Abstract
Several 400- to 800-kb murine chromosome domains switch from early to late replication during loss of pluripotency, accompanied by a stable form of gene silencing that is resistant to reprogramming. We found that, whereas enhanced nuclease accessibility correlated with early replication genome-wide, domains that switch replication timing during differentiation were exceptionally inaccessible even when early-replicating. Nonetheless, two domains studied in detail exhibited substantial changes in transcriptional activity and higher-order chromatin unfolding confined to the region of replication timing change. Chromosome conformation capture (4C) data revealed that in the unfolded state in embryonic stem cells, these domains interacted preferentially with the early-replicating chromatin compartment, rarely interacting even with flanking late-replicating domains, whereas after differentiation, these same domains preferentially associated with late-replicating chromatin, including flanking domains. In both configurations they retained local boundaries of self-interaction, supporting the replication domain model of replication-timing regulation. Our results reveal a principle of developmentally regulated, large-scale chromosome folding involving a subnuclear compartment switch of inaccessible chromatin. This unusual level of regulation may underlie resistance to reprogramming in replication-timing switch regions.
Keywords: development, epigenetics, chromosome organization
Cells undergo global changes in chromosome structure during mammalian development (1). In the cells of early embryos and embryonic stem cells (ESCs), chromatin fibers are uniformly distributed throughout nuclei, but during differentiation, many compact heterochromatin regions appear (2, 3). Such global changes in chromatin structure are presumed to be important for cell commitment by stabilizing gene expression programs. Committed somatic cells can be reprogrammed back to the pluripotent ESC state by forced expression of a specific set of transcription factors to create induced pluripotent stem cells (iPSCs) (4, 5). However, some genes are difficult to reprogram, resulting in the generation of partially reprogrammed (p)iPSCs (6, 7). These genes appear to be occluded from binding to the reprogramming transcription factors (6), suggesting the existence of an epigenetic barrier at these gene regions.
Chromatin fibers vary in their general sensitivity to nucleases (8, 9) and fold into higher-order structures that can be observed at the microscopic level (10) or probed molecularly by mapping sites of chromatin interaction (11). There is a global correlation between general nuclease sensitivity (quantified across many kilobases as opposed to local hypersensitivity), gene density, and higher-order chromatin folding (measured by the proximity of sequences detected by fluorescence in situ hybridization; FISH) (12, 13). This has led to the common assumption that distended chromatin is more “accessible,” but direct evidence supporting this model is lacking. In addition, the extent to which these higher-order packaging steps are regulated during development is poorly understood.
DNA replication in mammals is regulated in a spatiotemporal manner. Each genomic segment is replicated at a distinct time during S phase, which can be differentially regulated in the context of development and disease (14). Genome-wide analyses of replication timing have identified megabase-sized domains of coordinate replication timing (“replication domains”). Replication timing closely aligns with spatial maps of chromatin proximity (3, 15–17), demonstrating that early- and late-replicating chromatin resides in spatially segregated compartments defined by chromatin interactions. Early- and late-replicating regions also correlate with nuclease sensitivity (12, 18). Replication domains are reorganized during ESC differentiation coordinated with transcriptional changes (3, 15, 16). Intriguingly, piPSCs fail to reprogram replication timing of chromosomal domains that switch from early to late (EtoL) replication during early development, and these same domains harbor many of the genes with transcription that is also difficult to reprogram (3). Together, these results have suggested that shifts in replication timing represent or reflect stable changes in epigenetic states and that late replication may form a barrier to reprogramming.
Here, we have asked whether this epigenetic barrier reflects changes in nuclease sensitivity and large-scale chromosome structure, focusing on two domains harboring the developmental pluripotency-associated 2/4 (Dppa2/4), microrchidia 1 (Morc1), and reduced-expression 1 (Rex1) genes. These domains switch replication timing from early to late during ESC differentiation and are difficult to reprogram for both replication timing and gene expression. We find that these domains undergo large-scale chromatin reorganization and a chromatin-interaction compartment switch during differentiation that is confined to the boundaries of the replication-timing switch. Surprisingly, these large-scale events do not involve changes in nuclease sensitivity, with domains remaining apparently inaccessible despite dramatic unfolding. Our results provide insight into the control of chromatin structure at developmentally regulated chromosomal domains and demonstrate that large-scale changes in chromatin folding can occur without changes in nuclease sensitivity.
Results
Genome-Wide Analysis of Chromatin Sensitivity to Nuclease During Differentiation.
