Abstract
During peripheral immune activation caused by an infection or an inflammatory condition, the innate immune response signals to the brain and causes an up-regulation of central nervous system (CNS) cytokine production. Central actions of proinflammatory cytokines, in particular IL-1β, are pivotal for the induction of fever and fatigue. In the present study, the influence of peripheral chronic joint inflammatory disease in rheumatoid arthritis (RA) on CNS inflammation was investigated. Intrathecal interleukin (IL)-1β concentrations were markedly elevated in RA patients compared with controls or with patients with multiple sclerosis. Conversely, the anti-inflammatory IL-1 receptor antagonist and IL-4 were decreased in RA cerebrospinal fluid (CSF). Tumor necrosis factor and IL-6 levels in the CSF did not differ between patients and controls. Concerning IL-1β, CSF concentrations in RA patients were higher than in serum, indicating local production in the CNS, and there was a positive correlation between CSF IL-1β and fatigue assessments. Next, spinal inflammation in experimental arthritis was investigated. A marked increase of IL-1β, IL-18, and tumor necrosis factor, but not IL-6 mRNA production, in the spinal cord was observed, coinciding with increased arthritis scores in the KBxN serum transfer model. These data provide evidence that peripheral inflammation such as arthritis is associated with an immunological activation in the CNS in both humans and mice, suggesting a possible therapeutic target for centrally affecting conditions as fatigue in chronic inflammatory diseases, for which to date there are no specific treatments.
Keywords: cerebral, sleep
Inflammation in the central nervous system (CNS) may have numerous causes, including bacterial infections, multiple sclerosis, or other neuroimmune conditions (1). During cerebral infections, disruption of the blood–brain barrier (BBB) allows for the influx of inflammatory cells and tissue-destructive products. In addition, there are elevated inflammatory cytokines in both the serum and the CNS. Importantly, preclinical work has shown that certain cytokines (e.g., IL-1β and IL-6), through their effect on supraspinal neuraxial function, result in fever and fatigue (2). In contrast, peripheral immune activation in autoimmune diseases, such as joint inflammation in rheumatoid arthritis, is mirrored in the blood with moderate increases in certain inflammatory cytokines, for example, IL-6 (3), and these diseases are not associated with disruption of the BBB (4). Hence the major impact of peripheral inflammatory cytokines on the CNS are likely indirect, possibly through cytokine-mediated production of prostaglandins in brain endothelium (5). However, bidirectional neuro-immune pathways mediating inflammatory signals from the periphery to the CNS have been defined in both animals (6) and in humans (7), and, importantly, a link between peripheral inflammation and increased cytokine production in the CNS has been shown in animal models (2, 8). In the CNS, proinflammatory cytokines are produced by microglia and astrocytes, and these factors have a direct effect on neurotransmission, for example, increasing pain transmission (9, 10). Cytokine expression in glia cells in the CNS is increased subsequent to brain and spinal cord injury and following peripheral inflammatory stimuli. Important proinflammatory mediators in this respect are tumor necrosis factor (TNF) and IL-1β, which have been detected in the brain in response to peripheral endotoxin challenge (11, 12). Not only acute inflammatory stimuli, but also chronic peripheral inflammation has been shown to affect CNS mechanisms. For example, experimental peripheral joint inflammation has been associated with activation of astrocytes in the spinal cord (8). Additionally, both peripheral and central blocking of the action of proinflammatory cytokines attenuates pain-like behavior in experimental arthritis (13, 14).
Rheumatoid arthritis (RA) is a chronic disease, characterized by inflammation in the joints, subsequently leading to joint destruction. Features of this disease implicate regulation at least in part by the central nervous system. First, the joint inflammation is symmetric and involves the small joints of the hands and feet (15). Second, pain elicited by compression of the metacarpophalangeal joints of RA patients is rapidly reduced after administration of TNF blockade and associated with changes in CNS functional MRI signals (16). Third, RA is associated with increased prevalence of severe fatigue, which is also reported in a number of other diseases associated with systemic inflammation (17) and is linked to IL-1β–dependent sickness behavior in systemically inflamed rodents (18). In addition, cytokine-blocking treatments targeting TNF or IL-1β are known to partly decrease fatigue symptoms in RA (19, 20). The aim of the current study was to further investigate to what extent peripheral inflammation and joint disease may be connected to central nervous inflammatory mechanisms.
Results
Elevated Levels of IL-1β in the Cerebrospinal Fluid of RA Patients.
