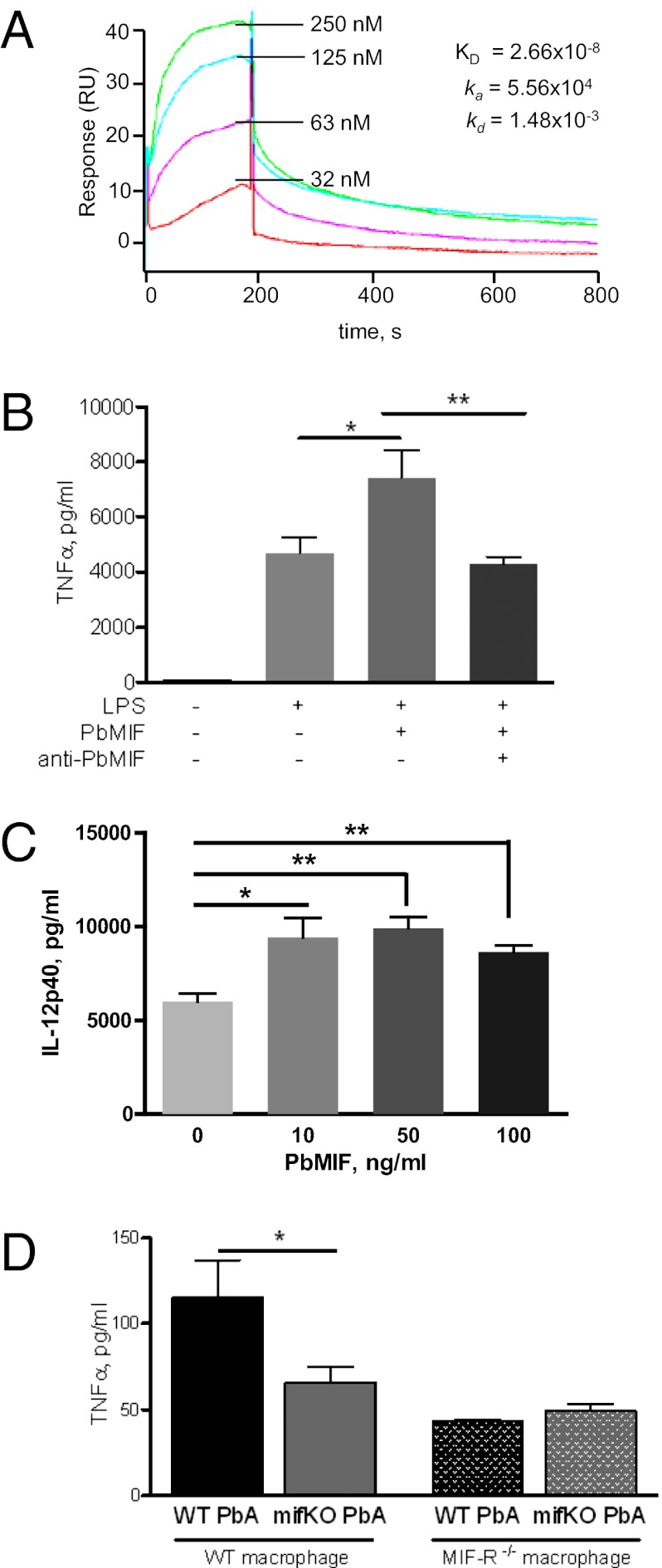
Mammalian MIF carries out many of its inflammatory effects by binding to the MIF receptor (MIF-R, also known as “CD74”) (35). To define the inflammatory function of PfMIF we expressed recombinant PfMIF and tested its equilibrium binding kinetics to recombinant MIF-R by surface plasmon resonance. We observed a high-affinity binding interaction between PfMIF and the MIF-R ectodomain (PfMIF Kd = 2.7 × 10−8 M) (Fig. 3A), comparable to the Kd observed between mammalian MIF and MIF-R [human MIF (HuMIF) Kd = 9.0 × 10−9 M] (35). These data also confirm a recent report of PMIF binding to host MIF-R by coimmunoprecipitation (31).
Fig. 3.
PMIF binds MIF-R and elicits enhanced TNF-α secretion from naive APCs. (A) High-affinity binding of recombinant PfMIF to the soluble ectodomain of the human MIF receptor (MIF-R73-232) measured by surface plasmon resonance (BIAcore). Analysis for human MIF binding revealed a Kd of 9.0 × 10−9M (35). (B) TNF-α secreted by bone marrow-derived dendritic cells into culture supernatant after 18 h stimulation with 10 ng/mL LPS, 100 ng/mL PbMIF, and/or 20 μg/mL polyclonal anti-PbMIF IgG. (C) IL-12p40 secreted by bone marrow-derived dendritic cells into culture supernatant after 10 h stimulation with 10 ng/mL LPS and 10, 50, or 100 ng/mL PbMIF. (D) TNF-α secreted by 106 peritoneal macrophages from WT BALB/c or MIF-R deficient (CD74-KO) BALB/c mice activated with 1 ng/mL IFN-γ and stimulated for 18 h with WT PbA- or mifKO PbA-infected RBCs (20 iRBCs per macrophage). Data are shown as mean ± SEM. *P < 0.05; **P < 0.01 by two-tailed t test.
MIF-R is highly expressed on activated APCs (35), which are important for initiating the pathogen-specific CD4 T-cell response during natural Plasmodium infection. We therefore assessed the effects of PMIF in vitro by stimulating APCs with PMIF. We noted an enhanced secretion of TNF-α and IL-12p40 when activated bone marrow-derived dendritic cells from naive mice were stimulated with recombinant P. berghei MIF (PbMIF) (Fig. 3 B and C). To investigate better the role of PbMIF during blood-stage malaria, we also studied the macrophage response to a strain of PbA with a genetic deletion in PbMIF (mifKO PbA) (36). Peritoneal macrophages were harvested from naive mice, activated with IFN-γ, and stimulated in vitro with magnetically purified PbA-infected red blood cells (iRBCs) obtained from WT PbA- or mifKO PbA-infected mice. Macrophages that were stimulated with WT PbA iRBCs produced more TNF-α than macrophages stimulated with mifKO PbA iRBCs, indicating that the presence of PbMIF increased inflammatory cytokine production. Furthermore, this effect was dependent on macrophage expression of the MIF receptor, supporting the idea that PMIF functions by signaling through the host MIF receptor (Fig. 3D).