As discussed in the introductory text, we previously showed that domains that switch their replication timing from EtoL during differentiation from ESCs to neural precursor cells (NPCs) are difficult to reprogram for both replication timing and gene expression (3). We investigated two of these domains, referred to as the Dppa2/4 and Rex1 domains (Fig. 1A). We used sex determining region Y-box 1 (Sox1)-GFP knock-in mouse ESCs, in which neural differentiation can be easily monitored (Fig. 1B) (19). Genes within these domains (Dppa2, Dppa4, Morc1, and Rex1) are highly expressed in ESCs but are down-regulated during differentiation (Fig. 1C). To test whether changes in replication timing coincide with nuclease sensitivity changes, chromatin from ESCs before and after differentiation to NPCs was digested with MNase and separated into more sensitive (low-molecular-mass DNA) and less sensitive (high-molecular-mass DNA) fractions by polyethylene glycol (PEG) precipitation (20). These two fractions were differentially labeled and hybridized to a mouse comparative genomic hybridization (CGH) array, and the signal ratio for each probe was plotted as a function of chromosomal position (Fig. 2 A and B). The same fractions were hybridized to mouse metaphase spreads to confirm that they derive from different parts of the genome (Fig. S1A).
Fig. 1.
Dppa2/4 and Rex1 chromosomal domains. (A) Replication timing of Dppa2/4 and Rex1 domains derived from genome-wide analyses (15). Briefly, BrdU-substituted DNA from early and late S-phase cells was differentially labeled and hybridized to a whole-genome oligonucleotide microarray. The signal ratio of early and late (log2 early/late) for each probe is plotted along the chromosomal position (Loess-smoothed data). All genomics data were assembled from the ReplicationDomain.org database (36). Regions referred to as the Dppa2/4 or Rex1 domains are highlighted in the red box. (B) ESC differentiation into neural progenitor cells was confirmed in each experiment by Oct4 immunofluorescence and GFP expression from a Sox1:GFP knock-in. (C) RT-PCR analysis of genes within replication-timing-switch regions in ESCs and NPCs.
Fig. 2.
Genome-wide analysis of chromatin sensitivity to nuclease. (A) Flowchart of the MNase sensitivity analysis. (B) Exemplary MNase-sensitivity plot of entire chromosome 11 (Upper) and its comparison with replication timing (Lower) in NPCs. The chromosomal position is shown on the x axis and relative enrichment of MNase sensitive chromatin (log2 sensitive/less sensitive; Loess-smoothed data as in Fig. 1) on the y axis. (C) Boxplots show MNase sensitivity data in early-to-early (EtoE), late-to-late (LtoL), and timing-switch domains (EtoL and LtoE) quantified in 200-kb windows. (D) MNase-sensitivity plots for Dppa2/4 and Rex1 replication domains in ESC and NPC. Replication timing-switch domains are indicated in the red box as in Fig. 1. Plots for chromosome 16 (62–65 Mb) are shown as an exemplary similar-sized chromosomal region that shows substantial MNase-sensitivity change but no replication-timing change. (E) MNase sensitivity in ESCs (y axis) is plotted against that in NPCs (x axis) in each category of replication domain (EtoE, LtoL, EtoL, LtoE). Each dot in the plots represents MNase sensitivity data quantified in a 200-kb genomic segment. Domains with ±0.20 or greater change in MNase sensitivity in NPCs are highlighted.
Consistent with previous reports (18, 21), early- and late-replicating chromosomal regions were associated with more and less sensitive chromatin, respectively (Fig. 2 B and C), and this correlation strengthened in NPCs (Fig. 2C). Surprisingly, both EtoL and late to early (LtoE) switching domains were exceptions to this global correlation, remaining packaged into relatively inaccessible chromatin in both ESCs and NPCs, and changes in replication timing were not accompanied by changes in nuclease sensitivity (Fig. 2C). In fact, EtoL regions were among the least sensitive chromatin in the genome, even when early replicating in ESCs. Similar results were obtained whether or not cells were first fixed in formaldehyde before MNase digestion (Fig. S1 B and C), or when a mixture of the restriction enzymes NlaIII/MspI was used as the nuclease instead of MNase (Fig. S1 B and C). Closer inspection of the EtoL Dppa2/4 and Rex1 domains confirmed that these regions were less sensitive both in ESCs and NPCs (Fig. 2D and Fig. S2). In contrast, replication domains that do significantly change nuclease sensitivity during differentiation were mainly found in constitutively early (EtoE) replication domains (Fig. 2E).
Large-Scale Chromatin Reorganization During Differentiation.