The clinical features of RA patients and healthy clinical controls are displayed in Table 1. No RA patient displayed any sign of neurological disease or generalized pain. In agreement with earlier data, RA patients had significantly higher levels in fatigue measurements and reduced sleep quality (21) compared with healthy controls. Lumbar puncture was performed in RA patients and presurgery in separate nonneurological, noninflammatory controls [cerebrospinal fluid (CSF) controls], and CSF was analyzed for cytokines previously described to be involved in sleep regulation and fatigue, i.e., TNF, IL-1β, and IL-6. In RA patients, IL-1β levels were markedly elevated (median 8.35 pg/mL) compared with controls (median 0.18 pg/mL) (Fig. 1). In RA, IL-1β levels in CSF were markedly higher compared with serum levels (8.35 ± 12.59 pg/mL vs. 0.00 ± 0.06; P < 0.005).
Table 1.
Clinical data in patients with rheumatoid arthritis and healthy controls
| RA (n = 14) | Healthy controls | P | |
| Age | 51 ± 7.2 | 44 ± 10.7 | NS |
| Swollen joints | 4.9 ± 3.8 | NA | NA |
| Tender joints | 4.2 ± 3.9 | NA | NA |
| DAS28 | 3.55 ± 1.3 | NA | NA |
| No. ACPA positive (%) | 12/14 (86) | NA | NA |
| No. RF positive (%) | 10/14 (71) | NA | NA |
| Global health VAS (mm) | 27.5 ± 16.9 | NA | NA |
| Pain VAS (mm) | 26.8 ± 18.1 | 0 ± 0 | P < 0.0001 |
| Fatigue VAS (mm) | 55.7 ± 25.6 | 2.4 ± 5.1 | P < 0.0001 |
| PSQI | 6.6 ± 3.0 | 1.8 ± 1.7 | P < 0.0001 |
Data are presented as the mean ± SD. ACPA, antibodies to citrullinated peptide antigens; DAS28, disease activity score for 28-joint count; NA, not applicable; NS, not significant; PSQI, Pittsburg Sleep Quality Index; RF, rheumatoid factor.
Fig. 1.
Levels of IL-1β in cerebrospinal fluid of RA patients (n = 14), CSF controls (n = 12), and MS patients (n = 14). ***P < 0.001. Results of post hoc tests are shown.
CSF from patients with multiple sclerosis (MS), a neuro-inflammatory disease, was analyzed for further comparison. MS is associated with cerebral immune and cytokine activation (1), and IL-1β release is prominent (22). Our study showed that IL-1β levels were markedly lower in MS patients in stable nonactive phase (median 0.54 pg/mL) (Fig. 1) compared with CSF from RA patients, indicating that RA is associated with significant immune activation despite no other signs of neurological disease. No significant differences in IL-1β levels were found between MS patients and CSF controls (Fig. 1).
Decrease of Anti-Inflammatory IL-1 Receptor Antagonist in RA CSF.
Next, we measured levels of the anti-inflammatory mediator IL-1 receptor antagonist (IL-1Ra) in RA CSF and controls. Concordant with the increased level of IL-1β, there was a decrease in IL-1Ra protein (median: 13.1 pg/mL) in RA CSF compared with controls (median 23.4 pg/mL) (Fig. 2A), suggesting a proinflammatory environment in RA CNS. The relationship between IL-1Ra and IL-1β has been documented as an adequate marker of inflammatory balance, for example, with a higher IL-1Ra:IL-1β ratio in the synovium of osteoarthritis patients compared with RA (23). A balance toward a proinflammatory state in the CNS of RA patients is further supported by the markedly decreased IL-1Ra:IL-1β ratio in RA patients compared with controls (Fig. 2B). In addition, RA CSF levels of IL-1Ra were markedly lower than serum levels [13.08 ± 9.82 pg/mL (CSF) vs. 271.3 ± 171.3 pg/mL (serum); P < 0.0001].
Fig. 2.
(A) IL-1Ra levels in CSF of RA patients (n = 14) and CSF controls (n = 9). Two controls had no detectable levels of IL-1Ra, and one outlier exceeding 2 SD was excluded. *P < 0.05. (B) Ratio between IL-1Ra and:IL-1β levels in CSF of RA patients and CSF controls. Values are the mean ± SEM (***P < 0.001). (C) Correlation between CSF IL-1β levels and fatigue in RA patients (n = 14). (R: 0.55; P < 0.05).
