Abstract
Human induced pluripotent stem cells (hiPSCs) are emerging as a tool for understanding human brain development at cellular, molecular, and genomic levels. Here we show that hiPSCs grown in suspension in the presence of rostral neuralizing factors can generate 3D structures containing polarized radial glia, intermediate progenitors, and a spectrum of layer-specific cortical neurons reminiscent of their organization in vivo. The hiPSC-derived multilayered structures express a gene expression profile typical of the embryonic telencephalon but not that of other CNS regions. Their transcriptome is highly enriched in transcription factors controlling the specification, growth, and patterning of the dorsal telencephalon and displays highest correlation with that of the early human cerebral cortical wall at 8–10 wk after conception. Thus, hiPSC are capable of enacting a transcriptional program specifying human telencephalic (pallial) development. This model will allow the study of human brain development as well as disorders of the human cerebral cortex.
Keywords: human embryonic stem cell, embryo, differentiation, cortical layer
Emerging data highlight the complexity and dynamic nature of gene expression in the central nervous system (CNS) and the divergence between human and other mammalian species, which is especially pronounced in the developing brain (1–4). Exploring such differences may reveal the genetic underpinnings of the larger size and complex architecture of the human brain and elucidate the molecular and cellular substrates of higher cognitive functions, as well as of our vulnerability to neurodevelopmental and neurodegenerative disorders. To understand the genetic programs that drive cell specification and differentiation in the human brain, it is important to develop model systems that recapitulate dynamic aspects of neural development, in addition to making inferences from commonly used models of lower mammalian species.
Recapitulating human neural development in vitro using human induced pluripotent stem cells (hiPSCs) can provide our first understanding of how genetic variation and disease-causing mutations influence neural development. Human iPSCs generated from reprogrammed cells can be differentiated into any tissue, including the CNS, while maintaining the genetic background of the individual of origin. These critical features have been exploited to model monogenic forms of neurodevelopmental disorders, such as Rett and Timothy syndromes, and even psychiatric disorders with complex inheritance, such as schizophrenia (5–7).
The brain and spinal cord develop according to distinct differentiation programs from the earliest stages of CNS development (i.e., at the progenitor stage during gastrulation) (8, 9). Regional differences in gene expression within stem and progenitor cells appear at the onset of the formation of both mouse (10, 11) and human CNS, as shown by recent studies of the human transcriptome using postmortem tissue (4).
Neural cells are thought to differentiate by “default” into an anterior, forebrain-like fate (12), and indeed monolayer neuronal cultures derived from hiPSCs and human embryonic stem cells (hESCs) can express some forebrain markers (6, 13, 14). However, they have not yet been shown to recapitulate the transcriptional program that gives rise to the mammalian telencephalon, and their regional specification remains unclear (15). Using a recently described method for neural differentiation that allows formation of serum-free, floating embryoid body-like, quick aggregates (SFEBq) (16) we derived neurally differentiated SFEBq-like multilayered structures from hiPSC. We also performed detailed immunophenotyping of these cultures, analyzed their transcriptome, and compared it with transcriptomes of hESCs, hiPSCs, human neuronal progenitors, and developing human brain tissue. These combined analyses demonstrate that hiPSC-derived multilayered structures display a gene expression profile typical of the embryonic telencephalon, and that neurons within these structures encompass both lower and upper cortical layer fates.
Results
Characterization of hiPSC Lines.
We used two hiPSC lines: PGP1-1 (17) and i03-01#9, a line derived in our laboratory. Both lines were established from healthy control adult male skin fibroblasts through retroviral reprogramming vectors expressing OCT4, SOX2, KLF4, and c-MYC (18). These hiPSC lines exhibited typical hESC morphology and expressed canonical pluripotency markers by immunocytochemistry as well as NANOG, DNMT3B, and other endogenous mRNAs that characterize pluripotent cells (Fig. S1 A and B). Pairwise correlations of global gene expression data obtained by microarrays (Dataset S1) showed high similarity in gene expression between PGP1-1 and the hESC line H1 (Pearson’s r > 0.978), i03-01#9 and H1 (Pearson’s r > 0.975), and PGP1-1 and i03-01#9 (Pearson’s r > 0.979).
We then tested the hiPSC lines with Pluritest (http://www.pluritest.org), an approach that classifies samples according to more than 450 genome-wide transcriptional profiles, including 223 hESC and 41 hiPSC lines from multiple laboratories (19). The i03-01#9 and PGP1-1 displayed Pluritest scores consistent with normal human pluripotent cells and clustered together with the H1 hESC line (green arrowhead, Fig. S1D). Taken together, these results confirm that PGP1-1 and i03-01#9 fulfill the established criteria for completely reprogrammed iPSC lines.