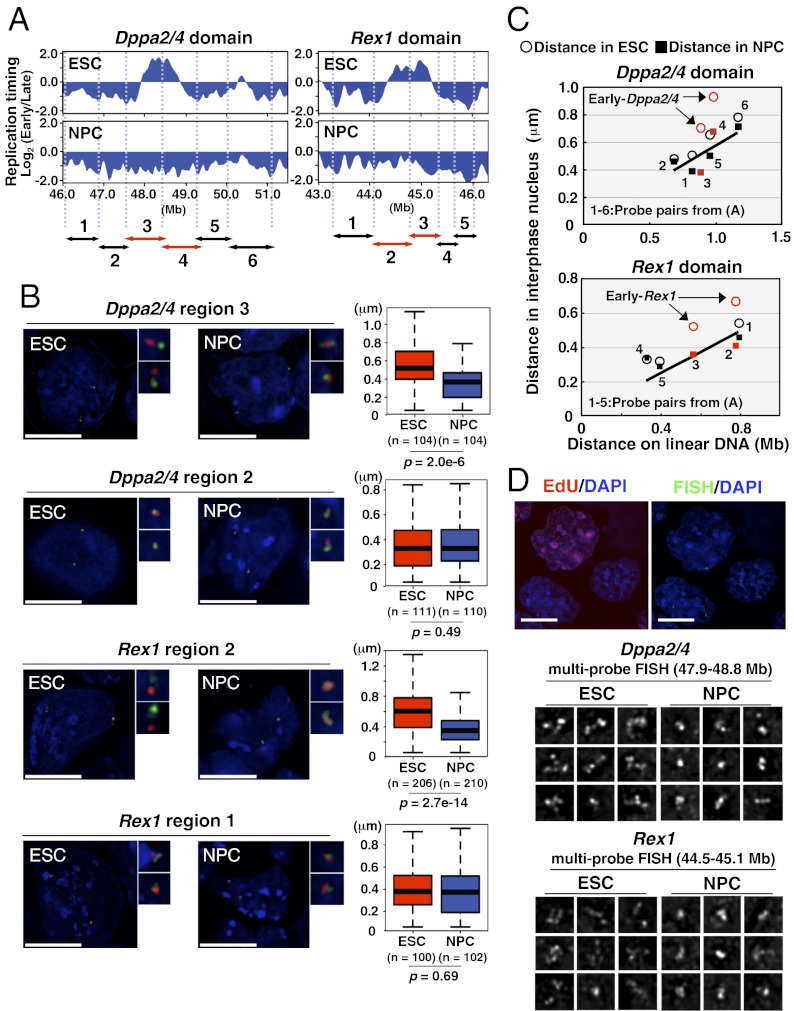
We next examined large-scale organization of these domains by measuring the interphase distance between pairs of FISH probes illustrated in Fig. 3A. First, we compared two pairs of probes, one each from the EtoL-switching domain and the adjacent constitutively late domain (LtoL) of Dppa2/4 and Rex1, using nuclei fixed in formaldehyde (3D FISH) to preserve 3D structure and mimic the MNase digestion conditions. At both loci, we detected a significant reduction in the distances between probes within EtoL domain after differentiation (Fig. 3B), whereas distances between control probes in the flanking domains did not change, suggesting that large-scale changes in chromatin organization were confined to the EtoL domain.
Fig. 3.
Chromatin reorganization of Dppa2/4 and Rex1 chromosomal domains during differentiation. (A) Positions of FISH probe pairs aligned to the replication-timing plots of Dppa2/4 and Rex1 domains. The center of each BAC probe is positioned at each arrowhead, and the regions between them are labeled as referred to in B and C. Regions in red overlap the EtoL-switching domains. (B) Chromatin organization of Dppa2/4 and Rex1 domains visualized by FISH with the probe pairs that demarcate each designated region, as indicated in A. FISH probes within (green) and outside (red) the EtoL-switching domains were visualized in paraformaldehyde (3D)-fixed ESCs and NPCs. FISH probe pairs in neighboring nonswitching regions were used as control. Box plots show the distances between centers of signals detected with each probe. (Scale bars: 10 μm.) (C) Chromatin organization across 4–5 Mb covering the regions shown in A, measured as the distance between FISH probes in nuclei from methanol/acetic acid (2D)-fixed cells and plotted vs. the physical distance between them on linear DNA. Open circles and filled squares represent the distance between FISH probes in ESC and NPC, respectively. The data points overlapping EtoL-switching domains are highlighted in red. The numbers nearest to each pair of data points (distances in ESC and NPC) correspond to the FISH probe pairs indicated in A. (D) Chromatin reorganization is not linked to DNA synthesis. (Top) Several FISH probes covering the entire switching domains of Dppa2/4 (47.9–48.8 Mb) and Rex1 (44.5–45.1 Mb) were hybridized and detected simultaneously by 3D FISH (green), whereas DNA synthesis was monitored by EdU incorporation (red). (Middle and Bottom) Examples of signal conformation predominantly observed in EdU-negative ESCs and NPCs. (Scale bars: 10 µm.)