Analysis of TNF levels in CSF displayed no significant differences between RA patients and controls (0.18 ± 0.25 pg/mL vs. 0.18 ± 0.11 pg/mL). For IL-6, there was a tendency to higher CSF levels in RA, but this did not reach statistical significance [2.59 ± 1.43 pg/mL (RA) vs. 1.69 ± 1.35 pg/mL (control)]. Unlike IL-1β, both TNF and IL-6 levels were markedly lower in CSF than in serum of RA patients [TNF: 0.18 ± 0.25 pg/mL (CSF) vs. 1.03 ± 0.97 pg/mL (serum), P = 0.001; IL-6: 2.59 ± 1.43 pg/mL vs. 6.25 ± 4.21 pg/mL, P = 0.01]. Additional experiments in which RA CSF was compared with a new CSF control group (due to lack of remaining CSF from the first control group) (Materials and Methods) showed decreased levels of anti-inflammatory IL-4 in RA CSF (0.06 ± 0.05 pg/mL vs. 0.23 ± 0.15 pg/mL) (Fig. S1), whereas IL-10 levels were below the detection limit in all patients and controls. Taken together, these data support the hypothesis that RA is accompanied not only by joint and systemic inflammation, but also by an immune activation in the CNS. Moreover, the cerebral IL-1 system seems to be activated in this process.
Correlation Between CSF IL-1β and Fatigue.
There were positive correlations between the increase in IL-1β and fatigue (R: 0.55, P < 0.05) (Fig. 2C), and a positive trend was observed for sleep quality (R: 0.46; P = 0.11), whereas no such correlation could be detected concerning pain (R: −0.15; P = 0.60) and tender joint count (R: −0.19; P = 0.52). No significant correlations could be detected between IL-1Ra, IL-6, or TNF and fatigue measurements.
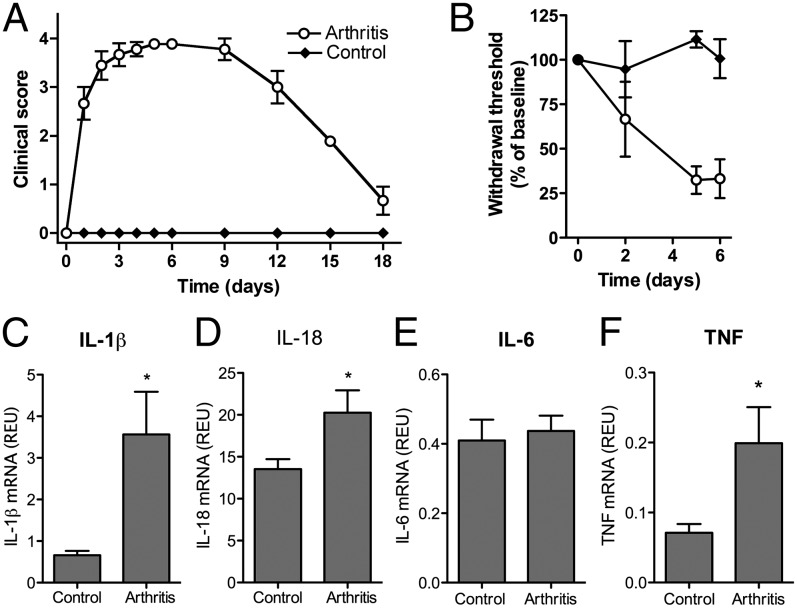
Immune Activation in Spinal Cord of Arthritic Mice.
To investigate if up-regulation of IL-1β in CNS is a general feature in arthritis, we analyzed spinal IL-1β production in serum-transferred K/BxN arthritis. This model is well described (24, 25) and characterized by a fast-developing arthritis with clinical signs of arthritis appearing after 24 h, peaking on day 6, and resolving after 15–20 d (Fig. 3A) with a concurrent induction of mechanical hypersensitivity (Fig. 3B). Lumbar spinal cords were collected the same day as the peak of arthritis (day 6), and mRNA was extracted. We detected a significant increase in the relative levels of IL-1β and IL-18 mRNA transcripts (Fig. 3 C and D) in the spinal cords of the arthritis group compared with controls. In contrast, no change in IL-6 mRNA levels were found (Fig. 3E), and IL-1Ra mRNA levels were below the detection limit in both arthritic and control mice. TNF mRNA levels were increased along with arthritis (Fig. 3F). There was no correlation between spinal IL-1β mRNA levels and mechanical hypersensitivity (R: 0.46, P = 0.36) on day 6.
Fig. 3.
(A) Disease severity was assessed by visual inspection of the front and hind paws using a scale of 0–4. (B) Tactile thresholds were assessed by von Frey filaments and expressed as the percentage of baseline following induction of K/BxN serum-transfer arthritis. Spinal cords were harvested on day 6. (C) IL-1β. (D) IL-18. (E) IL-6. (F) TNF mRNA levels assessed and expressed as relative expression units (REU). Each time point and bar represents mean ± SEM (n = 6–12 mice/group). *P < 0.05.
