Abstract
Although animals display a rich variety of shapes and patterns, the genetic changes that explain how complex forms arise are still unclear. Here we take advantage of the extensive diversity of Heliconius butterflies to identify a gene that causes adaptive variation of black wing patterns within and between species. Linkage mapping in two species groups, gene-expression analysis in seven species, and pharmacological treatments all indicate that cis-regulatory evolution of the WntA ligand underpins discrete changes in color pattern features across the Heliconius genus. These results illustrate how the direct modulation of morphogen sources can generate a wide array of unique morphologies, thus providing a link between natural genetic variation, pattern formation, and adaptation.
Keywords: Müllerian mimicry, Wnt pathway, Mendelian genetics, evolutionary-developmental biology
Cell fate induction by signaling molecules is a central characteristic of developmental pattern formation (1, 2), thus the evolution of genes encoding signaling molecules is expected to drive pattern evolution and contribute to morphological diversity (3, 4). An empirical test of this hypothesis relies on linking molecular and phenotypic evolution using forward genetic approaches (5–7), and indeed a handful of signaling gene variants underlying morphological differences in natural populations have been identified (8–14). Here we show that genetic variation linked to a Wnt-family signaling molecule drives the diversification of complex wing patterns in Heliconius erato and Heliconius melpomene mimetic butterflies (15). These species are unpalatable and each has undergone parallel adaptive radiations, such that the geographic range of each species is composed of a nearly identical patchwork of distinct geographic races (16, 17). Local populations of coexisting butterflies share common wing patterns that provide mutualistic protection (Müllerian mimicry) from avian predators (18, 19). With their extensive within-species variation (16, 20) and numerous cases of mimetic convergence between species, Heliconius wing patterns form an ideal model to study the repeatability of complex trait evolution (7, 15, 21–24).
Results
Wing Pattern Shape Variation Repeatedly Maps to WntA.
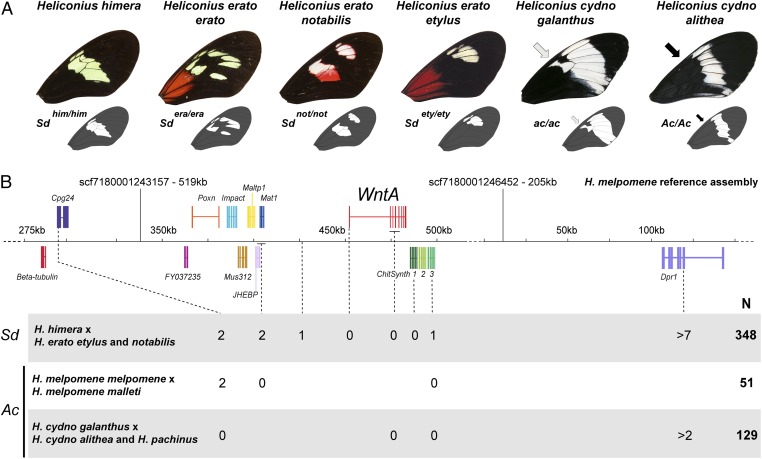
The great diversity in forewing band shape across the H. erato wing color pattern radiation is controlled by a single locus of large effect called Short band (Sd) (15, 22, 25). Pattern variation occurs through changes in the size and position of black pattern elements, which in turn define the shape of yellow and white areas of the forewing (Fig. 1A and SI Appendix, Fig. S1). We positionally cloned this locus using a combination of restriction-associated amplified DNA (RAD) markers (26) and traditional linkage mapping. Our RAD screen of a H. erato notabilis × Heliconius himera F2 brood pinpointed a single scaffold from the H. melpomene genome (27) that showed an enrichment of Sd-associated SNPs (SI Appendix, Table S1). Further fine-scale linkage mapping identified a 69-kb zero-recombinant interval centered on the gene encoding the signaling ligand WntA (Fig. 1B and SI Appendix, Tables S2–S4). Indeed, there was no recombination between black pattern phenotypes and WntA across 458 F2 offspring from crosses between H. himera and three different morphs of H. erato. Thus, natural Sd allelic variation controls at least four distinct pattern shape variants in H. erato (as shown in Fig. 1A). Furthermore, we found perfect linkage with a 1.1-cM resolution [∼200 kb (28)] between WntA and the Anterior cell spot (Ac) locus that controls variation of forewing pattern shape in the H. melpomene/Heliconius cydno clade (15, 22, 29). Pattern variation thus has a monogenic basis in two independent radiations, re-emphasizing that repeated evolution of butterfly color pattern mimicry is driven by a relatively small toolkit of large-effect loci (15, 21, 22, 24).