To further quantify the extent of chromatin unfolding, we extended this interprobe analysis further into the neighboring regions using the higher-throughput 2D FISH method in which nuclei are fixed in methanol:acetic acid (Fig. S3A). Because this method swells nuclei but is thought to retain relative distance relationships (22), we first confirmed the reproducibility of the changes observed with 3D FISH. We also confirmed that the distance between signals did not correlate with nuclear diameter, which is variable both within a sample preparation (e.g., cell cycle differences) and between samples (e.g., preparation artifacts) (Fig. S3 B and C). When the physical distance between probe pairs on the linear DNA molecule was plotted against its actual distance in the interphase nucleus, a good linear correlation was observed (Fig. 3C). However, ESC-specific early-replicating regions significantly deviated from this correlation (Fig. 3C, arrows), revealing a locally distended chromatin structure in ESCs, restricted to the region of replication timing change, that is restored to the local linear degree of chromatin organization after differentiation.
To further confirm our findings, we simultaneously hybridized three to four FISH probes spanning the entire Dppa2/4 or Rex1 and flanking domains, using 3D FISH (Fig. 3D). To exclude any effect of active DNA replication on FISH signal conformation, chromatin organization was analyzed in ethynyl deoxyuridine (EdU)-negative non–S phase nuclei. In ESCs, these probes formed dispersed signals, whereas these same probes produced compact signals in differentiated NPCs. We conclude that large-scale chromatin reorganization is confined to the region of replication timing change.
Chromatin Organization Is Not a Direct Consequence of Transcription.
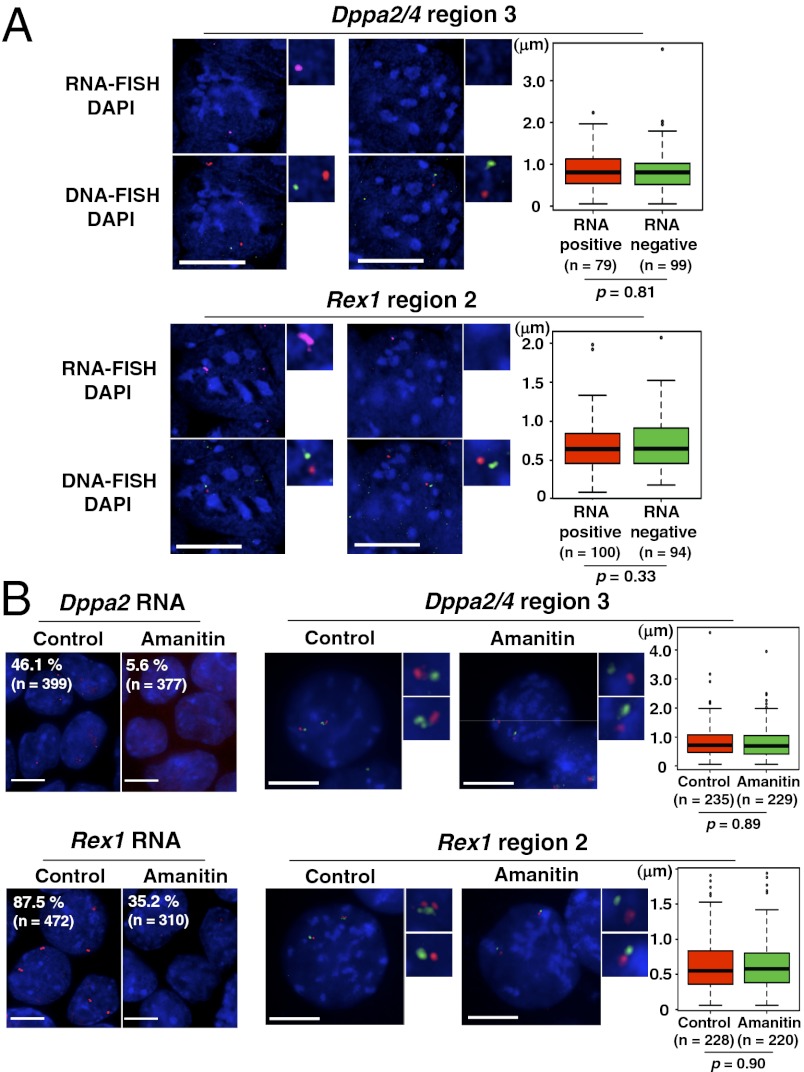
Because chromatin reorganization accompanies down-regulation of transcription within Dppa2/4 and Rex1 domains, we considered the possibility that changes in transcriptional activity could be driving chromatin reorganization of these domains, as suggested for gene-rich regions on the X chromosome (21) and homeobox (Hox) genes (23, 24). To test this, we analyzed chromatin organization in domains either expressing or not expressing Dppa2 or Rex1 using RNA/DNA-FISH. As expected, not all cells were positive for Dppa2 and Rex1 transcripts (Fig. 4), which reflects both heterogeneity of ESC populations and the stochastic nature of transcription (25–27). Rex1-negative populations were still positive for octamer-binding transcription factor 4 (Oct4) (hence undifferentiated) but showed reduced Nanog, demonstrating the ability of RNA-FISH to distinguish these previously described populations (Fig. S4). However, when chromatin organization was examined in these same cells by two-color DNA-FISH (Fig. 4A), we observed no difference in DNA-FISH probe distances between RNA-FISH–positive and –negative loci (Fig. 4A). To confirm this finding, cells were treated with the transcription inhibitor α-amanitin for 5 h before RNA-/DNA-FISH, which did not induce any significant change in chromatin organization of the Dppa2/4 and Rex1 domains in ESCs (Fig. 4B). Hence, chromatin organization of these domains is not directly dependent upon the act of transcription.