Excitatory Neuronal Precursor Cells Are Generated Within Multilayered Structures Derived from hiPSCs.
Undifferentiated PGP1-1 and i03-01#9 colonies were dissociated into single cells and cultured in suspension in the presence of FGF2 (5–20 ng/mL) and inhibitors of the bone morphogenetic protein (BMP), Wnt/β-catenin, and TGF-β/activin/nodal pathways to induce forebrain fate. The resulting 3D aggregates were then transferred onto a coated surface and maintained without any additional factors until day 45–70. On day 25, cells within the SFEBq-like structures expressed early neuroepithelial markers, including BLBP, N-CADHERIN, PAX6, and NESTIN (Fig. S1C).
Immunofluorescence analysis of the 3D structures at day 45–50 in serial cryosections revealed neural tube-like substructures within each aggregate, composed of radially arranged cells with apico-basal polarity, organized around a central lumen (Fig. 1). The radially arranged cells were PAX6+, NESTIN+, BLBP+, and GFAP+ and displayed N-CADHERIN immunoreactivity along their apical edge (Fig. 1 A–C, E, and F and Fig. S2 A–C). These radial glia-like progenitors expressed the neuroepithelial transcription factors (TFs) SOX1 and SOX2 (Fig. 1 H–L). Superficially to the radial glial layer were numerous neuronal precursor cells expressing T-box brain 2 (TBR2) (Fig. 1 C and F), a TF expressed by intermediate neuronal progenitors in the subventricular zone (SVZ) of the mammalian cerebral cortex (20). The proliferative markers Ki67 and phosphohistone H3 (pH3) were expressed by both radial glial cells and the surrounding intermediate precursors, and pH3 was expressed at the luminal (apical) surface, suggesting that radial glia undergo interkinetic nuclear migration (Fig. 1 M–O). More mature neurons (βIIITUBULIN+, MAP2+) also expressing T-box brain 1 (TBR1) (21), presumably progeny of TBR2+ precursors (20), were found superficially (basally) to the TBR2+ layer (Fig. 1 B, D, G, and P), reproducing the typical architecture of the developing mammalian cortex (22). In contrast, we found an almost complete absence of cells expressing ZIC1, a TF expressed in the hindbrain (Fig. 1 P and Q). Additionally, cells did not express ISLET1 and showed barely detectable levels of OLIG2 (Fig. 1R), TFs both expressed by more posterior and ventral regions of the brain and in the spinal cord.
Fig. 1.
Radial glia and excitatory progenitors differentiate from hiPSCs at day 50 in vitro. Day-50 forebrain-like structures contain radial glial cells immunoreactive for NESTIN, PAX6, and BLBP (A, B, and E), and βIIITUBULIN+ and TBR1+ neurons (B, D, and G). The radial glia express SOX1 and SOX2 (H–L), are mitotically active, as shown by Ki67 expression (M–O, red), and are polarized, displaying N-CADHERIN+ apical end-feet (C and F) as well as apical mitoses (M–O, arrowheads). A layer of TBR2+ intermediate progenitors (C and F) surrounds the radial glial layer and displays pH3+ basal mitoses (M–O, arrows). The neurons express MAP2 but rarely ZIC1 (P and Q) and do not express ISLET1 and OLIG2 (R). (Scale bars, 200 μm in A–D and M; 5 μm in E–G and I; 10 μm in H, J–L, N, and O.)
We next investigated the potential of these 3D multilayered aggregates to generate inhibitory neurons. On day 50 of neuronal differentiation, we found presence of GABAergic precursor cells (DLX1/2+, MASH1+) as well as more differentiated GAD-67+ inhibitory neurons (Fig. S3). Strongly positive DLX+ cells tended to be rounded and devoid of GAD-67+ processes (Fig. S3B, arrowheads), whereas cells weakly stained for DLX acquired long GAD-67+ processes (Fig. S3 A and C, arrows) and seemed to be migrating within the structure. Importantly, cells positive for inhibitory markers did not express the excitatory neuronal marker TBR1, suggesting that excitatory and inhibitory neuronal lineages are distinct and coexist in the preparation. The GABAergic component was always segregated from excitatory cells, in the center (Fig. S3A) or at the periphery (Fig. S3 D–F). The overall percentage of cells expressing GAD-67 was 6.9 ± 1.0 and 7.4 ± 0.7 in two different preparations.
Neuronal Connectivity in the Multilayered Structures Derived from hiPSCs.
