Abstract
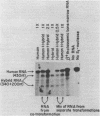
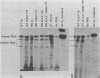
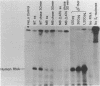
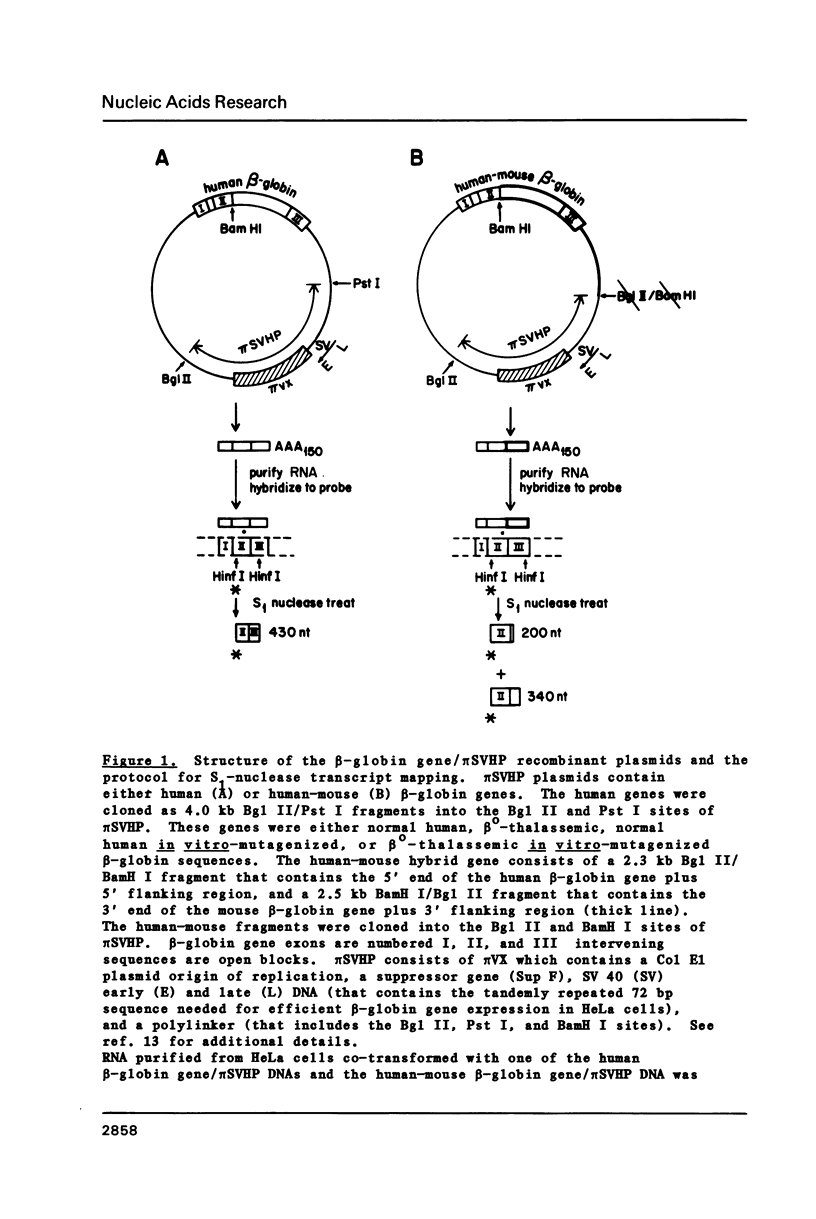
We have shown previously that a beta-globin RNA-deficient beta zero-thalassemia is caused by a single base-pair deletion in codon 44 of the human beta-globin gene1. The lack of beta-globin RNA in erythroid cells of these affected individuals is due to extreme beta-globin RNA instability (t 1/2 = 30 min)2. We have further investigated the mechanism of this extreme lability by transiently expressing the beta zero-thalassemic allele in HeLa cells and assaying the stability of the beta-globin RNA that is produced. Surprisingly, the beta zero-thalassemic RNA is much more stable in HeLa cells than in bone marrow cells. Apparently, developing erythroid cells have a mechanism for turning over this thalassemic RNA while cervical carcinoma cells do not. We also have assayed the stability of RNA derived from in vitro-mutagenized beta-globin genes. In HeLa cells, beta-globin RNAs harboring deletions in and around the translation initiation codon accumulate to steady-state levels that are similar to the level of normal beta-globin RNA.
