Abstract
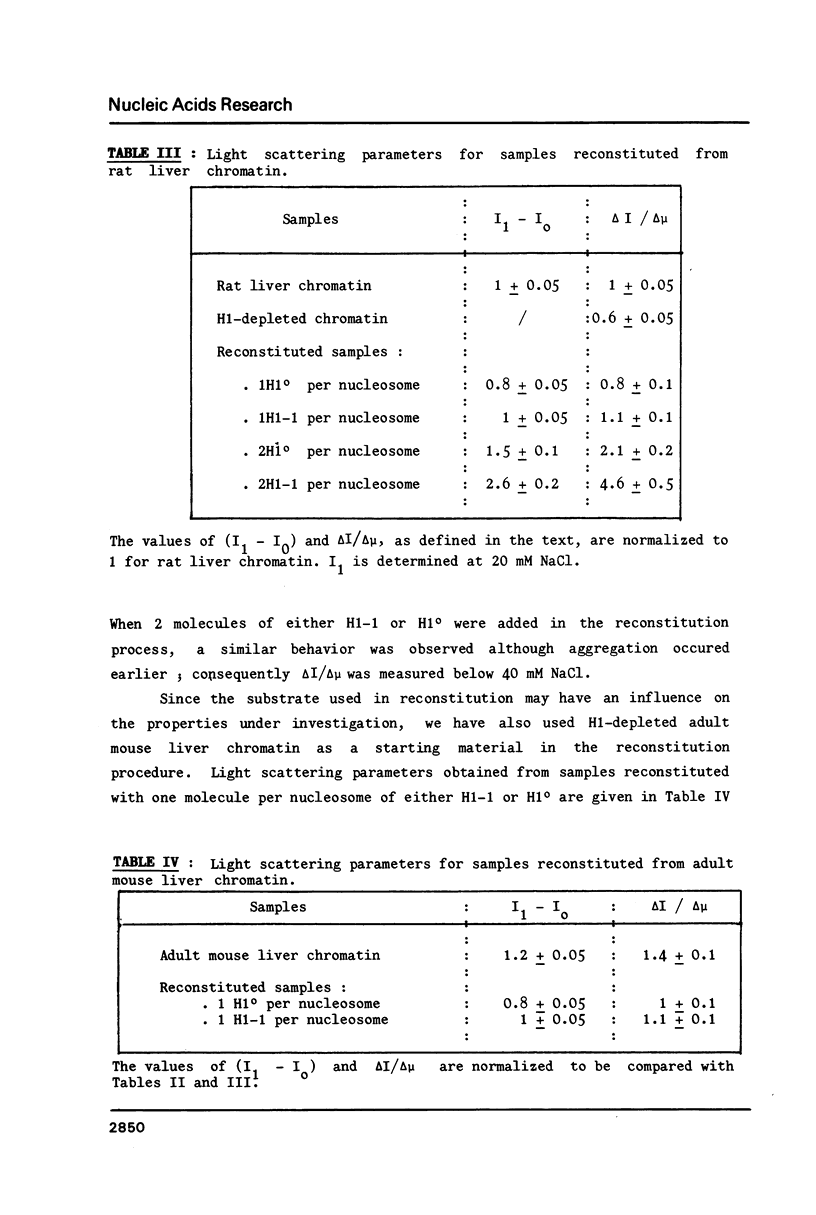
Micrococcal nuclease digestion and light scattering are used to compare native chromatins with various histone H1[0] contents. The experimental data show that the higher the H1[0] content, the greater the ability to form compact structures with increasing ionic strength, and the lower the DNA accessibility to micrococcal nuclease. On the contrary, reconstituted samples from H1-depleted chromatin and pure individual H1 fractions behave in such a way that samples reconstituted with pure H1 degree give rise to a looser structure, more accessible to nuclease than samples reconstituted with H1-1. This contradiction suggests that the effect of H1o on chromatin structure must originate from the interaction of this histone with other components in native chromatin among which other histone H1 subfractions are good candidates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Cowling G. J., Harborne N., Cattini P., Craigie R., Gould H. Regulation of the higher-order structure of chromatin by histones H1 and H5. J Cell Biol. 1981 Aug;90(2):279–288. doi: 10.1083/jcb.90.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausio J., Borochov N., Seger D., Eisenberg H. Interaction of chromatin with NaCl and MgCl2. Solubility and binding studies, transition to and characterization of the higher-order structure. J Mol Biol. 1984 Aug 15;177(3):373–398. doi: 10.1016/0022-2836(84)90291-2. [DOI] [PubMed] [Google Scholar]
- Biard-Roche J., Gorka C., Lawrence J. J. The structural role of histone H1: properties of reconstituted chromatin with various H1 subfractions (H1-1, H1-2, and H1o). EMBO J. 1982;1(12):1487–1492. doi: 10.1002/j.1460-2075.1982.tb01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashkevich V. K., Nikolaev L. G., Zlatanova J. S., Glotov B. O., Severin E. S. Chemical crosslinking of histone H1o to histone neighbours in nuclei and chromatin. FEBS Lett. 1983 Jul 25;158(2):276–280. doi: 10.1016/0014-5793(83)80594-8. [DOI] [PubMed] [Google Scholar]
- Gjerset R., Gorka C., Hasthorpe S., Lawrence J. J., Eisen H. Developmental and hormonal regulation of protein H1 degrees in rodents. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2333–2337. doi: 10.1073/pnas.79.7.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Klingholz R., Strätling W. H. Reassociation of histone H1 to H1-depleted polynucleosomes. J Biol Chem. 1982 Nov 10;257(21):13101–13107. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. J., Chan D. C., Piette L. H. Conformational state of DNA in chromatin subunits. Circular dichroism, melting, and ethidium bromide binding analysis. Nucleic Acids Res. 1976 Nov;3(11):2879–2893. doi: 10.1093/nar/3.11.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Modak S. P., Lawrence J. J., Gorka C. Selective removal of histone H1 from nucleosomes at low ionic strength. Mol Biol Rep. 1980 Dec 31;6(4):235–243. doi: 10.1007/BF00777531. [DOI] [PubMed] [Google Scholar]
- Nelson P. P., Albright S. C., Wiseman J. M., Garrard W. T. Reassociation of histone H1 with nucleosomes. J Biol Chem. 1979 Nov 25;254(22):11751–11760. [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Ring D., Cole R. D. Close contacts between H1 histone molecules in nuclei. J Biol Chem. 1983 Dec 25;258(24):15361–15364. [PubMed] [Google Scholar]
- Smith B. J., Johns E. W. Histone H1o: its location in chromatin. Nucleic Acids Res. 1980 Dec 20;8(24):6069–6079. doi: 10.1093/nar/8.24.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Künzler P. Histone H5 can correctly align randomly arranged nucleosomes in a defined in vitro system. Nature. 1983 Apr 7;302(5908):548–550. doi: 10.1038/302548a0. [DOI] [PubMed] [Google Scholar]
- Thoma F., Koller T. Unravelled nucleosomes, nucleosome beads and higher order structures of chromatin: influence of non-histone components and histone H1. J Mol Biol. 1981 Jul 15;149(4):709–733. doi: 10.1016/0022-2836(81)90354-5. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Khabaza A. J. Cross-linking of histone H1 in chromatin. Eur J Biochem. 1980 Dec;112(3):501–511. doi: 10.1111/j.1432-1033.1980.tb06113.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Histone-H1-dependent chromatin superstructures and the suppression of gene activity. Cell. 1984 Aug;38(1):17–27. doi: 10.1016/0092-8674(84)90522-1. [DOI] [PubMed] [Google Scholar]