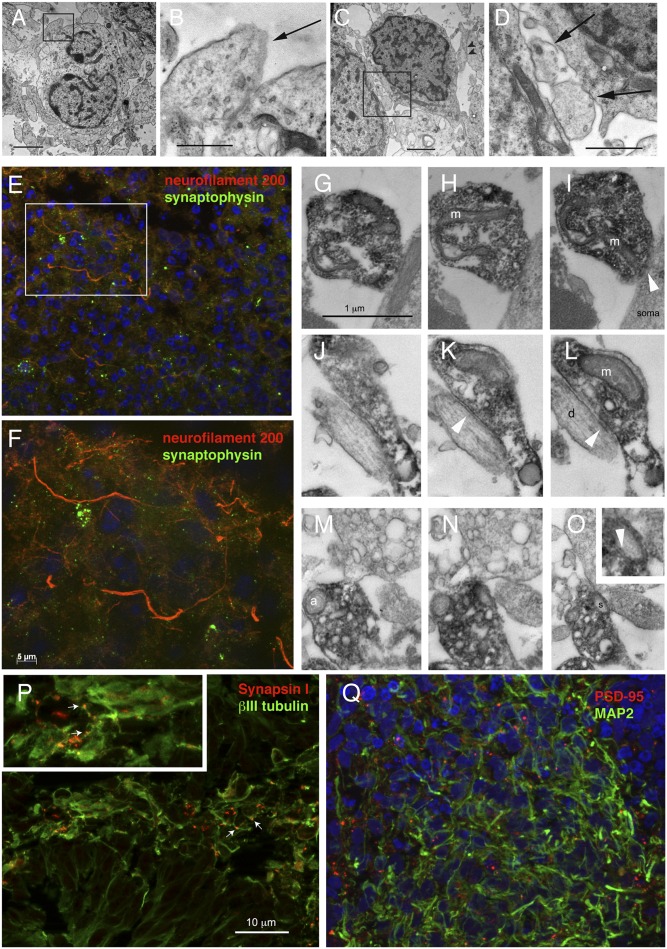
At day 70 of differentiation in vitro, the majority of polarized radial glial cells disappeared from the aggregates, together with the PAX6 and BLBP protein expression. Electron microscopy revealed the presence of immature synaptic boutons containing vesicles (Fig. 2 A–D, arrows), which contacted large neuronal cells via thickenings resembling immature synapses, in that the postsynaptic membrane specializations and associated structures did not appear fully developed. Immunostaining for the presynaptic markers synaptophysin (SYP) and synapsin I (SYN I), as well as postsynaptic density protein 95 (PSD-95), revealed extensive synaptic differentiation (Fig. 2 E, F, P, and Q). Immunoelectron microscopy demonstrated SYP immunoprecipitate within boutons containing synaptic vesicles (Fig. 2 G–O), which contacted dendrites and spines via synapses (Fig. 2 J–O, arrowheads). We also observed extensive immunostaining for the vesicular glutamate transporter vGLUT1 at both 50 and 70 d in culture, consistent with glutamatergic synapses differentiation (Fig. S4 I–K). By day 70, astroglial cells differentiated throughout the culture, as demonstrated by the presence of GFAP/S100β immunostained cells, particularly in the outer region (Fig. S2 D–I).
Fig. 2.
Evidence for synapses in hiPSC-derived multilayered structures at 50 and 70 d in vitro. (A–D) Day-70 multilayered structures analyzed by EM show examples of direct apposition between vesicles-containing putative axon terminals (black arrows) and neuronal cell bodies. (E and F) Synaptophysin/neurofilament 200 double immunostaining at day 70 showing numerous synaptophysin-labeled boutons decorating the neurites. (G–O) Immunoelectron microscopy showing synaptophysin-immunoprecipitate within putative axon terminals contacting cell bodies (soma) (G–I), dendrites (d) (J–L), and spines (s) (M–O). White arrowheads, synaptic thickening; m, mitochondia; a, autophagosome. (P and Q) Day-50 multilayered structures stained for SYN I and βIIITUBULIN (P) or PSD-95 and MAP2 (Q). White arrows in P point to SYN I-positive boutons. (Scale bars, 2 μm in A and C; 0.5 μm in B and D; 10 μm in E, P, and Q; 5 μm in F; 1 μm in G–O.)
Global Gene Expression Analysis Reveals the Implementation of a Forebrain Neuronal Gene Expression Program.