Fig. 4.
Chromatin reorganization is not dependent upon active transcription. (A) Chromatin organization at RNA-FISH–positive and –negative homologs in ESCs. Dppa2 and Rex1 transcripts were visualized by RNA-FISH (magenta), and chromatin organization was visualized simultaneously by DNA-FISH (green and red). The same probe pairs (Dppa2/4 region 3 and Rex1 region 2) shown in Fig. 3A were used for DNA-FISH. Boxplots show the distances between DNA-FISH signals measured in RNA-positive and -negative homologs. (Scale bars: 10 μm.) (B) Chromatin organization of ESCs after transcriptional inhibition by α-amanitin. Cells were treated with 100 μg/mL α-amanitin for 5 h, and Dppa2 and Rex1 transcriptional level was analyzed by RNA-FISH (Upper Left and Lower Left). Chromatin organization of Dppa2/4 and Rex1 domains was analyzed by measuring the distance between DNA-FISH probes spanning region 3 in Fig. 3A in 2D-fixed cells (Upper Right and Lower Right). Boxplots show the distance between DNA-FISH signals measured in control and α-amanitin–treated cells. (Scale bars: 10 μm.)
Changes in Spatial Compartmentalization During Differentiation.
The close correlation between replication timing and spatial compartmentalization of the genome revealed by Hi-C (16) suggested that the dramatic unfolding observed by FISH may accompany a subnuclear chromatin-interaction compartment switch. Hence, we investigated the interaction of the Dppa2/4 and Rex1 domains with the rest of the genome using chromosome conformation capture on chip (4C) (28) (Fig. 5A). As expected, frequent interaction near the bait region was observed in both ESCs and NPCs (Fig. S5) (24, 29). Close inspection of the region surrounding the replication-timing-switch domain revealed that the interaction frequencies aligned with the replication domain boundaries in ESCs and a neighboring early domain (Fig. 5B). Upon differentiation to NPCs, interactions with the neighboring early domain were strongly reduced, and those with the neighboring late domain were increased (Fig. 5B, highlighted in blue). Potential artifacts such as restriction fragment length and GC content (17) are not a concern when comparing two differentiation states because they are constant between datasets. Importantly, the sharp drop off in interaction frequencies aligning with the replication timing boundaries in ESCs (i.e., the boundaries of the main bait-proximal peak) were maintained after differentiation, suggesting that the domain of replication-timing change is a stable spatial unit of chromatin self-interaction in either nuclear compartment (Fig. 3C). As a control, a bait region positioned over the constitutively late-replicating β-globin locus retained preferential interaction with late-replicating regions before and after differentiation, including coordinated changes in its interaction with flanking domains that changed replication timing (Fig. 5B).
Fig. 5.
Changes in chromatin interaction maps of the Dppa2/4 and Rex1 chromosomal domains parallel replication-timing changes during differentiation. (A) Flowchart of the 4C method. In brief, chromatin from ESCs and NPCs was fixed in paraformaldehyde to preserve chromatin interactions in the nucleus, digested with a restriction enzyme, and ligated with other chromatin in spatial proximity. The resulting hybrid molecules were purified and used as a template for primer extension reaction using the bait-specific primer. This was followed by adaptor ligation, nested PCR, labeling with fluorescent dye, and hybridization to a custom microarrray (described in ref. 28). (B) Interaction profiles around the bait regions (Dppa2/4, Rex1, and control β-globin) were determined as the signal ratio of 4C sample to reference genomic DNA. Smoothed 4C values over a moving average of 39 probes (red) were plotted and compared with the replication-timing profile (blue). The gray dotted line in each plot shows a 95% confidence threshold [5% false-discover rate (FDR)]. Significance of interactions is shown as the range in color using multiscale analysis “domainograms” below each plot [colors range from black (P = 1) to yellow (P = 10−10)] (37). The regions undergo significant changes in 4C interaction during differentiation and are highlighted with blue bars (identified using both threshold and domainogram). (C) Analysis of replication-timing properties of interaction partners reveals that early- and late-replicating domains preferentially interact with early- and late-replicating chromosomal regions, respectively. Boxplots show the replication timing of Dppa2/4, Rex1, and β-globin domain-interacting partners before and after differentiation. (D) Model for chromatin reorganization associated with a subnuclear chromatin-interaction compartment switch. Taken together, FISH and 4C data demonstrate that in pluripotent stem cells, domains subjected to EtoL regulation partially unfold and move into the early-replicating subnuclear chromatin-interaction compartment, where they are suppressed from interacting with late-replicating chromatin, even neighboring late-replicating domains. During differentiation, these domains retract and acquire a similar spatial compaction to surrounding domains. In this configuration, they interact more frequently with late-replicating chromatin including neighboring domains, while, at the same time, retaining the identity of their self-interacting unit boundaries.