PMIF Increases Inflammatory Cytokine Production and CD4 T-Cell Activation During P. berghei Infection.
Like malaria patients, mice infected with WT PbA showed significant serum concentrations of PbMIF (Fig. S3A). PbMIF is expressed during the blood stages (36, 37) and accumulates in the spleen, the main organ associated with anti-Plasmodium immune responses (Fig. S3B). To confirm the effect of PMIF on inflammatory responses in vivo, we compared serum and splenic cytokine levels in mice infected with WT or mifKO PbA. Mice infected with WT PbA showed an increase in serum IL-12, IL-1β, TNF-α, and IL-6 concentrations compared with mifKO PbA-infected mice (Fig. 4A). Higher splenic levels of the inflammatory cytokines IFN-γ, IL-1β, and IL-6 also were detected in WT PbA-infected mice than in mifKO PbA-infected mice (Fig. 4B). The presence of PMIF did not alter the expression of host MIF (at day 5 post infection, mouse MIF = 32 ± 16 ng/mL in WT PbA infections and 28 ± 9.2 ng/mL in mifKO PbA infections, n = 5 per group, P = not significant), and no apparent differences in survival, parasitemia, and anemia were seen in BALB/c mice infected with WT PbA or mifKO PbA (Fig. S3C). These data support the interpretation that PbMIF increases the innate inflammatory response during acute blood-stage Plasmodium infection.
Fig. 4.
Increased inflammatory cytokines produced in WT PbA- versus mifKO PbA-infected mice. Serum (A) and spleen (B) lysate levels of the indicated cytokines detected in BALB/c mice infected with WT PbA (solid line) or mifKO PbA (dashed line). Data are shown as mean ± SEM and are representative of four independent experiments. n = 3–5 per group. *P < 0.05; **P < 0.01; ***P < 0.005 by two-tailed t test.
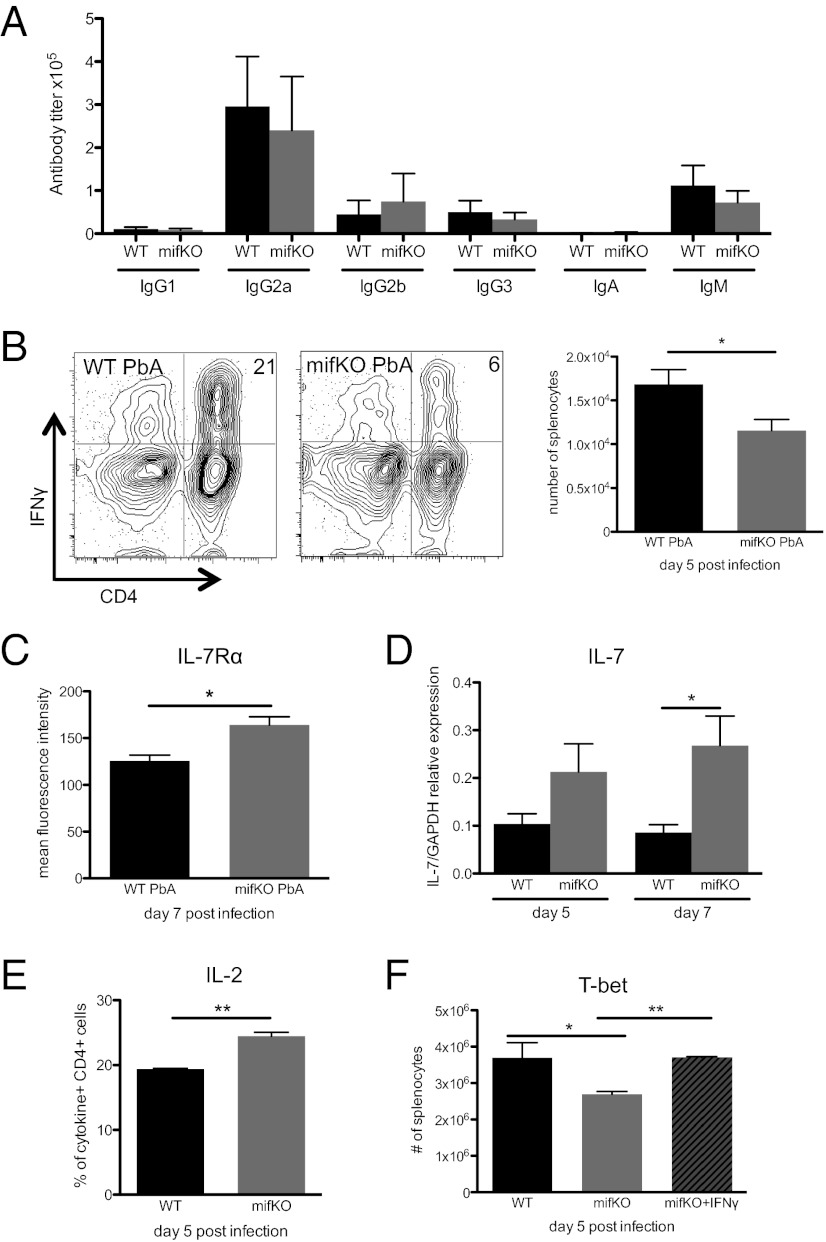
Next, we were interested in determining whether the ability of PbMIF to regulate the inflammatory milieu during acute blood-stage malaria affects the development of Plasmodium-specific adaptive immune responses. Both antibody and CD4 T-cell responses are involved in the control of blood-stage Plasmodium infection (2, 38), and we first analyzed whether PbMIF affects anti-Plasmodium antibody production. BALB/c mice were infected with WT PbA or mifKO PbA, and the infections were cured by chloroquine. At day 33 post infection, anti-PbA antibody titers were measured in serum samples, and we observed no significant differences between WT PbA- and mifKO PbA-infected mice in the titers of all antibody isotypes measured (Fig. 5A).