To ascertain whether these hiPSC-derived structures underwent a region-specific differentiation program, we assessed their gene expression profiles by microarrays. Whole-genome expression data analyzed by the Pluritest demonstrated progressively lower pluripotency scores and higher novelty scores, suggesting ongoing neuronal differentiation (Fig. S1D). Gene expression was compared between 3D multilayered structures at day 50 in vitro and their corresponding undifferentiated hiPSC lines PGP1-1 (one preparation) and i03-01#9 (four preparations). Hierarchical clustering of the samples demonstrated a highly consistent pattern of gene expression across all lines and preparations (Figs. S5 and S6). The combined dataset, including all hiPSC lines and different 3D preparations analyzed together, revealed that 3,363 genes were differentially expressed in day-50 multilayered structures compared with the undifferentiated hiPSC stage (P value ≤0.05 together with a fold change of ±2.0), of which 1,629 genes increased and 1,734 genes decreased in abundance. The top down-regulated molecules (between −9,110- and −1,384-fold) were LIN28A, DPPA4, RAB17, CDH1/E-CADHERIN, OCT4/POU5F1, ZSCAN10, and GAL, reflecting genes commonly expressed in hESCs and hiPSCs and epithelial-specific genes (boxed areas in Fig. S5A). In addition to OCT4, the expression of the other reprogramming factors (SOX2, KLF4, and c-MYC) as well as of other genes characterizing a pluripotent state (NANOG, DNMT3B, and JARID2) was also down-regulated (Dataset S1). Conversely, mRNAs encoding for known effectors of neuronal differentiation increased in abundance, including MAP2, βIII TUBULIN, ephrins and their receptors, semaphorins, and neural cell adhesion molecules (Table S1). Genes involved in neurotransmission were also up-regulated, including SNAP25, SNAP29, SNAP91, SYN I, SYP, Bassoon, PSD-95, and glutamate ionotropic and metabotropic receptors (Table S1).
Ingenuity pathway analysis for differentially expressed genes in day-50 3D structures revealed that the most significantly enriched biological function categories were Nervous System Development and Function, Cancer, Cellular Assembly and Organization, and Neurological Disease (Dataset S2). The most significant gene ontology terms under the Nervous System Development and Function category were Neurogenesis of Cerebral Cortex and Hippocampus, Guidance of Neurites, Brain Development, Hippocampal Development, Cortical Development, Neuronal and Glial Differentiation, and Synaptic Transmission, and most of these included up-regulated genes. In contrast, the Cancer category included predominantly down-regulated genes with known involvement in the control of cell cycle, suggesting that the majority of the neuronal cells at day 50 are likely already postmitotic (Dataset S2). Virtually all terms in the Cellular Assembly and Organization category involved neural cell functions, such as growth and fasciculation of neurites and synapse formation (Dataset S2). Significant Neurological Disease associations included genes implicated in disorders of the developing nervous system, such as schizophrenia, and neurodegenerative disorders, such as Alzheimer’s and Huntington disease (Dataset S2).
Consistent with the above characterization, the top DAVID gene ontology clusters were Neuron Differentiation/Morphogenesis, Synapse Formation, Forebrain/Pallium Development, and Regulation of Transcription (Dataset S3). Thus, differentially expressed genes in hiPSC-derived structures at day 50 in vitro converged toward telencephalic development, neurogenesis, neuronal process outgrowth, and synaptogenesis.
Comparison of gene expression in our hiPSC-derived cells with normal human neural progenitors (NHNP) derived from early human embryo of ∼8–19 gestational weeks and differentiated in vitro (23) revealed a significant similarity of gene expression patterns. Among the 4,427 genes significantly changed in NHNP between time 0 and 4 wk of neuronal differentiation in vitro (23) and the 3,363 genes significantly changed between the hiPSC and 50 d in vitro stage in our model, 1,100 genes were in common. The P value for such overlap is <10−12 (calculated using numerical simulations and tail probability estimation).
Regional Specificity of Multilayered Aggregates Is Most Consistent with a Dorsal Telencephalic Fate.
To further understand whether our 3D multilayered structures were regionally specified as reflected by their pattern of gene expression, we took into account published datasets of telencephalic gene expression profiles (24, 25), literature data, and the Allen brain atlas of gene expression database. Overall the expression pattern of the hiPSC-derived multilayered structures at day 50 in vitro was more typical of dorsal forebrain than ventral forebrain (Fig. 3A) and excluded hindbrain and spinal cord fates. For instance, genes that confer hindbrain and spinal cord fates, such as HOX genes, GBX2, HB8, NKX2.2, OLIG2, EVX1/2, ZIC1/2, and GCM1, and transcripts specific of olfactory bulb development, such TBX21, were not expressed or down-regulated (Fig. 3A and Dataset S1). Among the top 10 genes with the highest degree of up-regulation in the day-50 neural structures were those involved in specification and patterning of the mammalian neocortex, including LHX2, LEF1, TBR2, PAX6, FEZF2, and RELN (Fig. 3A, Table 1, and Fig. S5B, boxed area). Very high levels of up-regulation were also noted for COUP-TF1, EMX2, and EMX1 and for other genes involved in cortical architecture and layer identity, such as TBR1, CTIP2, CUX1, CUX2, POU2F2/BRN2, and TLX (Table 1). Some genes involved in GABAergic differentiation were also expressed (including MASH1 and ARX), but the basal telencephalon differentiation program did not seem to be fully implemented (Fig. 3A and Fig. S3). This observation is intriguing, given previous findings suggesting that a lineage of human neocortical GABAergic progenitors expressing MASH1 originates from the dorsal forebrain (26).