Discussion
The main finding of the present study is that peripheral inflammation in a chronic disease, such as RA, seems to be associated with an increase in spinal and, presumably, supraspinal immune activation, despite no focal neurological symptoms. IL-1β, previously known to be of importance in sickness response and sleep regulation (18), was markedly increased in lumbar CSF whereas other key cytokines such as TNF and IL-6 showed no significant difference. In addition, activation of the IL-1 system is a central mechanism in sickness response, shown in several studies (18, 26), and has been linked to fatigue in human disease (27). The powerful effects on fatigue of immunosuppressive biologic therapies in RA (19) suggest that inflammation, either peripheral or cerebral, is also involved in fatigue mechanisms in RA, and rapid improvement of fatigue has also been shown with agents that specifically block IL-1β action (20). Conversely, we found a decrease in the intrathecal IL-1Ra and IL-4 levels, and, together, these data demonstrate evidence for an activated IL-1 system not only in the joints, but also in the CNS of RA patients.
Notably, although no RA patient in the present study had any clinical signs of neurological involvement, CSF levels of IL-1β were in the same range as previously detected in the active phase of MS (22). How, then, does peripheral inflammation result in the up-regulation of IL-1β production in the CNS? It has been suggested that circulating cytokines can reach the CNS through direct passage in areas where the BBB is more permeable (e.g., the circumventricular organs, or CVOs) by specific transport systems or through release from immune cells entering the CNS. Alternatively, cytokines may activate receptors on endothelial cells, stimulating these to release inflammatory factors into the CNS parenchyma, or peripheral afferent neurons could be activated by cytokines, leading to activation of CNS glia cells and subsequent release of cytokines in the CNS (28). In the present study, a diffusion of IL-1β from the systemic circulation to the brain is possible, but for a number of reasons we believe this to be unlikely. First, it has been debated whether or not cytokines like IL-1β can actually cross the BBB. The BBB was intact in all RA patients (Materials and Methods), and therefore the main transport should be active mechanisms, which have also been described for IL-1 (28).
Second, CSF levels were 10-fold higher than serum IL-1β levels in the RA patients, making the elevated central concentrations a result of circulating IL-1β from systemic inflammation improbable. For the same reason, diffusion of IL-1β through the CVOs is unlikely as a source of the markedly increased levels in RA. Notably, although we found elevated numbers of mononuclear cells in CSF from MS patients, the mononuclear cell count was normal in RA CSF (Fig. S2), thus pointing to sources other than immune cells for the increased IL-1β levels in RA CSF. Instead, we speculate that systemic inflammatory mediators in RA induce prostaglandin-mediated signaling across the BBB through interaction with cerebral endothelium cytokine receptors, as suggested earlier (5). These mechanisms could then activate the resident nonneuronal cells in the CNS to produce proinflammatory mediators, such as IL-1β.
Microglial cells are known to produce IL-1β, IL-1R I, and IL-1Ra in response to inflammatory stimuli (29, 30). In addition, spinally produced IL-1β stimulates cyclo-oxygenase-2 activity and prostaglandin production, such as PGE2 (31). Increased production of PGE2 following peripheral inflammation has been reported in the CNS (32). The lack of significant up-regulation of TNF and IL-6 in CSF is in line with earlier data demonstrating low levels of these cytokines in CSF in general (33), and the discrepancy versus IL-1β levels further supports a possible separate regulation of the IL-1 system in RA CNS. Moreover, there were positive correlations between an increase in IL-1β and clinically defined fatigue, and the same positive trend was observed for sleep quality. In contrast, although we cannot rule out that pain is a contributing factor, no direct correlation could be detected between IL-1β levels in CSF and pain scores or the number of tender joints. Notably, although experimental arthritis leads to an increased production of IL-1β mRNA in the spinal cord, and mice with mechanical hypersensitivity had elevated levels of spinal Il-1β mRNA, no direct correlation between IL-1β levels and pain scores was detected, which matches the human data. Taken together, our results thereby differ from findings in noninflammatory chronic pain conditions in humans, where IL-1β levels in CSF showed a positive correlation with pain assessments (34). Our observations support earlier data indicating that IL-1β is involved in the mechanisms leading to fatigue (17), which seem to be differentiated from pain in the context of RA.
There were no obvious differences in CSF IL-1β levels between RA patients with different treatments, for example, TNF blockade and other disease-modifying antirheumatic drugs. Concerning IL-1Ra, a tendency toward higher levels in methotrexate (MTX)-treated patients could be detected, in line with MTX crossing the BBB and potentially exerting anti-inflammatory effects on IL-1Ra–producing cells in the CNS (35). However, the numbers in each subgroup were too low to draw any general conclusions. No similar tendencies were detected for CSF IL-1β levels.