Fig. 5.
PbMIF skews activated CD4 T cells toward a short-lived effector phenotype. (A) Mice were infected with WT PbA or mifKO PbA and were cured by i.p. injection of chloroquine (50 mg/kg) on days 7–10 post infection. Serum antibody titers were determined by direct ELISA, using purified PbA iRBC lysates as the coating antigen. Data shown are representative of two independent experiments. n = 4–5 animals per group. (B) CFSE-labeled splenocytes (2 × 107) from naive Thy1.1+ BALB/c donors were transferred into Thy1.2+ BALB/c recipients, which were then infected with PbA. PbA-responsive CD4 T cells are defined as CFSElo, Thy1.1+, CD4+ cells (see schematic, Fig. S4), and the number of IFN-γ–producing CFSElo, Thy1.1+, CD4+ PbA-responsive cells was determined. (C) IL-7Rα surface expression, shown as mean fluorescence intensity of Thy1.1+, CD4+ PbA-responsive cells. Data shown are representative of two independent experiments. n = 4 animals per group. (D) RT-PCR was performed on RNA extracted from homogenized spleens of mice infected with WT PbA or mifKO PbA at day 5 and day 7 post infection. Expression of IL-7 to relative GAPDH is shown. Data shown are representative of two independent experiments. n = 4 animals per group. (E) IL-2–producing cells as per cent of IFN-γ–, TNF-α–, and/or IL-2–producing malaria-responsive CD4 T cells. (F) Mice were infected with WT PbA or mifKO PbA. The mifkO PbA-infected mice were divided into two groups; one group was injected with PBS, and the other group was injected with 10 μg IFN-γ on days 0, 2, and 4 post infection. Intranuclear T-bet staining was performed on day 5 post infection. The number of T-bethi PbA-responsive cells (Ki67hi, CD4+) is shown. Data are shown as mean ± SEM and are representative of two independent experiments. n = 4 animals per group. *P < 0.05; **P < 0.01 by two-tailed t test.
A major contribution of CD4 T cells to the immune response against blood-stage malaria is to produce IFN-γ (23). Using a CFSE-based method for detecting CD4 T cells responding to blood-stage malaria, we found similar numbers of PbA-responsive CD4 T cells in WT PbA- and mifKO PbA-infected mice at day 5 post infection (Fig. S4), indicating comparable initiation of the anti-Plasmodium CD4 T-cell response. However, more PbA-responsive CD4 T cells produced IFN-γ in WT PbA-infected mice than in mifKO PbA-infected mice (Fig. 5B and Fig. S5). Compared with macrophages, few CD4 T cells express MIF-R (<2% of CD4+ splenocytes) (Fig. S6A) (35), indicating that PMIF signaling is likely APC dependent. The ability of PbMIF to increase IFN-γ production by CD4 T cells indeed appears to depend on signaling through the host MIF-R, because the addition of PbMIF to unstimulated anti-CD3/CD28 or phorbol 12-myristate 13-acetate (PMA)/ionomycin-stimulated naive CD4 T-cell cultures did not enhance production of IFN-γ by the CD4 T cells (Fig. S6B).
The effect of PbMIF on the host appears to be confined to proinflammatory cytokine production, because PbMIF had no discernible impact on IL-10 and IL-4 production or on regulatory T-cell formation; nor were there differences in the proportion of CD4 T-cell, CD8 T-cell, macrophage, or B-cell populations in WT PbA- or mifKO PbA-infected spleens (Fig. S7). However, we did observe a 45% increase in the proportion of PD-1+ PbA-responsive CD4 T cells in the presence of PbMIF (Fig. S8), suggesting that PbMIF may be involved in the recently described ability of malaria to induce CD4 T-cell exhaustion (39).
Decreased CD4 T-Cell Survival Signals in the Presence of PMIF.
We hypothesized that the ability of PMIF to augment the host inflammatory response may contribute to Plasmodium parasite fitness by hindering the development of host-protective responses. Because IL-12 and IFN-γ have been observed to interfere with memory T-cell formation by promoting effector cell death (Fig. 1) (12, 40), we explored the possibility that the up-regulation of inflammatory cytokines induced by PbMIF could lead to decreased long-term CD4 T-cell–mediated protection against malaria. One of the hallmarks of CD4 T-cell–mediated protection is that pathogen-specific CD4 T cells can survive the contraction phase to produce a rapid response during reexposure to the pathogen (10). We thus examined whether the presence of PMIF alters the expression of survival signals for PbA-responsive CD4 T cells. IL-7Rα is a receptor for the prosurvival cytokine IL-7 (41, 42) and has been correlated with increased memory CD4 T-cell development in protozoan infections (43, 44). Even though similar numbers of CD4 T cells responded to WT PbA or mifKO PbA infection (Fig. S4), more PbA-responsive CD4 T cells from WT PbA-infected mice down-regulated expression of IL-7Rα compared with mifKO PbA-infected mice (Fig. 5C). The expression of IL-7, the prosurvival cytokine ligand of IL-7Rα, also was decreased in the spleens of WT PbA-infected versus mifKO PbA-infected mice (Fig. 5D).
IL-2 is a prosurvival and mitogenic signal for T cells, and T cells that produce IL-2 are more likely to survive the contraction phase and become protective memory T cells (45). Notably, in recent studies of the RTS,S malaria vaccine, protected recipients had more IL-2–producing CD4 T cells than study participants who were not protected from malaria (5). In the presence of PbMIF, fewer cytokine-secreting CD4 T cells produce IL-2 (Fig. 5E), suggesting that fewer CD4 T cells will persist through the contraction phase and initiate a robust recall response. (Although Ly6C is a recently identified marker for memory CD4 T cells (46), available antibodies do not detect the Ly6C variant expressed by BALB/c CD4 T cells.) The differences in IL-7Rα, IL-7, and IL-2 levels between WT PbA and mifKO PbA infections suggest that PbMIF plays a role in determining whether responding CD4 T cells become long-lived memory cells or short-lived effector cells.