Fig. 1.
Linkage mapping of forewing pattern shape variation to WntA. (A) Examples of forewing pattern shapes found in Heliconius shown with their associated Sd and Ac genotypes. Mapped Ac polymorphisms in the H. melpomene/H. cydno clade consist of presence or absence of a proximal melanic patch (arrows). (B) Linkage-mapping of Sd and Ac. Crossing-over events between genetic variation and individual phenotypes out of N individuals are featured at each marker. The depicted region spans the halves of two scaffolds separated by an assembly gap; however, the reference H. melpomene genetic map (27) and the colinearity of the Impact-Dpr1 syntenic block with the silkworm genome assembly (46) provide independent evidence of contiguity between these two scaffolds (SI Appendix, Fig. S2).
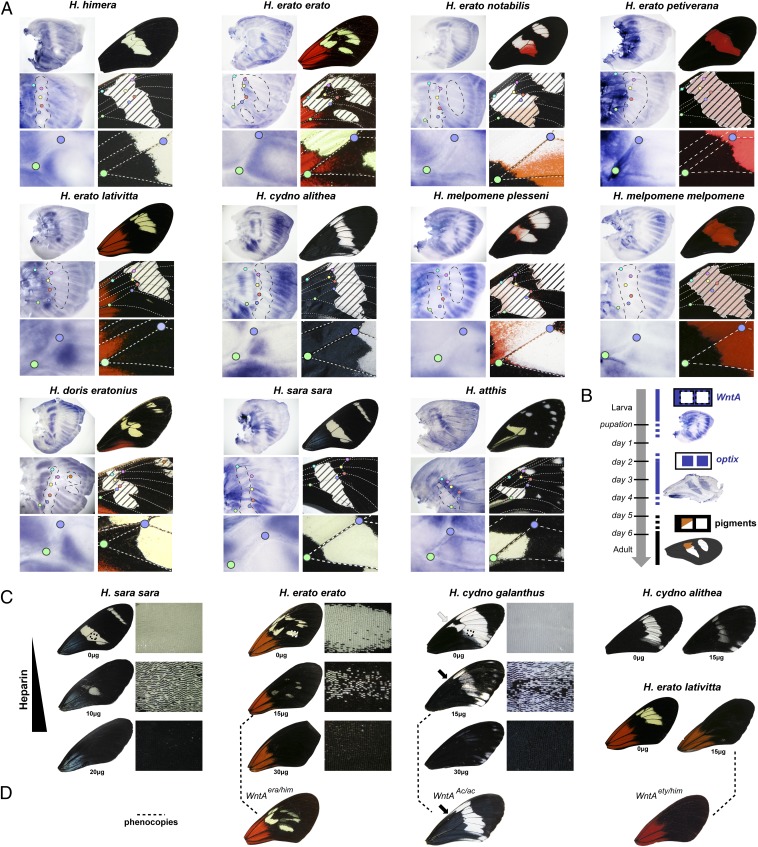
WntA Expression Is Variable and Marks Presumptive Black Patterns.
WntA is a member of the Wnt-family of signaling ligands (SI Appendix, Fig. S3), of which Wingless has previously been identified as a morphogen involved in Drosophila and lepidopteran wing pattern formation (30, 31). Genetic support for WntA as the source of adaptive variation in Heliconius wing patterns was corroborated by expression studies. WntA mRNA expression prefigured black patterns in a total of seven Heliconius species examined (Fig. 2A). For example, WntA distribution varied between the Sd-informative H. erato and H. himera morphs, correlating with the position of black color in the central portion of the wing; WntA expression also showed convergence between the comimics H. erato notabilis and H. melpomene plesseni by prefiguring the black stripe dividing their forewing color patches. It is noteworthy that this median split is also visible in the complementary pattern of optix expression that marks the H. melpomene plesseni red/white patterns at later stages (24) (Fig. 2B). The spatial association of WntA expression with intraspecific color pattern variation, combined with the genetic mapping data, strongly argues that WntA cis-regulatory evolution is driving wing pattern variation (6). Consistent with this conclusion, we observed no coding variation within the H. melpomene/H. cydno clade or between parapatric races of H. erato with divergent forewing band shapes (SI Appendix, Table S5).