Fig. 3.
Regional and temporal specification of hiPSC-derived neuronal cells. (A) Fold differences in neuronal gene expression compared with undifferentiated hiPSCs for dorsally and ventrally enriched telencephalic genes. The gene lists were manually curated according to published data (24, 25, 35). Orange, genes that are statistically different at P < 0.05 and fold change ±2.0; gray, genes that do not fulfill the minimum P value criterion. (B) Number of developing human brain samples in Kang et al. (4) correlating with the day-50 multilayered structures within 95% confidence interval of the maximal correlation. Human brain samples are displayed according to developmental stages (Upper) or brain regions (Lower). PCW, postconceptional week; M, months; Y, years; FC, frontal, PC, parietal, TC, temporal, and OC, occipital cerebral cortical wall; HIP, hippocampal anlage; AMY, amygdala; DIE, diencephalon; URL, upper rhombic lip.
Table 1.
Up-regulation of genes involved in cortical morphogenesis
| Gene symbol | F-I | P |
| Dorsal telencephalon specification & patterning | ||
| LHX2 | 7,477 | 0 |
| LEF1 | 2,016 | 0 |
| PAX6 | 970 | 0.00006 |
| FEZF2 | 846 | 0 |
| NR2F1/COUP-TF1 | 589 | 0.00667 |
| EMX2 | 243 | 0 |
| NEUROG2 | 180 | 0 |
| WNT3A | 52.1 | 0.00223 |
| NR2E1/TLX | 43.6 | 0 |
| GAS1 | 38.8 | 0 |
| ZEB2/ SIP-1/ ZFHX1B | 30.8 | 0.00631 |
| EMX1 | 25.9 | 0 |
| GLI3 | 16.4 | 0 |
| AHI1 | 6.80 | 0.00103 |
| BBS1 | 2.66 | 0.04026 |
| BBS2 | 2.56 | 0.03462 |
| Cortical progenitor proliferation | ||
| ID4 | 120 | 0 |
| ASTN1 | 77.6 | 0.00153 |
| FOXG1 | 29.1 | 0 |
| Cortical neuron migration and layer specification | ||
| EOMES/TBR2 | 1,093 | 0.00001 |
| RELN | 834 | 0 |
| FEZF2 | 846 | 0 |
| DCX | 297 | 0 |
| TBR1 | 73.0 | 0.04796 |
| SEMA3C | 61.4 | 0.00223 |
| NR2E1 (TLX) | 43.6 | 0 |
| NTSR1 | 49.9 | 0.00584 |
| BCL11B/CTIP2 | 35.5 | 0.04262 |
| POU3F2 /BRN2 | 16.0 | 0.02681 |
| CDK5R1 | 14.9 | 0.00077 |
| RORB | 13.0 | 0.00209 |
| ENC1 | 12.2 | 0 |
| EFNB2 | 11.8 | 0 |
| SSTR2 | 9.90 | 0.00006 |
| GPRIN1 | 6.02 | 0.00005 |
| FYN | 3.34 | 0.00606 |
| PAFAH1B1 | 4.14 | 0 |
| CUX2 | 3.94 | 0.00042 |
| CUX1 | 3.80 | 0.00282 |
| EPHB1 | 3.62 | 0.00073 |
| DPF1 | 3.53 | 0.03704 |
Manually curated list of genes significantly up-regulated (P values ≤0.05) in day-50 multilayered structures compared with undifferentiated hiPSC involved in mammalian neocortical development and/or expressed in a layer-specific manner in the human neocortex [see supplementary table 14 in Kang et al. (4)]. F-I, fold increase in gene expression.
Transcription Factors Expression Patterns Suggest Dynamic Acquisition of Cortical Layer Identities.
Using a panel of antibodies directed to TFs that control cortical layer fates during normal cortical development (22, 27–29), we next investigated whether the implementation of the neocortical transcriptional program resulted in the specification of cortical excitatory neuron subtypes. We found that MAP2+ neurons coexpressed TFs that specify a wide spectrum of upper and lower cortical layer identity. These included TBR1, CTIP2, TLE4 (cortical layers V–VI) and BRN2 and SATB2 (cortical layers II–IV) (Fig. 4). The proportion of neurons for lower layers was 30–40%, whereas that for upper layers was 10–20% (Fig. 4S). Double-staining experiments showed that TBR1 and TLE4 (layers V–VI) colocalized with CTIP2 (layer V) in many neurons, but cells displayed predominant expression of either one factor or another (Fig. 4 C–F and J–M and Fig. S4 A–D). In contrast, cells of lower layers (TBR1, CTIP2, and TLE4) showed more segregated pattern of expression from those of upper layers (SATB2 and BRN2) (Fig. 4 G–I, N–R, and S and Fig. S4 E–H and L). In addition to SATB2 and BRN2 proteins, a number of TFs required for cortical upper layer formation were up-regulated at the mRNA level: CUX1 and CUX2 (fourfold), BRN2 (14-fold) (Fig. 3A and Table 1), PAX6 (970-fold), and TLX (43-fold) (Table 1). No appreciable differences in neuronal differentiation and substructural organization were observed between the two iPSC lines (compare Fig. 4 and Fig. S4) and concentrations of FGF2 (Fig. 4S).