Because we noted that PbMIF contributes to an increase in the levels of two cytokines that induce T-bet expression, i.e., IFN-γ and IL-12 (Fig. 4) (47, 48), we next assessed T-bet levels in PbA-responsive CD4 T cells. We observed greater T-bet expression in PbA-responsive CD4 T cells from mice infected with WT PbA than from mice infected with mifKO PbA (Fig. 5F), further indicating that PbMIF promotes the terminal differentiation of effector cells.
The phenotype of CD4 T cells during PbA infection in IFN-γ/IL-12–neutralized animals appeared to be similar to those of mifKO PbA-infected animals (Fig. 1), suggesting that the ability of PMIF to induce IFN-γ and IL-12 may be responsible for the observed differences in PbA-responsive CD4 T cells during WT PbA and mifKO PbA infections. To test whether increased IFN-γ signaling is a mechanism by which PbMIF modulates the development of memory precursor CD4 T cells, we infected mice with mifKO PbA and administered 10 μg IFN-γ during infection. Because IFN-γ was administered extrinsically, we expected that IFN-γ production by CD4 T cells would not be influenced in the treated and control groups (Fig. S9), and we asked whether the increased systemic IFN-γ levels would alter PbA-responsive CD4 T cells. We observed that administration of IFN-γ during mifKO PbA infection caused the phenotype of PbA-responsive CD4 T cells to resemble that of CD4 T cells from WT PbA-infected mice. Treatment of mifKO PbA-infected mice with IFN-γ increased the expression of T-bet in PbA-responsive CD4 T cells to levels similar to those in PbA-responsive CD4 T cells from WT PbA infections (Fig. 5F). The observation that administration of IFN-γ recapitulates the CD4 T-cell phenotype of WT PbA-infected mice during mifKO PbA infection provides evidence that links the ability of PbMIF to induce IFN-γ production with a decrease in the numbers of long-lived memory precursor effector T cells.
Decreased T-Cell Survival in the Presence of PMIF.
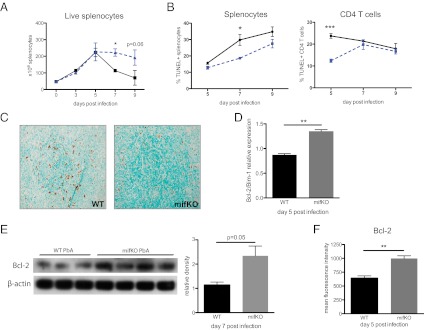
In the presence of IFN-γ, Plasmodium-specific CD4 T cells exhibit an increased susceptibility to undergo cell death in vivo (12, 16). Because of the PbMIF-mediated increase in IFN-γ and the increased numbers of CD4 T cells with a short-lived effector phenotype (10), we anticipated that more T-cell death occurs in the presence of PbMIF. The number of live splenocytes began to decline after day 5 in WT PbA-infected mice but not in mifKO PbA-infected mice (Fig. 6A). To investigate whether apoptotic cell death was responsible for this decline in live splenocyte numbers, we performed TUNEL staining and found increased apoptosis in both the total splenocyte population and the CD4 T-cell population of WT PbA-infected mice compared with mifKO PbA-infected mice (Fig. 6B). These results were confirmed by a spleen histologic TUNEL study (Fig. 6C).
Fig. 6.
Increased Bcl-2–associated apoptosis in the presence of PbMIF. (A) Number of live splenocytes in WT PbA (solid line) and mifKO PbA (dashed line) infections identified by Trypan blue exclusion. (B) TUNEL+ cells as per cent of splenocytes and TUNEL+ CD4 T cells as per cent of CD4 T cells from WT PbA- (solid line) and mifKO PbA- (dashed line) infected mice. (C) TUNEL staining of spleen histologic sections at day 7 post infection. (Magnification 20×.) n = 5 per group. (D) Expression of Bcl-2/Bim-1 was determined by RT-PCR of spleen lysates at day 5 post infection. Data are representative of two independent experiments. n = 4 animals per group. (E) Spleen lysates also were obtained from infected animals on day 7 post infection, and Bcl-2 protein was detected by immunoblotting (Left). Densitometry analysis is shown (Right). (F) Mean fluorescence intensity of Bcl-2 in PbA-responsive CD4 T cells. Data are representative of three experiments and are shown as mean ± SEM. n = 3–5 per group. *P < 0.05; **P < 0.01; ***P < 0.005 by two-tailed t test.
We then examined whether the increase in apoptosis during WT PbA infections, compared with mifKO PbA infections, is regulated by Bcl-2, as in the case of IFN-γ/IL-12–associated cell death (Fig. 1D). Bim-1 is a proapoptotic antagonist of Bcl-2 (49), and we noted a decrease, which is indicative of increased susceptibility to apoptosis, in the ratio of Bcl-2/Bim-1 expression levels in WT PbA-infected spleens (Fig. 6D) (49). Bcl-2 down-regulation in the presence of PbMIF was confirmed further by immunoblotting of spleen lysates from WT PbA- and mifKO PbA-infected mice at day 5 post infection (Fig. 6E). We then detected Bcl-2 levels in PbA-responsive CD4 T cells and found decreased intracellular levels of Bcl-2 in WT PbA- versus mifKO PbA-infected mice (Fig. 6F). We did not observe CD4 T-cell apoptosis to be associated with changes in Fas or Fas ligand signaling, a result that is in agreement with previous characterizations of CD4 T cells during blood-stage malaria (12).
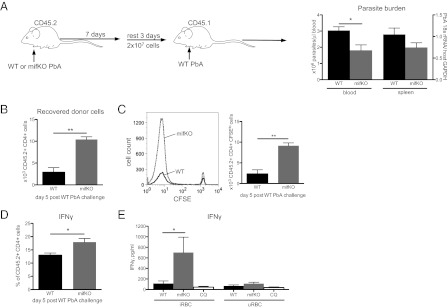
T Cells Activated in the Presence of PMIF Confer Less Protection During Challenge.