These 4C results confirm the FISH results shown in Fig. 3, indicating that the Dppa2/4 and Rex1 loci are distant from their neighboring late-replicating domains in ESCs and come in closer proximity after differentiation. However, the 4C results also reveal longer-range interactions that change during differentiation. To further confirm these interactions, we performed 3D FISH with five pairs of probes positioned up to 2.2 Mb from the Dppa2/4 or Rex1 bait regions (Fig. S6). Results confirmed that the frequency of long-range interactions revealed by 4C is recapitulated in the relative proximities of FISH probes.
We next examined the cis-interactions across the remainder of the chromosome, which revealed a strong correlation between the replication timing of the bait region and that of its interacting partners (Fig. 5C). In ESCs in which Dppa2/4 and Rex1 domains are early-replicating, these domains preferentially interact with other early-replicating domains, whereas in NPCs, late-replicating Dppa2/4 and Rex1 domains preferentially interact with late-replicating regions. Constitutively late β-globin interacts preferentially with late regions in both ESCs and NPCs. These results confirm that a subnuclear chromatin-interaction compartment change occurs at Dppa2/4 and Rex1 domains during differentiation, coinciding with the replication-timing switch and dramatic 3D unfolding in these domains.
Discussion
Our study demonstrates that Dppa2/4 and Rex1 domains form a locally unfolded chromatin configuration in ESCs that is delimited by the region of replication timing change and returns to the more compact state of flanking chromatin during differentiation. This change in chromatin folding accompanies a subnuclear chromatin-interaction compartment switch but does not involve a change in chromatin fiber nuclease sensitivity.
Our genome-wide MNase sensitivity analysis before and after ESC differentiation revealed a positive correlation between early replication and high nuclease sensitivity, consistent with previous studies (12, 18). However, this correlation did not apply to regions that change replication timing during differentiation. EtoL-switching regions were among the least-sensitive chromatin whether early- or late-replicating. Because these timing-switch domains contain AT-rich/long-interspersed nuclear element (LINE)-rich sequences that are generally seen in late-replicating heterochromatin regions (30, 31), it is possible that nuclease sensitivity of these switching regions is more affected by sequence composition rather than by epigenetic properties. In fact, these results are consistent with our previously proposed hypothesis that to change replication timing during development, domains must display sequence properties exhibited by late-replicating domains (30). Regardless, changes in replication timing and transcriptional activity clearly do not require changes in domain-wide nuclease sensitivity. It is important, however, to keep in mind that the general nuclease sensitivity measured here is independent of localized changes in hypersensitive sites or nucleosome occupancy (32). Hence, we cannot rule out localized changes in nucleosome occupancy or positioning.
Although there seems to be a general correlation between open chromatin structure (assessed by nuclease sensitivity) and chromatin decondensation at the cytological level (12), this does not apply to the EtoL domains Dppa2/4 and Rex1, where chromatin of lower nuclease sensitivity forms a cytologically decondensed higher-order chromatin structure detected by FISH. Our results suggest that higher-order chromatin unfolding accompanied by early replication is a transcriptionally permissive environment for genes that are nonetheless embedded in a nuclease-protected chromatin domain. Dppa2/4 and Rex1 are members of a class of genes that switch from EtoL during loss of pluripotency, remain late-replicating and silent throughout the rest of development, and are particularly difficult to reprogram after differentiation (3). A requirement for higher-order unfolding to activate these genes could explain their unusually stable silencing.