Fig. 4.
Specification of cortical layer fates in hiPSC-derived neurons. (A) Scheme of TF directing cortical layer fates. (B–R) Immunostaining for TFs in sections from 3D structures at day 50 in vitro. (B) Double immunostaining for MAP2 and CTIP2, showing CTIP2 nuclear localization in MAP2+ cells; (C–F) TBR1 and CTIP2; (G–I) TBR1 and SATB2; (J–M) TLE4 and CTIP2; (N–P) TLE4 and SATB2; and (Q and R) TBR1 and BRN2, showing differences in relative colocalization of these TFs in differentiating neurons. White arrowheads, cells stained for a single TF; white arrows, cells that colocalize two factors. Broken line in Q delimits the radial glial layer where BRN2 is also expressed but was excluded from quantification. (S) Stereological quantification of TF expression in 3D structures cultured with different amounts of FGF2 for the first 18 d. DAPI is in blue. (Scale bars, 200 μm in B and G; 100 μm in C–F and J; 25 μm in H and I; 20 μm in K–P.)
Correspondence with Patterns of Gene Expression in Developing Human Brain.
To verify the conclusion that the mRNA and protein expression patterns of our hiPSC-derived multilayered structures at day 50 are typical of the human dorsal forebrain, we performed a pairwise correlation analysis (details in SI Materials and Methods) between the global gene expression levels of our samples (including the hiPSC and the day-50 multilayered structures) and 1,340 postmortem human tissue samples at 15 different developmental stages, comprising the cerebellar cortex, mediodorsal nucleus of the thalamus, striatum, amygdala, hippocampus, and 11 areas of the neocortex (4). The day-50 multilayered aggregates from the i03-01#9 line showed the highest correlation coefficients with the human postmortem dataset, and correlated only (as estimated by the 95% confidence interval) with human cerebral cortex at 4–10 postconceptional weeks (PCW), with a prevalence for 8–10 PCW and frontal regions (Fig. 3B).
Discussion
We demonstrate that under conditions that mimic neural induction of early forebrain, aggregates of hiPSCs segregate into discrete layers containing radial glia, neuronal progenitors, and early neurons that enact a transcriptional program specifying initial stages of embryonic human dorsal telencephalic (pallial) development. The neurons form morphologically identifiable synapses and exhibit protein and gene expression profiles typical of mammalian excitatory cortical neurons encompassing both lower and upper layer fates, as well as GABAergic neurons of presumed dorsal telencephalic identity. Comparison with gene expression datasets from the human brain revealed that the transcriptome of the hiPSC-derived multilayered 3D structures reflects that of the human cerebral cortex at 8–10 wk after conception.
The hiPSC aggregates recapitulate in culture the in vivo cytoarchitecture that includes radial glial cells expressing neural progenitor proteins, intermediate progenitors, and maturing neurons. This spontaneous segregation also occurs in SFEBq cultures derived from mESC and hESC (16) but had not been previously shown for hiPSC. We demonstrate that the hiPSC-derived radial glia express, like human radial glia, GFAP; the intermediate progenitors express TBR2, and the neurons express MAP2 and a variety of TFs specifying cortical neuron identities. The multilayered structures at day 50 in vitro expressed the four TFs (LHX2, FOXG1, PAX6, and EMX2) that are fundamentally required for the formation of the six-layered dorsal pallium by suppressing dorsomedial and ventral telencephalic fates (30–32). These 3D structures also express many other TFs (FEZF2, BRN2, TBR1, CTIP2, and CUX1) and extracellular signaling molecules (RELN and EFNB2) known to direct cortical layer formation and identities. At the protein level, TFs known to specify lower-layer identity (i.e., TBR1, CTIP2, and TLE-4) and upper-layer identity (i.e., SATB2 and BRN2) were expressed in a mostly nonoverlapping fashion by developing neurons. Together, our combined morphological, immunocytochemical, and transcriptional analyses reveal that it is possible to recapitulate initial stages of development of the human dorsal telencephalon from hiPSCs and that laminar segregation of progenitors may be crucial for directing advanced features of cortical neuron differentiation.