As detailed above, and in line with the human data, we could detect increased mRNA levels of IL-1β in the spinal cord of arthritic animals. As mentioned above, activation of spinal glia cells have been detected earlier during arthritis. We used another approach for investigating the segmental spinal cord cytokine production. Spinal TNF mRNA levels were increased, and the peaks of these cytokines coincided with the peak of arthritis severity. These data suggest that afferent signaling from joints affected by inflammation may give rise to CNS immune activation, which is similar to findings in other models of joint inflammation (36, 37). IL-1β is synthesized as the inactive precursor pro-IL-1β, which is cleaved to the active from by caspase-1 (38). IL-18, another important member of the IL-1 family of proinflammatory cytokines, is matured by the same enzyme. Interestingly, IL-18 involvement in human RA pathology has been discussed (39, 40), and data from animal models suggest that IL-18 is functioning as a glia modulator with a regulatory role in spinal pain processing (41). Thus, we assessed IL-18 in the K/BxN model and found increased spinal IL-18 mRNA levels subsequent to induction of arthritis, which is in line with mutual production mechanisms with IL-1β. From our data it is not evident which cell types are responsible for the increased spinal cytokine production, but earlier data indicate that cells of glial origin have the capacity to produce both IL-1β and IL-18 (29, 42) and have proven to be activated in response to experimental arthritis (8). The difference in spinal TNF mRNA levels in experimental arthritis compared with unchanged TNF levels in RA CSF may be explained by the known discrepancies concerning production of cell-bound, versus soluble, TNF in the CNS (43). Thus, activated microglial cells are known to produce mainly cell-bound TNF, which mediates cell–cell interactions, whereas the release of the soluble form of TNF, which may be detected in CSF, is marginal. In contrast to TNF, IL-6 displayed no differences in production in either RA CSF or mice spinal cord, suggesting minor importance of central IL-6 production in the context of peripheral inflammatory action on CNS mechanisms.
In summary, we present evidence for a central activation of inflammatory mechanisms, and mainly the IL-1 system, in both patients with RA and experimental arthritis, indicating that cerebral immune activation is a general phenomenon in peripheral joint disease. Together with previous data on the importance of IL-1β in sleep regulation and fatigue, our results also implicate the IL-1 system as a possible therapeutic target for centrally affecting conditions such as fatigue in chronic inflammatory diseases, for which to date there is no specific treatment.
Materials and Methods
Patients.
RA, CSF controls, or clinical controls had no present or concurrent neurological disease. No analgesics were allowed 24 h before pain and fatigue assessments. All patients gave their informed consent to participate. The study was carried out in compliance with the Helsinki Declaration and approved by the regional ethics committee at the Karolinska Institute.
RA Patients.
Fourteen female patients all fulfilled both the 1987 and the 2010 American College of Rheumatology criteria for RA (44, 45), and none fulfilled the American College of Rheumatology criteria for fibromyalgia (46). Clinical baseline data are presented in Table 1. Seven patients were on MTX monotherapy, two were on MTX combined with etanercept or infliximab, one was on adalimumab monotherapy, three were on sulfasalazine, and one was on hydroxychloroquine. Three patients were also on low-dose prednisone (all below 7.5 mg/d). No non-steroidal anti-inflammatory drug (NSAID) was administered within 24 h before CSF sampling and pain and fatigue assessments.
Healthy Clinical Controls.
Healthy women who did not fulfill the criteria for RA or fibromyalgia were assessed by the visual analog scale (VAS) of fatigue, and the Pittsburgh Sleep Quality Index (PSQI). None received medication with NSAID. No control was assessed with chronic pain, defined as VAS pain > 40 mm.
Multiple Sclerosis Patients.
Fourteen female patients (46 ± 5 y; P = NS compared with RA patients and CSF controls) all fulfilled criteria for multiple sclerosis (47) and brain magnetic resonance imaging showing typical findings. One patient had primary progressive MS, and the rest of the patients had relapsing remittent MS in remission, defined as a stable status on the Expanded Disability Status Scale for >3 mo before sampling. At the time of lumbar puncture, no MS patient had current treatment with any immune modulating agent. Two patients had previously been treated with Copaxone, one patient had previous treatment with Novantrone, one with Rebif, and one patient had previous treatment with β-IFN. All treatments were ended at least 4 wk before sampling.
CSF Controls.
The CSF samples from 12 women (55 ± 9 y) served as a control group (CSF controls). The samples therefore were obtained from patients who needed spinal anesthesia for surgical procedures and who had no neurological, immunological, or malignant disease. There were no significant differences in age between RA patients and control subjects. None of the controls fulfilled RA or fibromyalgia criteria. No control was assessed with chronic pain, defined as VAS pain > 40 mm. Due to limited amounts of the original control CSF, for the analysis of CSF IL-10 and IL-4 only, a second group of CSF controls was used for comparisons with RA. This second group was composed of nine women (44 ± 14 y; P = NS compared with RA patients) with non-inflammatory neurological symptoms.