Full text
PDF



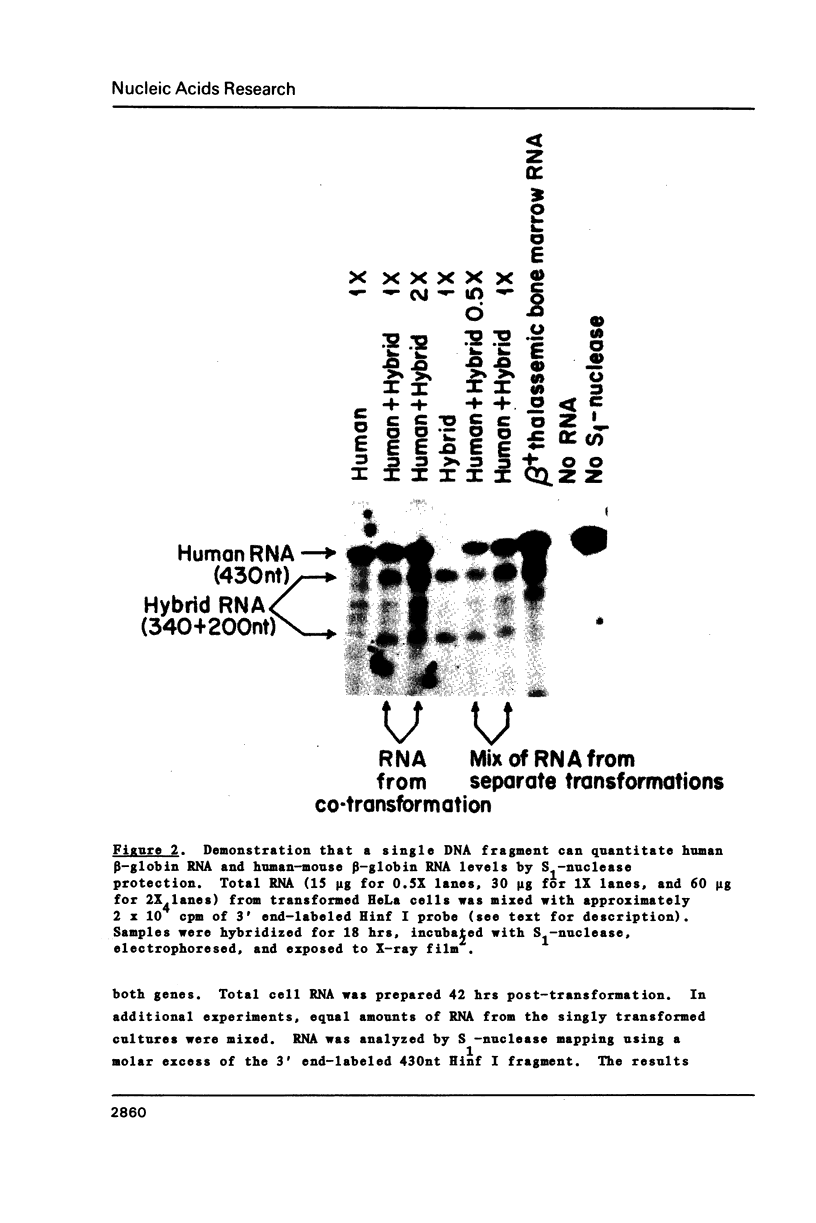


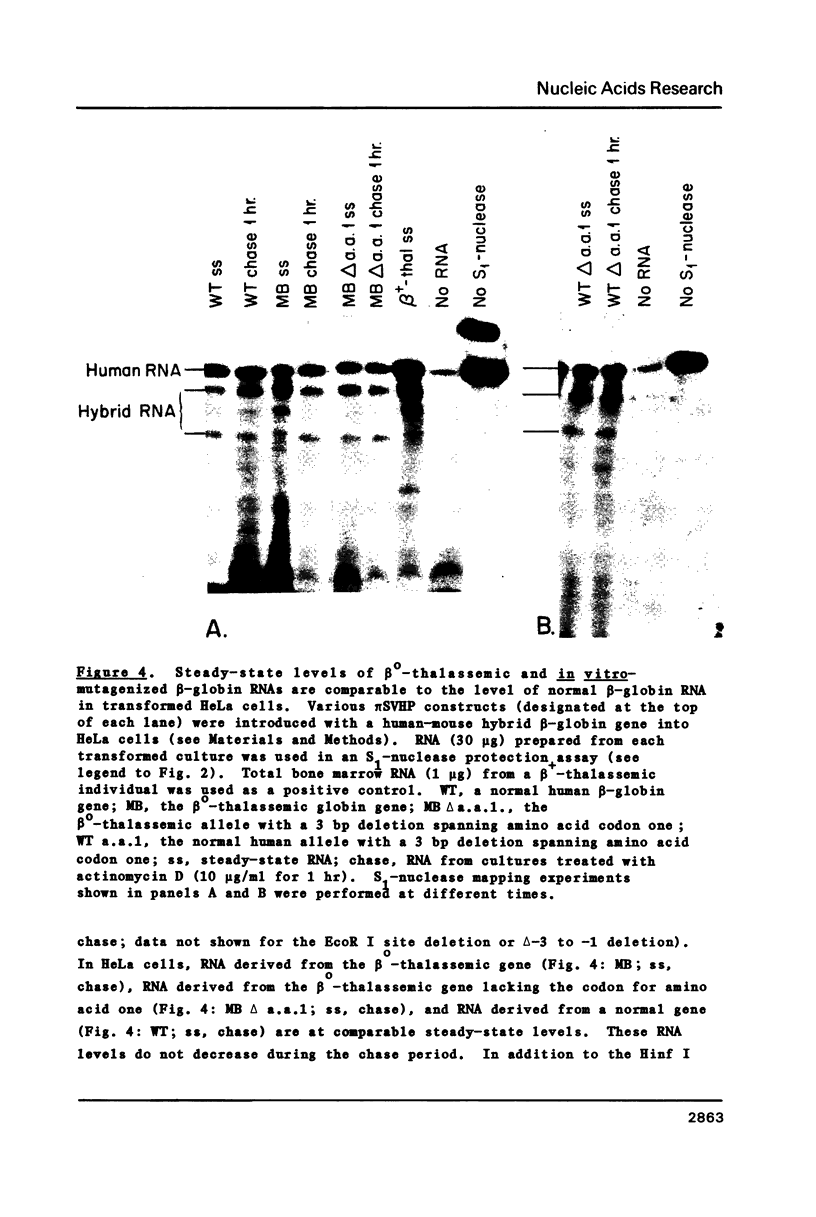
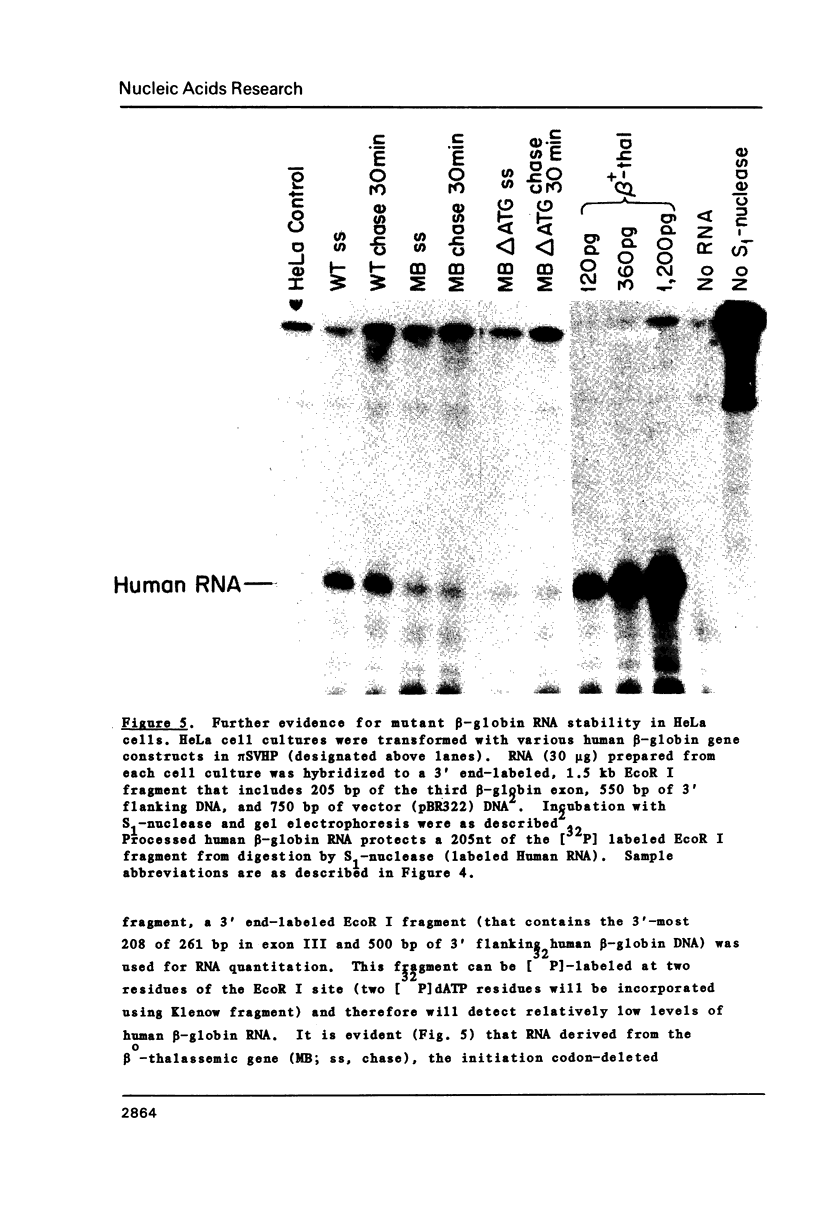



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N. A., Robert B., Jacquet M., Buckingham M. E., Gros F. Changes in gene expression during myogenic differentiation. I. Regulation of messenger RNA sequences expressed during myotube formation. J Mol Biol. 1980 Jul 15;140(4):441–458. doi: 10.1016/0022-2836(80)90264-8. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. E., Caput D., Cohen A., Whalen R. G., Gros F. The synthesis and stability of cytoplasmic messenger RNA during myoblast differentiation in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1466–1470. doi: 10.1073/pnas.71.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Wilson M. C., Darnell J. E., Jr Chinese hamster polyadenylated messenger ribonucleic acid: relationship to non-polyadenylated sequences and relative conservation during messenger ribonucleic acid processing. Mol Cell Biol. 1981 Feb;1(2):188–198. doi: 10.1128/mcb.1.