Although these data demonstrate dynamic acquisition of correct cortical neuron subtype identities within the 3D structures, cortical layer architecture was not fully recapitulated. For example, upper-layer neurons in the human cortex outnumber those of lower layers, whereas in our 3D structures at day 50 the proportions of lower-layer neurons were higher than those of upper layers, likely owing to the early stage of our preparation. Indeed, at day 50 in vitro the 3D structures displayed a gene expression profile typical of the dorsal telencephalon at an early stage, including FOXG1, which controls the specification of the neocortex from archicortex (33, 34), LHX2, which specifies the dorsal pallium as distinct from the cortical hem (31), and GLI3, which specifies dorsal from ventral telencephalon (35). Other highly expressed TFs reflect the acquisition of rostral (frontal) cortical area identities [i.e., PAX6 (32, 36)], which agrees with the high correlation found between gene expression in our 3D structures and the human frontal cortical primordium (see below).
Previous work (37, 38) revealed that neuronal progenitors derived from mouse ES cells seem to be intrinsically able to produce cortical neurons in vitro, recapitulating the normal sequence of cortical layer fates. Differently, dissociated progenitors derived from human ESC/hiPSC by at least some of the recent embryoid bodies-based methods (13) do not permit an advanced degree of region-specific gene expression and cell differentiation. Although Shi et al. (39) were able to achieve cortical neuron differentiation from hESC/hiPSC cultures using a dissociated culture system similar to that of Gaspard et al. (38), their protocol involves the addition of retinoic acid, a molecule that antagonizes forebrain development (40), and it will be eventually important to show that global gene expression analysis is consistent with the cellular phenotype. Classic embryological and genetic work has shown that early forebrain specification occurs via production of Lefty1, Dickkopf, and Cerberus in the anterior visceral endoderm, which inhibit the BMP, TGF-β/activin/nodal, and Wnt/β-catenin pathways at the beginning of gastrulation (9). Following the protocol of Eiraku et al. (16), we have recapitulated this triple inhibition in vitro, and we have added moderate concentrations of FGF2, a molecule that synergizes with the early telencephalic inducers (12, 41). Indeed, we show not only a concerted up-regulation of dorsal telencephalic genes in our preparation but also the absence of molecules that are up-regulated in caudal regions of the CNS.
Global gene expression profiles have not been previously reported in either mESC- or hESC-derived SFEBq cultures, and hence the ability of this 3D culture systems to truly recapitulate telencephalic neural differentiation programs had not been deciphered. The global comparison between transcriptomes of all our samples and those obtained from the postmortem human brain at several stages of development revealed a striking correspondence in gene expression between our day-50 3D structures and the cerebral cortical wall at 8–10 wk after conception, particularly the frontal cortical primordium. This stage is characterized by formation of deep-layer cortical neurons and of the SVZ, as well as axonal extension through the major commissures and descending pathways (4). These processes are reflected in our 3D structures by significant enrichment in genes with functions related to cortical neurogenesis, growth and axon guidance; genes expressed in the SVZ (TBR2, TLX, CUX1/2); as well genes involved in the establishment of laminar structures such as the dorsal pallium. Thus, the dynamic changes in transcriptional profile that the hiPSC undergo as they differentiate under these conditions reflect those present in the early stages of human dorsal pallium development in vivo.
We also found ultrastructural, transcriptional, and molecular evidence for early synapse formation, including components of synaptic vesicles, neurotransmitter receptors, and transporters, concomitantly with evidence of astroglial differentiation. This may represent either an aspect of neural development that is particularly accelerated in vitro, or it may simply reflect the peculiarities of human cortical development, which is characterized by early synapses of cortical neurons with the subplate (42, 43), which appear as early as 10–11 PCW.
In summary, we were able to generate 3D self-organized structures from human iPSCs that recapitulate the program of early dorsal telencephalic development in humans. Using patient-specific iPSCs this model may offer novel insights into the pathophysiology of a large variety of disorders presenting primarily with cortical dysfunction, including numerous neurodevelopmental and late-onset neurodegenerative conditions.
Materials and Methods
hiPSC and Neuronal Differentiation.
Details of the protocol for generating and characterizing the hiPSC are listed in SI Materials and Methods. Three-dimensional cell aggregates known as SFEBq were generated according to Eiraku et al. 2008 (16), with some modifications as described in SI Materials and Methods.
Biostatistics.
We performed pairwise comparisons between the global gene expression levels of all our samples (undifferentiated hiPSCs and day-50 multilayered structures) and all of the samples of Kang et al. (4) and estimated the Spearman correlation coefficients for all of the comparisons. We then selected the sample in Kang et al. (4) showing the highest correlation coefficient with one of our samples, estimated its 95% confidence interval, and included any other sample in the postmortem human tissue dataset whose correlation coefficient would fall within this interval (SI Materials and Methods).