Our findings support the conclusion that a strong inflammatory response leads to decreased survival of Plasmodium-specific T cells. To assess the functional significance of these observations, we examined the effect of PbMIF on the ability of T cells to confer protection against a second PbA challenge infection that more closely models human disease in regions of high malaria transmission. CD45.2+ mice were infected with either WT PbA or mifKO PbA, and at day 7 post infection splenocytes were isolated, CFSE-labeled, and transferred into congenic CD45.1+ mice. All recipients were challenged with WT PbA at day 3 post transfer. Mice that received splenocytes from WT PbA-infected donors had higher circulating parasite burdens after the challenge infection than mice that received splenocytes from mifKO PbA-infected donors (Fig. 7A). The diminished control of parasites by recipients of cells from WT PbA-infected donors may be attributed both to fewer surviving donor CD4 T cells (Fig. 7B) and to decreased anti-Plasmodium responses in the remaining donor CD4 T cells (Fig. 7 C and D). Fewer CD4 T cells from WT PbA-infected donors than from mifKO PbA-infected donors proliferated and produced IFN-γ after the challenge infection (Fig. 7 C and D).
Fig. 7.
CD4 T cells activated in the presence of PbMIF confer decreased protection to homologous challenge. (A) CD45.2+ BALB/c mice were infected with WT PbA or mifKO PbA, and splenocytes were isolated at day 7 post infection. Single-cell suspensions of splenocytes were CFSE labeled and were incubated with 10 μM chloroquine for 2 h at 37 °C. Then 2 × 107 splenocytes were adoptively transferred i.v. into naive CD45.1+ BALB/c recipient mice. All CD45.1+ recipient mice were challenged with WT PbA at day 3 post transfer (see schematic). Parasite burden was measured by quantitative PCR detection of P. berghei 18S rRNA copies/μL of peripheral blood, and splenic parasite burden was measured by expression of 18S rRNA relative to host GAPDH. Peripheral parasite burdens on day 17 post challenge are shown. A 30–40% increase in peripheral parasite burdens starting at day 9 post challenge and a 40% increase in spleen parasite burden at day 19 in recipients of splenocytes from WT PbA infections were observed. (B) Number of CD4+ CD45.2+ donor cells from WT PbA- or mifKO PbA-infected mice recovered in recipient CD45.1+ mice at day 5 post challenge. (C) Proliferation of CD4 T cells from WT PbA- or mifKO PbA-infected CD45.2+ donors at day 5 post challenge of CD45.1+ recipients, measured by CFSE dilution. (D) Per cent of CD4 T cells secreting IFN-γ from WT PbA- or mifKO PbA-infected CD45.2+ donors detected at day 5 post challenge in CD45.1+ recipients. (E) BALB/c mice were infected with WT PbA or mifKO PbA and were treated with i.p injection of 50 mg/kg chloroquine on days 7–10. Mice were killed at day 21 post treatment, and 105 CD4 T cells enriched from spleens were cocultured with 105 naive splenocytes and uninfected or infected RBC lysates (10 RBCs per naive splenocyte) for 3 d. Then IFN-γ levels were measured in the culture supernatant. Data are shown as mean ± SEM and are representative of two independent experiments. n = 5 mice per group. *P < 0.05; **P < 0.01 by two-tailed t test.
We confirmed these findings by a second experimental design to test the long-term protection conferred by CD4 T cells that previously were exposed to either WT PbA or mifKO PbA infections. Mice were infected with WT PbA or mifKO PbA and were cured by 4 d of chloroquine treatment starting on day 7 post infection. Three weeks after chloroquine treatment, CD4 T cells were isolated and restimulated ex vivo by coculturing with naive APCs and PbA antigens from iRBC lysates. As shown in Fig. 7E, CD4 T cells from mice previously infected with WT PbA showed lower proliferation and IFN-γ production than cells from mice infected with mifKO PbA. Thus, not only do fewer malaria-responsive CD4 T cells survive in the presence of PbMIF, but the remaining cells also are less capable of mediating a robust anti-Plasmodium recall response.
Discussion
Individuals in endemic areas remain at risk for Plasmodium reinfection despite the acquisition of partial immunity and tolerance to disease manifestations. A better understanding of why acquired immunity to Plasmodium is slow to develop, incomplete, and short lived is essential to improving strategies for malaria control (50). Malaria is characterized by a highly inflammatory cytokine milieu, and the lack of effective protection against infection suggests that memory T cells are not adequately formed or maintained during infection (51). Expression of IFN-γ during blood-stage malaria may direct the contraction of responding CD4 T cells (12, 16); therefore, the role of IFN-γ in mediating CD4 T-cell death is of particular interest, because it may affect directly the formation of immunological memory.
We show here that IFN-γ and IL-12 regulate the contraction phase of the anti-Plasmodium blood-stage CD4 T-cell response. IFN-γ and IL-12 signaling through CD4 T cells results in the up-regulation of T-bet and a concurrent down-regulation of Bcl-2 and IL-7Rα. These changes in T-bet and Bcl-2 promote the development of antigen-experienced CD4 T cells into short-lived terminal effector cells rather than long-lived memory cells. Terminally differentiated effector T cells are more susceptible to the contraction phase that occurs in the adaptive immune cells after the peak of the immune response, whereas long-lived memory precursor effector T cells are preserved in the memory T-cell pool (10). Fewer surviving memory CD4 T cells result in decreased control of Plasmodia during reinfection.