Fig. 2.
Variable expression of WntA explains pattern shape diversity in Heliconius. (A) Larval wing disk in situ hybridizations showing WntA expression in presumptive black territories, and delineating pattern boundaries. Vein landmarks define homologous positions between larval and adult wings. In the intermediate panels, dashed lines corresponding to the presumptive position of the light-color patterns (hashed on adult wings) were added to facilitate the comparison of larval and adult wing topologies. (B) WntA marks presumptive optix-negative territories in H. melpomene plesseni. Expression of optix was reproduced from ref. 24. (C) Heparin injections result in dose-dependent expansions of black patterns that phenocopy the effects of WntA heterozygosity (D, dashed lines). The absence of effect on basal iridescent (H. sara sara) and red (H. erato erato and H. erato lativitta) patterns rules out a generic effect of heparin on wing scale phenotypes. H. erato lativitta resembles H. erato etylus (used in mapping crosses) and both originate from Eastern Ecuador low-lands.
Heparin-Sensitive Signal Determines Pattern Boundaries.
Our results imply that WntA controls pattern formation and variation, suggesting that modulating WntA protein distribution should result in pattern alterations. To test this prediction, we injected heparin into early pupae of a variety of Heliconius morphs. Heparin is an analog of heparan sulfate proteoglycans, a class of extracellular matrix compounds that play a key role in morphogen gradient formation (32). In particular, heparin-like chains bind Wnt-family ligands and promote their mobility in epithelial tissues, resulting in the expansion of their extracellular gradients (32–41). There is precedent for this effect in the butterfly Junonia coenia, where heparin injections resulted in specific expansions of the wing patterns associated with wingless expression (30, 42). We found that heparin injections had dose-dependent effects on WntA-associated Heliconius patterns (Fig. 2C and SI Appendix, Fig. S4). High dosages resulted in complete melanization of the central wing, and intermediate dosages caused partial expansion of WntA-associated black patterns with fields of peppered scales along expanding pattern boundaries. We thus conclude that an extracellular heparin-sensitive signal determines scale cell fate in a concentration-dependent manner. By themselves, these pharmacological results might be explained by possible heparin effects on a number of ligands (32); it is therefore important to note that heparin injections produced phenocopies of WntA-heterozygous phenotypes observed in H. erato and H. cydno hybrids (Fig. 2D). WntA heterozygosity is predicted to spread WntA distribution by driving its expression in adjacent domains, and similarly, heparin injections are expected to promote Wnt-ligand spread (32–41). The comparable outcomes of heparin injections and WntA heterozygosity thus suggest that heparin-induced pattern expansions are largely mediated by WntA (SI Appendix, Fig. S5).
Discussion
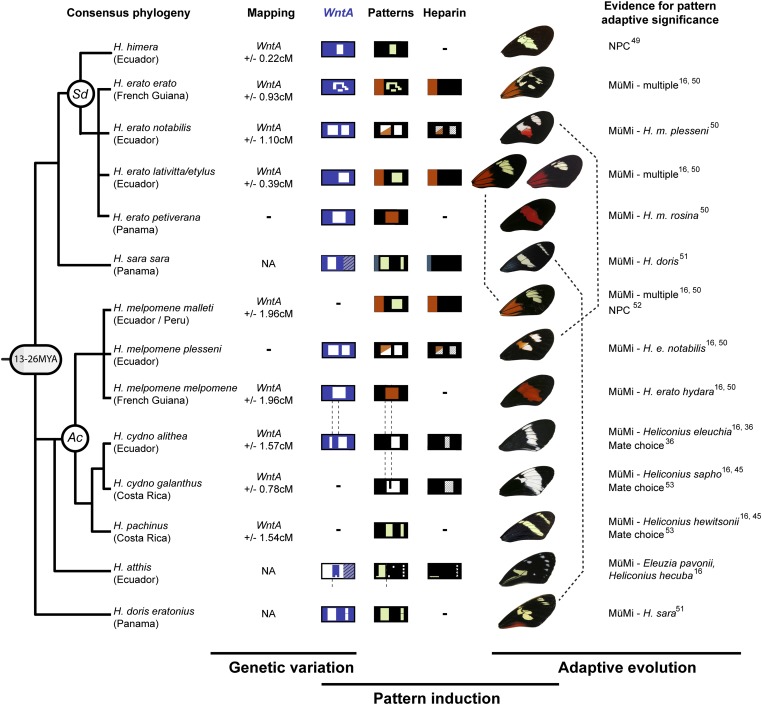
In this study we provide phylogenetically replicated genetic, expression, and pharmacological data that implicate WntA in pattern induction and adaptive evolution of black wing patterns in Heliconius butterflies (Fig. 3). It is worth noting the caveat, however, that the strongest support for the role of a specific gene in a developmental process is targeted loss- or gain-of-function work, which we lack because of technical limitations of our study system. This said, our heparin injection results were entirely consistent with the expectations of a targeted Wnt gain-of-function experiment (32–41). Heparin may interact with secreted molecules other than WntA, but it is too large to cross cell membranes and none of the WntA-neighboring genes encode extracellular products that could interact with heparin to explain its effects (SI Appendix, Table S4). Thus, although we are unable to formally rule out wing patterning functions for genes genetically linked to WntA, all results positively indicate that cis-regulatory variation at WntA is sufficient to explain the natural wing pattern variation considered in this study.