Lumbar Puncture, Cytokine, and Clinical Measurements.
Lumbar puncture was performed within 24 h after pain and fatigue assessments. CSF was centrifuged, and supernatants were frozen and stored at −80 °C until use.
Cytokine levels (IL-1β, IL-1Ra, TNF, IL-6, IL-4, and IL-10) were analyzed by ELISA (R&D Systems, high-sensitivity Quantikine). Fatigue and pain were assessed by the VAS as previously described (48). Measurement of sleep disturbance and sleep quality was performed using the PSQI (49). Disease activity scores in RA were calculated using a 28-joint count as previously described (50).
BBB in Patients.
Damage of the BBB was calculated as a serum albumin/CSF albumin ratio (albumin index). The median albumin index was 5.26 × 10−3 in RA. All RA patients had normal albumin index in relation to age (51), indicating no breech of the BBB. In MS patients, the median albumin index was 5.90 × 10−3. Two MS patients had an abnormal albumin index in relation to age. The median CSF IgG index in RA patients was 0.47 compared with 0.84 in MS patients (P < 0.0001). For CSF control patients, data on CSF albumin levels and the IgG index were not available. No control had any sign of neurological disease as mentioned above.
Animals.
All experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego (under the Guide for Care and Use of Laboratory Animals, National Institutes of Health publication 85–23) (52).
Mice were housed up to four per standard cage at room temperature, maintained on a 12-h light/dark cycle. Testing was performed during the light cycle. Food and water were available ad libitum. C57BL/6 mice (male, 25–30 g) were purchased from Harlan. KRN T-cell receptor transgenic mice were a gift from D. Mathis and C. Benoist (Harvard Medical School, Boston, MA) and from the Institut de Génétique et de Biologie Moléculaire et Cellulaire and were maintained on a C57BL/6 background (K/B). Arthritic mice were obtained by crossing K/B with NOD/Lt (N) animals (K/BxN). NOD/Lt mice were purchased from the Jackson Laboratory.
Blood from arthritic adult K/BxN mice was collected, centrifuged at 10,000 × g for 10 min, and the sera were pooled. Recipient C57BL/6 mice received 100 μL sera by i.p. injection on day 0 and day 2 (total volume 200 μL). Control animals were injected with pooled sera from naive C57BL/6 mice. Clinical arthritis scores were evaluated for 18 d using a scale of 0–4 where for each swollen paw, 1 point was given, resulting in a maximum score of 4 per mouse.
Assessment of Mechanical Hypersensitivity.
Animals were habituated to the testing environment before baseline testing. On test days, mice were placed in individual compartments on top of a wire mesh surface and allowed to acclimatize for 1–2 h before testing. Withdrawal thresholds were assessed with calibrated von Frey optiHair filaments (Marstock OptiHair) with logarithmically incremental stiffness (0.5, 1, 2, 4, 8, 16, 32 mN). Each filament was pressed perpendicularly against the mid hind paw and held for ∼3 s. A positive response was noted if the paw was withdrawn. The threshold with a 50% probability of withdrawal was calculated as previously described (24). Withdrawal thresholds for both hind paws were averaged and expressed as percentage of baseline.
Quantitative Real-Time PCR.
On day 6, lumbar spinal cords were dissected and immediately frozen and stored at −80 °C until use. mRNA was extracted using TRIzol (Invitrogen) according to the manufacturer’s protocol. cDNA was prepared and quantitative real-time PCR was performed with TaqMan Gene Expression Assays (Applied Biosystems) according to the manufacturer’s instructions to determine relative mRNA levels, using the GeneAmp 7500 Fast Sequence Detection system (Applied Biosystems). Predeveloped specific primers were used to detect IL-1β (Assay ID Mm00446185-m1), IL-6 (Mm00446190_m1), intracellular/soluble IL-Ra (Assay ID Mm00446185-m1), IL-18 (Assay ID Mm00434226_m1), TNF (Assay ID Mm00443258_m1), and HPRT1 (Assay ID Mm00446968_m1) (Applied Biosystems). Sample threshold cycle (Ct) values in standard curve samples (mouse RAW 264.7 cells stimulated with LPS for 4 h) containing IL-1β, IL-1Ra, IL-6, IL-18, and HPRT1 mRNA were used to calculate the cDNA concentration equivalents in the spinal cord samples. The IL-1β, IL-6, IL-18, and IL1-Ra data were then normalized to HPRT gene expression to obtain relative concentrations and presented as relative expression units.