Our studies indicate that inflammatory cytokines critically influence the formation of immunological memory to malaria by modulating the survival of Plasmodium-responsive T cells. We identified a role for PMIF in the up-regulation of host inflammatory cytokines. This observation was unexpected and perhaps contrary to the expectation that Plasmodium-encoded factors evolved to diminish or subvert the host inflammatory response (52). Nevertheless, we observed that, when PbMIF is present, the increase in inflammatory cytokine production leads to up-regulation of T-bet and down-regulation of CD62L, IL-7Rα, and Bcl-2 in Plasmodium-responsive CD4 T cells. These PbMIF-induced changes cause more responding CD4 T-cells to develop into terminally differentiated effector T cells. As in the case of PbA infection in control IgG- and anti–IFN-γ/IL-12–treated mice, terminally differentiated effector T cells are susceptible to apoptosis and thus do not survive the contraction phase and are not present in the memory T-cell population when the host again is exposed to Plasmodium infection. Without an adequate anti-Plasmodium memory CD4 T-cell population, the host is not protected against subsequent malaria infection. In the absence of PMIF signaling, more responding CD4 T cells develop into memory precursor CD4 T cells, which persist and confer enhanced protection against future infections.
Plasmodium-specific CD4 T cells not only are important as a source of IFN-γ but also are essential for helping activate both CD8 T cells and B cells, which enhance the host anti-Plasmodium response (53). Although anti-Plasmodium antibody titers and CD8 T-cell populations were not affected by the presence of PMIF during a primary infection, it is not known whether PMIF also alters the ability of CD4 T cells to provide help in activating these adaptive immune cell populations. Differences in the virulence of murine Plasmodium models may complicate further the outcome of immunomodulation by the Plasmodium MIF ortholog, as suggested by study of the Plasmodium yoelii MIF variant (54, 55).
This study implicates the immunomodulatory action of a Plasmodium protein in interfering with the establishment of protective immunity. Plasmodium species have coevolved with their hosts for more than 100,000 y, and several strategies have been identified by which these parasites evade immune destruction to ensure persistence (50, 56). Most immunomodulatory mechanisms that have been described to date involve blockade of different components of the innate immune response (52), and the possibility that malaria parasites may actively direct the inflammatory response to interfere with the development of protective immunity had not been explored closely.
It is interesting to consider that the low protection rates of many Plasmodium blood-stage vaccines in endemic populations (57) may be caused by the active interference of adaptive immune responses by PMIF rather than by the immunogenicity of the candidate antigens. Notably, parasite MIF orthologs have been identified in several evolutionarily distant species of helminthic and protozoan pathogens (29). The close structural similarities between these parasite orthologs and mammalian MIF, together with evidence that these orthologs bind the MIF receptor (58, 59), suggest that parasite proteins also may act to modulate the adaptive immune responses of their hosts.
Malaria is a major global health challenge, and several vaccine initiatives are under way. Without natural acquisition of sterilizing immunity, the stimuli necessary for developing effective anti-malaria immunity remain unknown. The present findings indicating that Plasmodia actively modulate the host immune response to prevent the development of effective memory CD4 T cells have implications for the therapeutic immunomodulation of malaria infection and for vaccine development.
Experimental Methods
Patient Samples and Measurement of PMIF and Anti-PMIF Antibody Titer.
Sera from a well-characterized cohort of P. falciparum-infected patients from Zambia were used in our study (33).
We developed ELISAs to measure PfMIF and PbMIF levels from serum and spleen lysates. Briefly, polyclonal antibodies against PfMIF or PbMIF were produced in rabbits (PFR&L), and antibody specificity was verified by both Western blotting and ELISA, as described recently (60). IgG antibody fractions were purified and used to coat microtiter plates (Nunc) at 1 μg/mL anti-PfMIF or anti-PbMIF IgG overnight. The plates then were washed and blocked in assay diluent (eBioscience) for 1 h. Patient or mouse sera were added and incubated for 2 h at room temperature. Bound PfMIF or PbMIF was detected by adding biotinylated versions of the rabbit polyclonal IgG, followed by streptavidin-HRP (eBioscience) and developed with TMB substrate (Dako).
Anti-PbA antibody titers were determined by ELISA. Microtiter plates (Nunc) were coated with 100 ng/mL of PbA-iRBC lysate overnight. Plates then were blocked in assay diluent (eBioscience) for 1 h, and mouse sera were serially diluted and added to the wells for 2 h. Antibody binding was determined by adding HRP-labeled goat anti-mouse antibodies (Southern Biotech) and were developed with TMB substrate (Dako).
Mice.
WT, Thy1.1, and CD45.1 BALB/c mice were purchased from Jackson Laboratories. All procedures performed in these experiments complied with federal and Yale University guidelines.
P. berghei Infection, Cytokine Depletion, and Adoptive Transfer of Splenocytes.
BALB/c mice were infected with WT PbA or mifKO PbA by i.p. injection of 106 iRBCs. In some experiments, mice were challenged by i.p. injection of 106 GFP-expressing PbA. The absence of PbMIF in the mifKO PbA strain was verified by ELISA with a specific PbMIF antibody.
IL-12 and IFN-γ were depleted by i.p. injection of 0.25 mg of antibody (clones C17.8 and XMG1.2, respectively) on days −1, 1, 3, and 5 post infection (61). IFN-γ was administered by injection of 10 μg IFN-γ (BioLegend) in PBS on days 0, 2, and 4 post infection.
Splenocytes were isolated from CD45.2+ BALB/c mice at day 0 or day 7 post infection. Single-cell suspensions were obtained, and red blood cells were lysed. In some experiments, splenocytes were labeled with 0.5 μM CFSE (Invitrogen) before transfer. Splenocytes from infected mice then were incubated with 10 μM chloroquine for 2 h at 37 °C, and 2 × 107 splenocytes were adoptively transferred into recipient Thy1.1+ BALB/c or CD45.1+ BALB/c mice.
PfMIF Recombinant Protein and Receptor Binding.
cDNA for PfMIF and PbMIF were synthesized (GenScript), and PfMIF and PbMIF were expressed and purified following a previously described methodology with <20 pg endotoxin/mg protein as assessed by the PyroGene Recombinant Factor C assay (Cambrex BioScience) (58). Real-time binding interaction of PfMIF to the soluble ectodomain of CD74 was analyzed using the BIAcore 2000 biosensor (60).
Flow Cytometry.