Fig. 3.
Summary of phylogenetic replication for linkage mapping of forewing pattern variation (Sd and Ac loci), WntA in situ hybridizations, and heparin injections presented in this study. Multiple lines of evidence suggest that forewing pattern shapes are adaptive across the genus (16, 36, 45, 49–53). NA, not applicable; NPC, narrow phenotypic cline; MüMi: Müllerian mimicry; —, not assessed because of stock limitations or unavailability. Note that H. erato lativitta/H. melpomene malleti, and H. erato notabilis/H. melpomene plesseni are convergent comimics that occur in Eastern Ecuador low- and highlands, respectively (dashed lines). Phylogenetic relationships between species are derived from a recent molecular phylogeny (47) after retaining nodes with bootstrap support ≥0.98. Estimated age of Heliconius radiation is derived from a recent molecular clock study (48). Only a subset of Heliconius wing pattern diversity is represented here.
This first discovery of a Wnt-pathway gene driving variation in natural populations complements developmental evolution studies [e.g., the previous reports of wing color patterning by wingless (30, 31)] with one important difference: we show that genetic changes at a Wnt locus itself are responsible for pattern variation. WntA is deployed early during wing development and may determine pattern boundaries and identities rather than acting directly as a melanic activator, as suggested by its complementarity with pattern-specific optix expression at later stages (Fig. 2B). As such, linking a patterning molecule to the evolution of a protean, highly variable trait fills an empirical gap between the genetics of adaptive change (6, 43) and the developmental pathways that generate complex phenotypic diversity (3, 4). Because of its repeated association with mimetic phenotypes in two independent color pattern radiations, cis-regulatory changes of WntA expression also appear to represent a path of least resistance in evolution of novel wing patterns. Spatial shifts of morphogen sources may thus be a key mechanism for generating phenotypic novelty through quantum leaps across the landscape of possible morphologies.
Methods
Mapping Crosses.
The H. himera × H. erato and H. melpomene malleti × H. melpomene melpomene families are described elsewhere (25, 28, 44). Segregation of the recessive ac allele in the H. melpomene cross is only visible if a yellow color of the forewing band is inherited from H. melpomene malleti, because of epistasis of Ac with the N locus (28, 29). Therefore, [ac/ac] vs. [Ac/−] segregating phenotypes were scored based on the presence vs. absence of a yellow “hourglass” pattern in the discal cell, respectively, and nonyellow banded individuals were not included in the analysis. The H. cydno galanthus (ac/ac) × H. pachinus (Ac/Ac) (one F2 cross) and H. cydno galanthus (ac/ac) × H. cydno alithea (Ac/Ac) (four male-informative backcross) families are described elsewhere (22, 45).
RAD Mapping.
Preparation of a RAD sequencing library followed the canonical protocol (26) with adaptations to a bulked segregant analysis design. In brief, genomic DNA from 25 Sdnot/not and 25 Sdhim/him F2 individuals from a single H. himera × H. erato notabilis family were combined equimolarly (50 ng per individual) into two separate Sdnot/not and Sdhim/him DNA pools. Each pool was digested with the restriction enzyme NcoI, ligated to barcoded adapters, and sequenced on a single lane of an Illumina Genome Analyzer GAII generating 72-bp paired-end reads. The resulting reads were sorted by barcode and aligned to the reference assembly of the H. melpomene genome. A custom script was used to generate a table of 46,236 SNPs that each mapped to a H. melpomene scaffold with a median coverage of 86×. Library preparation and data analysis details are included in SI Appendix, SI Methods.