Statistics.
The Kruskall–Wallis nonparametrical test was used for comparisons between the groups in the clinical study. For direct comparisons, the Mann–Whitney U test was used, and for paired analyses, the Wilcoxon Signed Rank was used. Figures represent raw data, and the corresponding data are presented as median values in the text. Results that are presented only in numerical form in the text are expressed as median ± SD. Student’s t test was used for comparisons of spinal mRNA levels in the K/BxN serum transfer model, and correlation between tactile thresholds and spinal IL1β mRNA levels was assessed by the Spearman rank correlation test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Rosmarie Johnson and Seija Johansson for excellent assistance in clinical assessments and lumbar puncture. We also thank Tomas Hökfelt for critical reading of the manuscript. This work was supported by grants from the National Institute of Health, the Swedish Research Council, the Swedish Rheumatism Association, the Swedish Foundation for Strategic Research, the Arthritis Foundation, and King Gustaf V’s 80-Year Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118748109/-/DCSupplemental.
References
- 1.Prendergast CT, Anderton SM. Immune cell entry to central nervous system: Current understanding and prospective therapeutic targets. Endocr Metab Immune Disord Drug Targets. 2009;9:315–327. doi: 10.2174/187153009789839219. [DOI] [PubMed] [Google Scholar]
- 2.Watkins LR, Maier SF. Immune regulation of central nervous system functions: From sickness responses to pathological pain. J Intern Med. 2005;257(2):1–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 3.Emery P, Luqmani R. The validity of surrogate markers in rheumatic disease. Br J Rheumatol. 1993;32(Suppl 3):3–8. doi: 10.1093/rheumatology/32.suppl_3.3. [DOI] [PubMed] [Google Scholar]
- 4.Eskandari F, Webster JI, Sternberg EM. Neural immune pathways and their connection to inflammatory diseases. Arthritis Res Ther. 2003;5:251–265. doi: 10.1186/ar1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engblom D, et al. Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med (Berl) 2002;80(1):5–15. doi: 10.1007/s00109-001-0289-z. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein RS, et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13:210–215. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inglis JJ, et al. Collagen-induced arthritis as a model of hyperalgesia: Functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis Rheum. 2007;56:4015–4023. doi: 10.1002/art.23063. [DOI] [PubMed] [Google Scholar]
- 9.Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. 2005;14(4):166–174. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson MR, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dam AM, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: A pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- 12.Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J Neuroimmunol. 1994;49(1–2):125–134. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 13.Boettger MK, et al. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: Evidence of a neuronal target. Arthritis Rheum. 2008;58:2368–2378. doi: 10.1002/art.23608. [DOI] [PubMed] [Google Scholar]
- 14.Boettger MK, et al. Spinal tumor necrosis factor alpha neutralization reduces peripheral inflammation and hyperalgesia and suppresses autonomic responses in experimental arthritis: A role for spinal tumor necrosis factor alpha during induction and maintenance of peripheral inflammation. Arthritis Rheum. 2010;62:1308–1318. doi: 10.1002/art.27380. [DOI] [PubMed] [Google Scholar]
- 15.Cutolo M, Straub RH, Bijlsma JW. Neuroendocrine-immune interactions in synovitis. Nat Clin Pract Rheumatol. 2007;3:627–634. doi: 10.1038/ncprheum0601. [DOI] [PubMed] [Google Scholar]
- 16.Hess A, et al. Blockade of TNF-α rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci USA. 2011;108:3731–3736. doi: 10.1073/pnas.1011774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology (Oxford) 2011;50:1009–1018. doi: 10.1093/rheumatology/keq454. [DOI] [PubMed] [Google Scholar]
- 18.Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yount S, et al. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapies alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:838–846. [PubMed] [Google Scholar]
- 20.Omdal R, Gunnarsson R. The effect of interleukin-1 blockade on fatigue in rheumatoid arthritis: A pilot study. Rheumatol Int. 2005;25:481–484. doi: 10.1007/s00296-004-0463-z. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe F, Michaud K, Li T. Sleep disturbance in patients with rheumatoid arthritis: Evaluation by medical outcomes study and visual analog sleep scales. J Rheumatol. 2006;33:1942–1951. [PubMed] [Google Scholar]