Acknowledgments
We thank Arif Kocabas for expert technical help, Anita Huttner for preparation of fibroblasts from the skin biopsy specimen, In-Hyun Park for advice in the characterization of iPSC lines and the gift of the iPSC PGP1-1, and Stephen A. Duncan for the gift of the K3 hiPSC line. We are grateful to Nenad Sestan and Feng Cheng for help with the comparison with the human brain datasets and to members of the F.M.V. laboratory and the Program in Neurodevelopment and Regeneration for insightful discussions. We are supported by Grants MH087879, MH089176 and DK006850 from the National Institutes of Health, by the State of Connecticut, and by the Simons Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202944109/-/DCSupplemental.
References
- 1.Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geschwind DH. Neurodevelopmental disorders: Hope for a new beginning. Curr Opin Neurol. 2011;24:95–97. doi: 10.1097/WCO.0b013e328344cd78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson MB, et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang HJ, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennand KJ, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchetto MC, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paşca SP, et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam PP. Regionalisation of the mouse embryonic ectoderm: Allocation of prospective ectodermal tissues during gastrulation. Development. 1989;107:55–67. doi: 10.1242/dev.107.1.55. [DOI] [PubMed] [Google Scholar]
- 9.Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28:641–651. doi: 10.1016/s0896-6273(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein JLR, Martinez S, Shimamura K, Puelles L. The embryonic vertebrate forebrain: The prosomeric model. Science. 1994;266:578–580. doi: 10.1126/science.7939711. [DOI] [PubMed] [Google Scholar]
- 11.Rhinn M, Picker A, Brand M. Global and local mechanisms of forebrain and midbrain patterning. Curr Opin Neurobiol. 2006;16:5–12. doi: 10.1016/j.conb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Levine AJ, Brivanlou AH. Proposal of a model of mammalian neural induction. Dev Biol. 2007;308:247–256. doi: 10.1016/j.ydbio.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JE, et al. Investigating synapse formation and function using human pluripotent stem cell-derived neurons. Proc Natl Acad Sci USA. 2011;108:3005–3010. doi: 10.1073/pnas.1007753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu BY, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen DV, Rubenstein JL, Kriegstein AR. Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron. 2011;70:645–660. doi: 10.1016/j.neuron.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eiraku M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Ball MP, et al. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nat Biotechnol. 2009;27:361–368. doi: 10.1038/nbt.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Müller FJ, et al. A bioinformatic assay for pluripotency in human cells. Nat Methods. 2011;8:315–317. doi: 10.1038/nmeth.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Englund C, et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hevner RF, et al. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 22.Hevner RF. Layer-specific markers as probes for neuron type identity in human neocortex and malformations of cortical development. J Neuropathol Exp Neurol. 2007;66:101–109. doi: 10.1097/nen.0b013e3180301c06. [DOI] [PubMed] [Google Scholar]
- 23.Konopka G, et al. Modeling the functional genomics of autism using human neurons. Mol Psychiatry. 2012;17:202–214. doi: 10.1038/mp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long JE, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb Cortex. 2009;19(Suppl 1):i96–i106. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batista-Brito R, Machold R, Klein C, Fishell G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex. 2008;18:2306–2317. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 27.Molnár Z, et al. Comparative aspects of cerebral cortical development. Eur J Neurosci. 2006;23:921–934. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molyneaux BJ, et al. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci. 2009;29:12343–12354. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britanova O, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 30.Chou SJ, Perez-Garcia CG, Kroll TT, O’Leary DD. Lhx2 specifies regional fate in Emx1 lineage of telencephalic progenitors generating cerebral cortex. Nat Neurosci. 2009;12:1381–1389. doi: 10.1038/nn.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangale VS, et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muzio L, et al. Emx2 and Pax6 control regionalization of the pre-neuronogenic cortical primordium. Cereb Cortex. 2002;12:129–139. doi: 10.1093/cercor/12.2.129. [DOI] [PubMed] [Google Scholar]
- 33.Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- 34.Muzio L, Mallamaci A. Foxg1 confines Cajal-Retzius neuronogenesis and hippocampal morphogenesis to the dorsomedial pallium. J Neurosci. 2005;25:4435–4441. doi: 10.1523/JNEUROSCI.4804-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rallu M, et al. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- 36.Bishop KM, Rubenstein JL, O’Leary DD. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Q, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 38.Gaspard N, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486, S1. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simeone A, et al. Retinoic acid induces stage-specific antero-posterior transformation of rostral central nervous system. Mech Dev. 1995;51:83–98. doi: 10.1016/0925-4773(95)96241-m. [DOI] [PubMed] [Google Scholar]
- 41.Stern CD. Neural induction: Old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- 42.Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: Its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- 43.Kostović I, Judas M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 2010;99:1119–1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]