Individual Genotyping.
The H. melpomene reference scaffold scf7180001243157 was referenced for linkage mapping of the Sd and Ac traits. PCR primers and genotyping methods are detailed in SI Appendix, Table S2.
Animals, Gene Cloning, and in Situ Hybridizations.
The butterflies used for expression analysis and sequence comparison originated from phenotypically pure stocks (i.e., homozygous for wing pattern alleles) maintained in the outdoor insectaries at the Heliconius Stock and Rearing Center at the Smithsonian Tropical Research Institute in Gamboa, Panama. Isolation of WntA cDNA and in situ hybridization was performed as previously described (24), and primer sequences and minor modifications are included in SI Appendix, SI Methods.
Heparin Injections.
Heparin sodium salt (Sigma-Aldrich) was dissolved in sterile H2O at a concentration of 5 μg/μL, aliquoted, and kept frozen. Pupae aged 12–16 h after pupation were injected with 5 μg/μL heparin or sterile H2O using a pulled glass micropipette mounted on a 10-μL cut pipette tip and a 2- to 20-μL pipette. Pupae were surface-sterilized with ethanol and injected on the left side in an interstice that separates the baso-posterior parts of the developing forewing and hindwing. As in a previous report (42), H2O controls showed no effects, heparin had systemic effects on both left and right wing patterns, and control and heparin injections did not produce local damage artifacts. All of the results presented in Fig. 2C were replicated at least three times per morph and dosage (including H2O control), but, because of stock limitations, the results presented in SI Appendix, Fig. S4 were replicated in only two individuals per morph and without H2O control.
Supplementary Material
Acknowledgments
We thank D. Kapan, S. Rusza, M. Abanto, P. Etter, J. Davey, H. Hines, A. Morrison, N. Gillingham, T. Douglas, J. Olander, A. Tapia, C. Pardo-Diaz, L. Maroja, and L. Gilbert for laboratory help and breeding work; and governmental agencies from Panama, Costa Rica, Ecuador, and French Guiana for permits. This work was funded by National Science Foundation Grants DEB-0844244, DEB-1020355, and IOS-1052541; and Biotechnology and Biological Sciences Research Council Grant BB/H01439X/1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RAD-mapping raw reads were deposited in the Short Read Archive (accession no. SRA045716.2); cDNA sequences were deposited in the GenBank database (accession nos. JN944582–JN944589); and genomic sequences can be accessed on the GenBank database (accession nos. HE668478 and HE669520) and on the Heliconius Genome Database, www.butterflygenome.org (scf180001243157 and scf7180001246452).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204800109/-/DCSupplemental.
References
- 1.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 2.Salazar-Ciudad I, Jernvall J, Newman SA. Mechanisms of pattern formation in development and evolution. Development. 2003;130:2027–2037. doi: 10.1242/dev.00425. [DOI] [PubMed] [Google Scholar]
- 3.Carroll SB, Grenier JK, Weatherbee SD. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design, Second Edition. Malden, MA: Blackwell Scientific; 2004. [Google Scholar]
- 4.Wilkins AS. The Evolution of Developmental Pathways. Sunderland: Sinauer Associates; 2002. [Google Scholar]
- 5.Stern DL. Evolutionary developmental biology and the problem of variation. Evolution. 2000;54:1079–1091. doi: 10.1111/j.0014-3820.2000.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 6.Stern DL, Orgogozo V. The loci of evolution: How predictable is genetic evolution? Evolution. 2008;62:2155–2177. doi: 10.1111/j.1558-5646.2008.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp A. Metamodels and phylogenetic replication: A systematic approach to the evolution of developmental pathways. Evolution. 2009;63:2771–2789. doi: 10.1111/j.1558-5646.2009.00761.x. [DOI] [PubMed] [Google Scholar]
- 8.Albertson RC, Streelman JT, Kocher TD, Yelick PC. Integration and evolution of the cichlid mandible: The molecular basis of alternate feeding strategies. Proc Natl Acad Sci USA. 2005;102:16287–16292. doi: 10.1073/pnas.0506649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonilla C, et al. The 8818G allele of the agouti signaling protein (ASIP) gene is ancestral and is associated with darker skin color in African Americans. Hum Genet. 2005;116:402–406. doi: 10.1007/s00439-004-1251-2. [DOI] [PubMed] [Google Scholar]