- 22.Dujmovic I, et al. The analysis of IL-1 beta and its naturally occurring inhibitors in multiple sclerosis: The elevation of IL-1 receptor antagonist and IL-1 receptor type II after steroid therapy. J Neuroimmunol. 2009;207(1–2):101–106. doi: 10.1016/j.jneuroim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Richette P, et al. A high interleukin 1 receptor antagonist/IL-1beta ratio occurs naturally in knee osteoarthritis. J Rheumatol. 2008;35(8):1650–1654. [PubMed] [Google Scholar]
- 24.Christianson CA, et al. Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain. 2010;151:394–403. doi: 10.1016/j.pain.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monach P, et al. The K/BxN mouse model of inflammatory arthritis: Theory and practice. Methods Mol Med. 2007;136:269–282. doi: 10.1007/978-1-59745-402-5_20. [DOI] [PubMed] [Google Scholar]
- 26.Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harboe E, et al. Fatigue in primary Sjögren’s syndrome: A link to sickness behaviour in animals? Brain Behav Immun. 2009;23:1104–1108. doi: 10.1016/j.bbi.2009.06.151. [DOI] [PubMed] [Google Scholar]
- 28.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Guo W, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinteaux E, Parker LC, Rothwell NJ, Luheshi GN. Expression of interleukin-1 receptors and their role in interleukin-1 actions in murine microglial cells. J Neurochem. 2002;83:754–763. doi: 10.1046/j.1471-4159.2002.01184.x. [DOI] [PubMed] [Google Scholar]
- 31.Samad TA, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi M, Martucci C, Ferrario P, Franchi S, Sacerdote P. Increased tumor necrosis factor-alpha and prostaglandin E2 concentrations in the cerebrospinal fluid of rats with inflammatory hyperalgesia: The effects of analgesic drugs. Anesth Analg. 2007;104:949–954. doi: 10.1213/01.ane.0000258060.89380.27. [DOI] [PubMed] [Google Scholar]
- 33.Vladić A, Horvat G, Vukadin S, Sucić Z, Simaga S. Cerebrospinal fluid and serum protein levels of tumour necrosis factor-alpha (TNF-alpha) interleukin-6 (IL-6) and soluble interleukin-6 receptor (sIL-6R gp80) in multiple sclerosis patients. Cytokine. 2002;20(2):86–89. doi: 10.1006/cyto.2002.1984. [DOI] [PubMed] [Google Scholar]
- 34.Backonja MM, Coe CL, Muller DA, Schell K. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol. 2008;195(1–2):157–163. doi: 10.1016/j.jneuroim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Cutolo M. Effects of DMARDs on IL-1Ra levels in rheumatoid arthritis: Is there any evidence? Clin Exp Rheumatol. 2002;20(5) Suppl 27:S26–S31. [PubMed] [Google Scholar]
- 36.del Rey A, et al. Disrupted brain-immune system-joint communication during experimental arthritis. Arthritis Rheum. 2008;58:3090–3099. doi: 10.1002/art.23869. [DOI] [PubMed] [Google Scholar]
- 37.Fiorentino PM, et al. Spinal interleukin-1beta in a mouse model of arthritis and joint pain. Arthritis Rheum. 2008;58:3100–3109. doi: 10.1002/art.23866. [DOI] [PubMed] [Google Scholar]
- 38.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 39.Bresnihan B, et al. Serum interleukin 18 and interleukin 18 binding protein in rheumatoid arthritis. Ann Rheum Dis. 2002;61:726–729. doi: 10.1136/ard.61.8.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rooney T, et al. Synovial tissue interleukin-18 expression and the response to treatment in patients with inflammatory arthritis. Ann Rheum Dis. 2004;63:1393–1398. doi: 10.1136/ard.2003.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci. 2008;28:12775–12787. doi: 10.1523/JNEUROSCI.3512-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conti B, et al. Cultures of astrocytes and microglia express interleukin 18. Brain Res Mol Brain Res. 1999;67(1):46–52. doi: 10.1016/s0169-328x(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 43.Ricciardi-Castagnoli P, et al. Cellular sources and effects of tumor necrosis factor-alpha on pituitary cells and in the central nervous system. Ann N Y Acad Sci. 1990;594:156–168. doi: 10.1111/j.1749-6632.1990.tb40476.x. [DOI] [PubMed] [Google Scholar]
- 44.Arnett FC, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 45.Aletaha D, et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 46.Wolfe F, et al. Report of the Multicenter Criteria Committee The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 47.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe F. Fatigue assessments in rheumatoid arthritis: Comparative performance of visual analog scales and longer fatigue questionnaires in 7760 patients. J Rheumatol. 2004;31:1896–1902. [PubMed] [Google Scholar]
- 49.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 50.Prevoo ML, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 51.Pakulski C, Drobnik L, Millo B. Age and sex as factors modifying the function of the blood-cerebrospinal fluid barrier. Med Sci Monit. 2000;6:314–318. [PubMed] [Google Scholar]
- 52.Committee on Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Bethesda: Natl Inst Health; 2011. DHHS Publ No (NIH) 85–23. [Google Scholar]
- 53.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.