Spleens were harvested at the indicated days post infection, homogenized, and passed through a 70-μm strainer to obtain single-cell suspensions. Red blood cells were lysed with ACK lysis buffer, and splenocytes were stained with Ki67, Bcl-2 (BD Biosciences), CD4, CD8, IL-7Rα, CD62L, IL-2, IFN-γ, T-bet, CD11a, CD45.2, Thy1.1 (eBioscience), or TUNEL (BioVision). The Foxp3 Staining Buffer Kit (eBioscience) was used for Ki67, T-bet, and Bcl-2 staining.
For intracellular cytokine staining, cells were stimulated ex vivo by coculturing with an 18 h prior coculture of iRBC lysates and naive CD45.1 splenocytes and with anti-CD3/CD28–coated beads (1 μL per 106 splenocytes) (Invitrogen) for 5 h in the presence of 1 μg/mL Brefeldin A (BD Bioscience). In some experiments, splenocytes were stimulated with 50 ng/mL PMA and 1 μg/mL ionomycin for 3 h in the presence of 1 μg/mL Brefeldin A. Stained cells were analyzed on LSR II or FACSCalibur flow cytometers (BD Bioscience). Data were analyzed with FlowJo software (TreeStar).
Quantification of Parasite Burden.
DNA was purified from blood samples obtained from the tail vein on the indicated days (Qiagen DNeasy), and P. berghei 18S rRNA copies were determined by quantitative PCR (Bio-Rad) (62). In some experiments, 18S rRNA expression relative to mouse GAPDH expression in RNA purified from spleens was analyzed by real-time PCR to determine spleen parasite burden (62). In animals challenged with GFP-expressing PbA, parasitemia was determined by analysis of blood samples with LSRII flow cytometers (BD Biosciences) (63). Parasitemia also was confirmed by counting iRBCs on blood smears obtained from tail vein samples stained with HEMA-3 (Fisher).
Cytokine Measurements and Histology.
Cytokine levels in serum, spleen lysates, or culture supernatant were measured by ELISA (eBioscience) or multiplex Luminex bead assays (Bio-Rad). Spleen lysates were obtained by freezing spleen sections immediately after harvesting, followed by homogenization in 1% TritonX-100 in Tris-buffered saline. Protein concentrations were determined from the supernatant fraction of the lysates, and ELISAs were conducted with 1 mg per well. Culture supernatants were collected from 106 splenocytes isolated from mice and were cultured for 18 h ex vivo or from 2.5 × 105 bone marrow-derived dendritic cells cultured for 10–24 h.
In some experiments, spleens were removed from infected mice, and TUNEL was performed on histologic sections (Yale University Research Histology Core Facility).
Cell Culture.
Bone marrow cells were isolated from naive BALB/c mice and matured into dendritic cells by incubation with 1 μg/mL GM-CSF for 7 d. Peritoneal elicited macrophages were obtained by injection of naive WT and MIF-R–deficient (64) BALB/c mice with 4% (wt/vol) thioglycollate. Peritoneal cells were recovered by washing the peritoneal cavity with 15 mL PBS. iRBCs were isolated from infected mice by exsanguination followed by purification of blood over magnetic LD columns (Miltenyi Biotec), and lysates were obtained by sonication. CD4 T cells were purified from naive mice by magnetic selection (Miltenyi).
Statistical Analysis.
Patient samples were analyzed by Pearson’s correlation and a Mann–Whitney test as indicated. All other data were analyzed by unpaired two-tailed Student’s t test. P values <0.05 were considered significant. Error bars indicate SE.
Supplementary Material
Acknowledgments
We thank S. M. Kaech, A. Iwasaki, and C. Ben Mamoun for valuable discussions and H. Shin for critical reading of the manuscript. T.S. is a recipient of a National Science Foundation Graduate Research Fellowship, and T.H. is funded by the National Institutes of Health Training Grant 5732A1007404-20. This work was funded by Netherlands Organization for Scientific Research Grant 816.02.001 (to K.A.) and National Institutes of Health Grants AI 5R01-51306-05 and AI 2R01-042310-12 (to R.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Author Summary on page 12280 (volume 109, number 31).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206573109/-/DCSupplemental.
References
- 1.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: More questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 2.Grun JL, Weidanz WP. Antibody-independent immunity to reinfection malaria in B-cell-deficient mice. Infect Immun. 1983;41:1197–1204. doi: 10.1128/iai.41.3.1197-1204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor-Robinson AW, Phillips RS, Severn A, Moncada S, Liew FY. The role of TH1 and TH2 cells in a rodent malaria infection. Science. 1993;260:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Good MF, Dontfraid F, Vinetz JM, Miller LH. Interdependence of CD4+ T cells and malarial spleen in immunity to Plasmodium vinckei vinckei. Relevance to vaccine development. J Immunol. 1989;143:2017–2023. [PubMed] [Google Scholar]
- 5.Lumsden JM, et al. Protective immunity induced with the RTS,S/AS vaccine is associated with IL-2 and TNF-α producing effector and central memory CD4 T cells. PLoS ONE. 2011;6:e20775. doi: 10.1371/journal.pone.0020775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens R, et al. Malaria-specific transgenic CD4(+) T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood. 2005;106:1676–1684. doi: 10.1182/blood-2004-10-4047. [DOI] [PubMed] [Google Scholar]
- 7.Pombo DJ, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360:610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 8.Good MF, Xu H, Wykes M, Engwerda CR. Development and regulation of cell-mediated immune responses to the blood stages of malaria: Implications for vaccine research. Annu Rev Immunol. 2005;23:69–99. doi: 10.1146/annurev.immunol.23.021704.115638. [DOI] [PubMed] [Google Scholar]
- 9.Todryk SM, et al. Multiple functions of human T cells generated by experimental malaria challenge. Eur J Immunol. 2009;39:3042–3051. doi: 10.1002/eji.200939434. [DOI] [PubMed] [Google Scholar]
- 10.Taylor JJ, Jenkins MK. CD4+ memory T cell survival. Curr Opin Immunol. 2011;23:319–323. doi: 10.1016/j.coi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, et al. The mechanism and significance of deletion of parasite-specific CD4(+) T cells in malaria infection. J Exp Med. 2002;195:881–892. doi: 10.1084/jem.20011174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luty AJ, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis. 1999;179:980–988. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 14.Schofield L, et al. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 15.Obar JJ, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villegas-Mendez A, et al. Heterogeneous and tissue-specific regulation of effector T cell responses by IFN-gamma during Plasmodium berghei ANKA infection. J Immunol. 2011;187:2885–2897. doi: 10.4049/jimmunol.1100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bejon P, et al. The induction and persistence of T cell IFN-gamma responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J Immunol. 2007;179:4193–4201. doi: 10.4049/jimmunol.179.6.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Kossodo S, Grau GE. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J Immunol. 1993;151:4811–4820. [PubMed] [Google Scholar]