- 10.Manceau M, Domingues VS, Mallarino R, Hoekstra HE. The developmental role of Agouti in color pattern evolution. Science. 2011;331:1062–1065. doi: 10.1126/science.1200684. [DOI] [PubMed] [Google Scholar]
- 11.Anderson TM, et al. Molecular and evolutionary history of melanism in North American gray wolves. Science. 2009;323:1339–1343. doi: 10.1126/science.1165448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colosimo PF, et al. Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science. 2005;307:1928–1933. doi: 10.1126/science.1107239. [DOI] [PubMed] [Google Scholar]
- 13.Miller CT, et al. cis-Regulatory changes in Kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell. 2007;131:1179–1189. doi: 10.1016/j.cell.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loehlin DW, Werren JH. Evolution of shape by multiple regulatory changes to a growth gene. Science. 2012;335:943–947. doi: 10.1126/science.1215193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papa R, Martin A, Reed RD. Genomic hotspots of adaptation in butterfly wing pattern evolution. Curr Opin Genet Dev. 2008;18:559–564. doi: 10.1016/j.gde.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Brown KS., Jr . Eco-geography and evolution in neotropical forests. Brazil: Universidade Estadual de Campinas; 1979. PhD thesis. [Google Scholar]
- 17.Hines HM, et al. Wing patterning gene redefines the mimetic history of Heliconius butterflies. Proc Natl Acad Sci USA. 2011;108:19666–19671. doi: 10.1073/pnas.1110096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapan DD. Three-butterfly system provides a field test of Müllerian mimicry. Nature. 2001;409:338–340. doi: 10.1038/35053066. [DOI] [PubMed] [Google Scholar]
- 19.Langham GM. Specialized avian predators repeatedly attack novel color morphs of Heliconius butterflies. Evolution. 2004;58:2783–2787. doi: 10.1111/j.0014-3820.2004.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert LE. In: Ecology and Evolution Taking Flight: Butterflies as Model Systems. Boggs CL, Watt WB, Ehrlich PR, editors. Chicago, IL: Univ of Chicago Press; 2003. pp. 281–318. [Google Scholar]
- 21.Joron M, et al. A conserved supergene locus controls colour pattern diversity in Heliconius butterflies. PLoS Biol. 2006;4:e303. doi: 10.1371/journal.pbio.0040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kronforst MR, Kapan DD, Gilbert LE. Parallel genetic architecture of parallel adaptive radiations in mimetic Heliconius butterflies. Genetics. 2006;174:535–539. doi: 10.1534/genetics.106.059527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baxter SW, et al. Convergent evolution in the genetic basis of Müllerian mimicry in Heliconius butterflies. Genetics. 2008;180:1567–1577. doi: 10.1534/genetics.107.082982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed RD, et al. optix drives the repeated convergent evolution of butterfly wing pattern mimicry. Science. 2011;333:1137–1141. doi: 10.1126/science.1208227. [DOI] [PubMed] [Google Scholar]
- 25.Kapan DD, et al. Localization of Müllerian mimicry genes on a dense linkage map of Heliconius erato. Genetics. 2006;173:735–757. doi: 10.1534/genetics.106.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etter PD, Bassham S, Hohenlohe PA, Johnson EA, Cresko WA. In: Molecular Methods for Evolutionary Genetics. Orgogozo V, Rockman MV, editors. New York, NY: Humana Press; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Heliconius Genome Consortium A butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature. 2012;487:94–98. doi: 10.1038/nature11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiggins CD, et al. A genetic linkage map of the mimetic butterfly Heliconius melpomene. Genetics. 2005;171:557–570. doi: 10.1534/genetics.104.034686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naisbit RE, Jiggins CD, Mallet J. Mimicry: Developmental genes that contribute to speciation. Evol Dev. 2003;5:269–280. doi: 10.1046/j.1525-142x.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin A, Reed RD. Wingless and aristaless 2 define a developmental ground plan for moth and butterfly wing pattern evolution. Mol Biol Evol. 2010;27:2864–2878. doi: 10.1093/molbev/msq173. [DOI] [PubMed] [Google Scholar]
- 31.Werner T, Koshikawa S, Williams TM, Carroll SB. Generation of a novel wing colour pattern by the Wingless morphogen. Nature. 2010;464:1143–1148. doi: 10.1038/nature08896. [DOI] [PubMed] [Google Scholar]