We previously demonstrated a close correlation between replication timing and long-range chromatin interactions found in human lymphocytes (16). However, we have identified many other correlations between chromosome properties and replication timing (3, 15), even those that correlate both spatially and temporally (33), that do not coordinately change with replication timing during differentiation. FISH analyses demonstrate a clear change in chromosome folding that is restricted to the region of replication-timing change, confirmed molecularly by 4C analyses. In fact, changes in interacting partners paralleled their replication timing during differentiation. This suggests that early- and late-replication domains are organized within chromosome territories to form distinct interacting compartments (Fig. 5D). The reduced proximity of early-replicating Dppa2/4 and Rex1 domains with their neighboring late-replicating domains, visualized by FISH (Fig. 3), correlates with their differential compartmentalization when they are in the early-replicating configuration. Given that early-replicating Dppa2/4 and Rex1 domains are surrounded by large late-replicating heterochromatin domains, the ESC-specific higher-order chromatin conformation may facilitate eviction of these domains from the late-replicating and transcriptionally repressive compartment of the nucleus and facilitate interactions with a compartment specialized for early replication and permissive for transcription. The fact that frequencies of chromatin interactions still drop off sharply at the replication-timing-switch domain boundaries, even when the domain is in the late-replicating configuration and able to interact with its neighbors (Fig. 5B), supports a model in which replication domains are units of regulation of self-interacting chromosome domains.
In conclusion, our results unravel the dynamic behavior of chromosomal domains that are known to be difficult to reprogram. Dissecting factors involved in these processes will be relevant to our understanding of cellular differentiation and reprogramming.
Materials and Methods
ESC Culture.
Mouse 46C ESCs on a gelatinized culture dish were maintained in the presence of leukemia inhibitory factor (LIF) as described previously (15). Neural differentiation was performed as described previously (19), and NPC samples were collected after 6 d. For replication labeling in Fig. 3, ESCs were cultured for 1 h in the presence of 100 μM EdU before harvesting.
RT-PCR.
RNA was isolated from ESCs and NPCs with RNeasy (Qiagen), according to the instructions of the manufacturer. First-strand cDNA was synthesized with the SuperScript III first-strand synthesis system (Invitrogen). Primer sequences are available upon request.
FISH-Based Chromatin Assay.
BAC clones used in this study were obtained from BACPAC Resource Center (http://bacpac.chori.org). FISH was performed as described previously (34). See SI Materials and Methods for details.
Chromatin-Sensitivity Profiling by Microarray.
MNase digestion was performed as described previously with slight modifications (35). See SI Materials and Methods for details.
Chromosome Conformation Capture on Chip.
The 4C experiments and analyses were performed as described previously with slight modifications (28). See SI Materials and Methods for details.
Statistical Analysis.
Statistical significance was examined by Wilcox test.
Supplementary Material
Acknowledgments
We thank P. Fraser and C. Osborne for advice on the 4C experiments and T. Sexton, B. Leblanc, and E. de Wit for advice on the 4C data analysis. We also thank B. Pope for help with the bioinformatics analysis. This work was supported by National Institutes of Health Grant GM085354 (to D.M.G.) and a postdoctoral fellowship from The Uehara Memorial Foundation (to S.-i.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the ReplicationDomain database, www.replicationdomain.org (Chip IDs: MNase ESC, MNase NPC, 4C-Dppa2/4 ESC, 4C-Dppa2/4 NPC, 4C-Rex1 ESC, 4C-Rex1 NPC, 4C-Beta-globin ESC, and 4C-Beta-globin NPC).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207185109/-/DCSupplemental.
References
- 1.Fisher CL, Fisher AG. Chromatin states in pluripotent, differentiated, and reprogrammed cells. Curr Opin Genet Dev. 2011;21:140–146. doi: 10.1016/j.gde.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed K, et al. Global chromatin architecture reflects pluripotency and lineage commitment in the early mouse embryo. PLoS ONE. 2010;5:e10531. doi: 10.1371/journal.pone.0010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiratani I, et al. Genome-wide dynamics of replication timing revealed by in vitro models of mouse embryogenesis. Genome Res. 2010;20:155–169. doi: 10.1101/gr.099796.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka S. Pluripotency and nuclear reprogramming. Philos Trans R Soc Lond B Biol Sci. 2008;363:2079–2087. doi: 10.1098/rstb.2008.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sridharan R, et al. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikkelsen TS, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fussner E, Ching RW, Bazett-Jones DP. Living without 30nm chromatin fibers. Trends Biochem Sci. 2011;36:1–6. doi: 10.1016/j.tibs.