- 19.Macatonia SE, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 20.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 22.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson MM, Tam MF, Wolf SF, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 24.Yoshimoto T, et al. A pathogenic role of IL-12 in blood-stage murine malaria lethal strain Plasmodium berghei NK65 infection. J Immunol. 1998;160:5500–5505. [PubMed] [Google Scholar]
- 25.Griffith JW, et al. Toll-like receptor modulation of murine cerebral malaria is dependent on the genetic background of the host. J Infect Dis. 2007;196:1553–1564. doi: 10.1086/522865. [DOI] [PubMed] [Google Scholar]
- 26.Chandele A, Mukerjee P, Das G, Ahmed R, Chauhan VS. Phenotypic and functional profiling of malaria-induced CD8 and CD4 T cells during blood-stage infection with Plasmodium yoelii. Immunology. 2011;132:273–286. doi: 10.1111/j.1365-2567.2010.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo SJ, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci USA. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermeire JJ, Cho Y, Lolis E, Bucala R, Cappello M. Orthologs of macrophage migration inhibitory factor from parasitic nematodes. Trends Parasitol. 2008;24:355–363. doi: 10.1016/j.pt.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobson SE, et al. The crystal structures of macrophage migration inhibitory factor from Plasmodium falciparum and Plasmodium berghei. Protein Sci. 2009;18:2578–2591. doi: 10.1002/pro.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: A study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 33.Thuma PE, et al. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J Infect Dis. 2011;203:211–219. doi: 10.1093/infdis/jiq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwiatkowski D, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 35.Leng L, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Augustijn KD, et al. Functional characterization of the Plasmodium falciparum and P. berghei homologues of macrophage migration inhibitory factor. Infect Immun. 2007;75:1116–1128. doi: 10.1128/IAI.00902-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordery DV, et al. Characterization of a Plasmodium falciparum macrophage-migration inhibitory factor homologue. J Infect Dis. 2007;195:905–912. doi: 10.1086/511309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von der Weid T, Honarvar N, Langhorne J. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J Immunol. 1996;156:2510–2516. [PubMed] [Google Scholar]
- 39.Butler NS, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 43.Colpitts SL, Dalton NM, Scott P. IL-7 receptor expression provides the potential for long-term survival of both CD62Lhigh central memory T cells and Th1 effector cells during Leishmania major infection. J Immunol. 2009;182:5702–5711. doi: 10.4049/jimmunol.0803450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephens R, Langhorne J. Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog. 2010;6:e1001208. doi: 10.1371/journal.ppat.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darrah PA, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 46.Marshall HD, et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agnello D, et al. Cytokines and transcription factors that regulate T helper cell differentiation: New players and new insights. J Clin Immunol. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 48.Lighvani AA, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci USA. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wojciechowski S, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierce SK, Miller LH. World Malaria Day 2009: What malaria knows about the immune system that immunologists still do not. J Immunol. 2009;182:5171–5177. doi: 10.4049/jimmunol.0804153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Good MF, Stanisic D, Xu H, Elliott S, Wykes M. The immunological challenge to developing a vaccine to the blood stages of malaria parasites. Immunol Rev. 2004;201:254–267. doi: 10.1111/j.0105-2896.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 52.Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 53.Langhorne J, et al. Dendritic cells, pro-inflammatory responses, and antigen presentation in a rodent malaria infection. Immunol Rev. 2004;201:35–47. doi: 10.1111/j.0105-2896.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 54.Thorat S, Daly TM, Bergman LW, Burns JM., Jr Elevated levels of the Plasmodium yoelii homologue of macrophage migration inhibitory factor attenuate blood-stage malaria. Infect Immun. 2010;78:5151–5162. doi: 10.1128/IAI.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller JL, Harupa A, Kappe SH, Mikolajczak SA. Plasmodium yoelii macrophage migration inhibitory factor is necessary for efficient liver-stage development. Infect Immun. 2012;80:1399–1407. doi: 10.1128/IAI.05861-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hedrick PW. Population genetics of malaria resistance in humans. Heredity (Edinb) 2011;107:283–304. doi: 10.1038/hdy.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thera MA, Plowe CV. Vaccines for malaria: How close are we? Annu Rev Med. 2012;63:345–357. doi: 10.1146/annurev-med-022411-192402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamir D, et al. A Leishmania ortholog of macrophage migration inhibitory factor modulates host macrophage responses. J Immunol. 2008;180:8250–8261. doi: 10.4049/jimmunol.180.12.8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho Y, et al. Structural and functional characterization of a secreted hookworm Macrophage Migration Inhibitory Factor (MIF) that interacts with the human MIF receptor CD74. J Biol Chem. 2007;282:23447–23456. doi: 10.1074/jbc.M702950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merk M, et al. The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor (MIF) Proc Natl Acad Sci USA. 2011;108:E577–E585. doi: 10.1073/pnas.1102941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 62.Kumar KA, Oliveira GA, Edelman R, Nardin E, Nussenzweig V. Quantitative Plasmodium sporozoite neutralization assay (TSNA) J Immunol Methods. 2004;292:157–164. doi: 10.1016/j.jim.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Franke-Fayard B, et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Shi X, et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]