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinniburgh A. J., Maquat L. E., Schedl T., Rachmilewitz E., Ross J. mRNA-deficient beta o-thalassemia results from a single nucleotide deletion. Nucleic Acids Res. 1982 Sep 25;10(18):5421–5427. doi: 10.1093/nar/10.18.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Ceccarelli A., Lodish H. F. Cyclic AMP stabilizes a class of developmentally regulated Dictyostelium discoideum mRNAs. Nature. 1983 Feb 17;301(5901):616–618. doi: 10.1038/301616a0. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Beach L. R., Honig G. R., Lazerson J., Ershler W. B., Ross J. Processing of human beta-globin mRNA precursor to mRNA is defective in three patients with beta+-thalassemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4287–4291. doi: 10.1073/pnas.77.7.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Rachmilewitz E. A., Ross J. Unstable beta-globin mRNA in mRNA-deficient beta o thalassemia. Cell. 1981 Dec;27(3 Pt 2):543–553. doi: 10.1016/0092-8674(81)90396-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moschonas N., de Boer E., Grosveld F. G., Dahl H. H., Wright S., Shewmaker C. K., Flavell R. A. Structure and expression of a cloned beta o thalassaemic globin gene. Nucleic Acids Res. 1981 Sep 11;9(17):4391–4401. doi: 10.1093/nar/9.17.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Puckett L., Darnell J. E. Essential factors in the kinetic analysis of RNA synthesis in HeLa cells. J Cell Physiol. 1977 Mar;90(3):521–534. doi: 10.1002/jcp.1040900315. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Volloch V., Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981 Feb;23(2):509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]