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Maeshima K, Hihara S, Eltsov M. Chromatin structure: Does the 30-nm fibre exist in vivo? Curr Opin Cell Biol. 2010;22:291–297. doi: 10.1016/j.ceb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Sinclair P, Bian Q, Plutz M, Heard E, Belmont AS. Dynamic plasticity of large-scale chromatin structure revealed by self-assembly of engineered chromosome regions. J Cell Biol. 2010;190:761–776. doi: 10.1083/jcb.200912167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieberman-Aiden E, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert N, et al. Chromatin architecture of the human genome: Gene-rich domains are enriched in open chromatin fibers. Cell. 2004;118:555–566. doi: 10.1016/j.cell.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Mateos-Langerak J, et al. Spatially confined folding of chromatin in the interphase nucleus. Proc Natl Acad Sci USA. 2009;106:3812–3817. doi: 10.1073/pnas.0809501106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Méndez J. Temporal regulation of DNA replication in mammalian cells. Crit Rev Biochem Mol Biol. 2009;44:343–351. doi: 10.1080/10409230903232618. [DOI] [PubMed] [Google Scholar]
- 15.Hiratani I, et al. Global reorganization of replication domains during embryonic stem cell differentiation. PLoS Biol. 2008;6:e245. doi: 10.1371/journal.pbio.0060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryba T, et al. Evolutionarily conserved replication timing profiles predict long-range chromatin interactions and distinguish closely related cell types. Genome Res. 2010;20:761–770. doi: 10.1101/gr.099655.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaffe E, Tanay A. Probabilistic modeling of Hi-C contact maps eliminates systematic biases to characterize global chromosomal architecture. Nat Genet. 2011;43:1059–1065. doi: 10.1038/ng.947. [DOI] [PubMed] [Google Scholar]
- 18.Bell O, et al. Accessibility of the Drosophila genome discriminates PcG repression, H4K16 acetylation and replication timing. Nat Struct Mol Biol. 2010;17:894–900. doi: 10.1038/nsmb.1825. [DOI] [PubMed] [Google Scholar]
- 19.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 20.Lis JT, Schleif R. Size fractionation of double-stranded DNA by precipitation with polyethylene glycol. Nucleic Acids Res. 1975;2:383–389. doi: 10.1093/nar/2.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naughton C, Sproul D, Hamilton C, Gilbert N. Analysis of active and inactive X chromosome architecture reveals the independent organization of 30 nm and large-scale chromatin structures. Mol Cell. 2010;40:397–409. doi: 10.1016/j.molcel.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskeland R, et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell. 2010;38:452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambeyron S, Bickmore WA. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 2004;18:1119–1130. doi: 10.1101/gad.292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noordermeer D, et al. The dynamic architecture of Hox gene clusters. Science. 2011;334:222–225. doi: 10.1126/science.1207194. [DOI] [PubMed] [Google Scholar]
- 25.Levsky JM, Shenoy SM, Pezo RC, Singer RH. Single-cell gene expression profiling. Science. 2002;297:836–840. doi: 10.1126/science.1072241. [DOI] [PubMed] [Google Scholar]
- 26.Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- 27.Suter DM, et al. Mammalian genes are transcribed with widely different bursting kinetics. Science. 2011;332:472–474. doi: 10.1126/science.1198817. [DOI] [PubMed] [Google Scholar]
- 28.Schoenfelder S, et al. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat Genet. 2010;42:53–61. doi: 10.1038/ng.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simonis M, et al. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C) Nat Genet. 2006;38:1348–1354. doi: 10.1038/ng1896. [DOI] [PubMed] [Google Scholar]
- 30.Hiratani I, Leskovar A, Gilbert DM. Differentiation-induced replication-timing changes are restricted to AT-rich/long interspersed nuclear element (LINE)-rich isochores. Proc Natl Acad Sci USA. 2004;101:16861–16866. doi: 10.1073/pnas.0406687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodfine K, et al. Replication timing of the human genome. Hum Mol Genet. 2004;13:191–202. doi: 10.1093/hmg/ddh016. [DOI] [PubMed] [Google Scholar]
- 32.Keene MA, Elgin SC. Micrococcal nuclease as a probe of DNA sequence organization and chromatin structure. Cell. 1981;27:57–64. doi: 10.1016/0092-8674(81)90360-3. [DOI] [PubMed] [Google Scholar]
- 33.Yokochi T, et al. G9a selectively represses a class of late-replicating genes at the nuclear periphery. Proc Natl Acad Sci USA. 2009;106:19363–19368. doi: 10.1073/pnas.0906142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takebayashi S, et al. Regulation of replication at the R/G chromosomal band boundary and pericentromeric heterochromatin of mammalian cells. Exp Cell Res. 2005;304:162–174. doi: 10.1016/j.yexcr.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Dennis JH, et al. Independent and complementary methods for large-scale structural analysis of mammalian chromatin. Genome Res. 2007;17:928–939. doi: 10.1101/gr.5636607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weddington N, et al. ReplicationDomain: A visualization tool and comparative database for genome-wide replication timing data. BMC Bioinformatics. 2008;9:530. doi: 10.1186/1471-2105-9-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noordermeer D, et al. Variegated gene expression caused by cell-specific long-range DNA interactions. Nat Cell Biol. 2011;13:944–951. doi: 10.1038/ncb2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.