- 32.Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley RS, Brown AMC. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990;9:1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reichsman F, Smith L, Cumberledge S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J Cell Biol. 1996;135:819–827. doi: 10.1083/jcb.135.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binari RC, et al. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–2632. doi: 10.1242/dev.124.13.2623. [DOI] [PubMed] [Google Scholar]
- 36.Greco V, Hannus M, Eaton S. Argosomes: A potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–645. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- 37.Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- 38.Hufnagel L, Kreuger J, Cohen SM, Shraiman BI. On the role of glypicans in the process of morphogen gradient formation. Dev Biol. 2006;300:512–522. doi: 10.1016/j.ydbio.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 39.Colombres M, et al. Heparin activates Wnt signaling for neuronal morphogenesis. J Cell Physiol. 2008;216:805–815. doi: 10.1002/jcp.21465. [DOI] [PubMed] [Google Scholar]
- 40.Fuerer C, Habib SJ, Nusse R. A study on the interactions between heparan sulfate proteoglycans and Wnt proteins. Dev Dyn. 2010;239:184–190. doi: 10.1002/dvdy.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling L, et al. Synergism between Wnt3a and heparin enhances osteogenesis via a phosphoinositide 3-kinase/Akt/RUNX2 pathway. J Biol Chem. 2010;285:26233–26244. doi: 10.1074/jbc.M110.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serfas MS, Carroll SB. Pharmacologic approaches to butterfly wing patterning: Sulfated polysaccharides mimic or antagonize cold shock and alter the interpretation of gradients of positional information. Dev Biol. 2005;287:416–424. doi: 10.1016/j.ydbio.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Prud’homme B, Gompel N, Carroll SB. Emerging principles of regulatory evolution. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8605–8612. doi: 10.1073/pnas.0700488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papa R, et al. Highly conserved gene order and numerous novel repetitive elements in genomic regions linked to wing pattern variation in Heliconius butterflies. BMC Genomics. 2008;9:345. doi: 10.1186/1471-2164-9-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chamberlain NL, Hill RI, Kapan DD, Gilbert LE, Kronforst MR. Polymorphic butterfly reveals the missing link in ecological speciation. Science. 2009;326:847–850. doi: 10.1126/science.1179141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan J, et al. SilkDB v2.0: A platform for silkworm (Bombyx mori) genome biology. Nucleic Acids Res. 2010;38(Database issue):D453–D456. doi: 10.1093/nar/gkp801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beltran M, Jiggins CD, Brower AVZ, Bermingham E, Mallet J. Do pollen feeding, pupal-mating and larval gregariousness have a single origin in Heliconius butterflies? Inferences from multilocus DNA sequence data. Biol J Linn Soc Lond. 2007;92:221–239. [Google Scholar]
- 48.Pohl N, Sison-Mangus MP, Yee EN, Liswi SW, Briscoe AD. Impact of duplicate gene copies on phylogenetic analysis and divergence time estimates in butterflies. BMC Evol Biol. 2009;9:99. doi: 10.1186/1471-2148-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiggins CD, McMillan WO, Neukirchen W, Mallet J. What can hybrid zones tell us about speciation? The case of Heliconius erato and H. himera (Lepidoptera:Nymphalidae) Biol J Linn Soc Lond. 1996;59:221–242. [Google Scholar]
- 50.Mallet J. In: Hybrid Zones and the Evolutionary Process. Harrison RG, editor. New York, NY: Oxford University Press; 1993. pp. 226–260. [Google Scholar]
- 51.Mallet J, Gilbert LE. Why are there so many mimicry rings—Correlations between habitat, behavior and mimicry in Heliconius butterflies. Biol J Linn Soc Lond. 1995;55:159–180. [Google Scholar]
- 52.Mallet J, et al. Estimates of selection and gene flow from measures of cline width and linkage disequilibrium in heliconius hybrid zones. Genetics. 1990;124:921–936. doi: 10.1093/genetics/124.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kronforst MR, Young LG, Gilbert LE. Reinforcement of mate preference among hybridizing Heliconius butterflies. J Evol Biol. 2007;20:278–285. doi: 10.1111/j.1420-9101.2006.01198.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.