Abstract
Increases in species diversity and density from higher to lower latitudes are well documented. Nevertheless, the consequences of these changes in diversity for structuring ecological communities and influencing biotic evolution are largely unknown. It is widely believed that this increase in species diversity is associated with increased intensity of ecological interactions closer to the equator. For plant–herbivore interactions in particular, the predictions are that, at lower latitudes, plants will be attacked by more individual herbivores, more herbivore species, and more specialized herbivores and, therefore, will suffer greater damage. We used a large-scale latitudinal transect from Mexico to Bolivia to quantify changes in leaf damage, diversity, and abundance of lepidopteran larvae on two widely distributed host species of the genus Piper (Piperaceae). We show that both density and species richness of herbivores were highest at the equator and decreased with increasing latitude, both northward and southward. Contrary to expectation, however, this increase in herbivore diversity was attributable to the addition of generalist not specialist species. Finally, and again contrary to expectation, the increase in herbivore density with decreasing latitude did not produce a corresponding damage gradient. We propose that the lack of a latitudinal concordance between increases in herbivore density and diversity with decreasing latitude, and the resulting herbivore damage, supports the hypothesis of better plant antiherbivore defenses at lower latitudes. Furthermore, the changes in the relative abundance of generalist vs. specialist species suggest that the nature of the selective pressure is intrinsically different between higher and lower latitudes.
Keywords: herbivory, latitudinal gradient, Lepidoptera
The vast body of qualitative and quantitative evidence on changes in species diversity across the latitudinal gradient has raised much speculation on the possible ecological and evolutionary consequences of this pattern. A longstanding hypothesis in community ecology predicts that, as species diversity and species abundance increase, the intensity and frequency of ecological interactions between species increase (1). Therefore, competition, predation, and parasitism are all thought to be more intense at lower latitudes (1, 2). Similarly, current theory predicts that this increase in species interactions at lower latitudes imposes strong natural selection for mechanisms that will reduce the intensity or frequency of negative interactions. As a result, species at lower latitudes are likely to have higher levels of specialization to lessen competition and greater defenses to reduce predation (2). For plant–herbivore interactions in particular, it is expected that at lower (tropical) latitudes, abundance and species richness of insect herbivores will be higher, and, therefore, lower latitudes will also have more specialized herbivores and plants will suffer greater biomass loss to herbivory (3–5). Consequently, this herbivore species latitudinal gradient is likely to impose stronger herbivore pressure on plants at lower latitudes (6–8).
Herbivore pressure can be defined as the strength of natural selection imposed by herbivores for plant phenotypes that have greater fitness in the presence of those herbivores. Herbivore pressure is expected to increase with greater tissue loss when damage reduces growth and reproduction (quantitatively) (9) but also with changes in the identity of the herbivore species (qualitatively) (7). Increased number of species attacking a given plant host should result in a qualitatively different set of selective pressures compared with attack by fewer species because each herbivore reacts idiosyncratically to the defensive arsenal of a plant and may, therefore, influence plant fitness in different ways (10–13). From the point of view of the plant, each combination of traits embodied by a specific herbivore species may represent an additional and potentially unique evolutionary hurdle that the plant may need to overcome and may be manifested as a tradeoff in defense allocation.
Furthermore, to accurately assess qualitative changes in herbivore pressure, it is not only critical to document the taxonomic identity of the herbivores but also to examine patterns of host use by those herbivores (e.g., whether the herbivores are specialists or generalists) (14). Because specialists are likely to be better-adapted to specific qualitative defense compound types such as alkaloids, and generalists are expected to be better equipped to overcome quantitative digestibility-reducing compounds like polyphenols, current theory predicts that specialist and generalist herbivores will exert different and perhaps contrasting selective pressures (14–16).
Herbivore pressure traditionally has been measured as the amount of tissue loss to herbivores, overlooking the identity of the herbivores causing that damage. We argue that neither by itself is sufficient to characterize the selection pressure imposed by herbivores; measuring total damage alone does not reveal the identity of the causative agents, and simply identifying the herbivore fauna is insufficient because total tissue loss is not always related to the number of insect species causing that damage (17). Moreover, different herbivore species often create different patterns of damage because of species-specific differences in size, phenology, and feeding behaviors, resulting in differential effects on growth and reproduction (18, 19).
Despite an abundance of theoretical work, few studies have systematically explored the consequences of the latitudinal diversity gradient on plant–herbivore interactions and none has quantified both quantitative and qualitative changes in herbivore pressure within the tropical realm (6). Finally, no studies of terrestrial systems have included sites from both sides of the equator (20).
Here, we used a large-scale latitudinal transect from Mexico to Bolivia to quantify changes in diversity and abundance of lepidopteran larvae on two widely distributed host species, Piper aduncum and Piper aequale (a pioneer and a primary forest species respectively). Piper (Piperaceae) is a genus of shrubs, vines, and small trees represented by some 1,500 species in the New World (21). Piper represents a common and diverse component of Neotropical wet forest understories, with up to 64 species in a single lowland forest location (22).
Each host-plant species was sampled in five locations distributed along the latitudinal gradient (Los Tuxtlas, Mexico, 18° north; La Tirimbina, Costa Rica, 10° north; Jatun Sacha, Ecuador, 0°; Yanamono, Peru, 3° south; Madidi, Bolivia, 15° south). Herbivores were collected, reared to adults, and then classified into three categories depending on their diet: true specialists, herbivore species that feed on only one host plant species (in this case P. aduncum or P. aequale); genus specialists, herbivores that feed on two or more plant species from the genus Piper; and generalists, herbivores that feed on two or more host species from different plant families. In addition, we assessed the amount of leaf area removed by herbivores on each sampled Piper plant. Finally, we quantified their average leaf toughness as a measure of leaf mechanical defenses.
Results and Discussion
We found that total herbivore species richness per square meter of foliage sampled increased significantly with decreasing latitude (F = 12.81, P < 0.0001 for P. aequale; F = 8.35, P = 0.0001 for P. aduncum) (Fig. 1 and Table S1). Additionally, herbivore diversity was additive approaching the equator, because most species of herbivores found at higher latitudes were also found at lower latitude sites but not vice versa. Thus, patterns of increasing insect herbivore species at lower latitude, documented previously at the community level (4), are mirrored by those at the individual plant level for these two species of Piper.
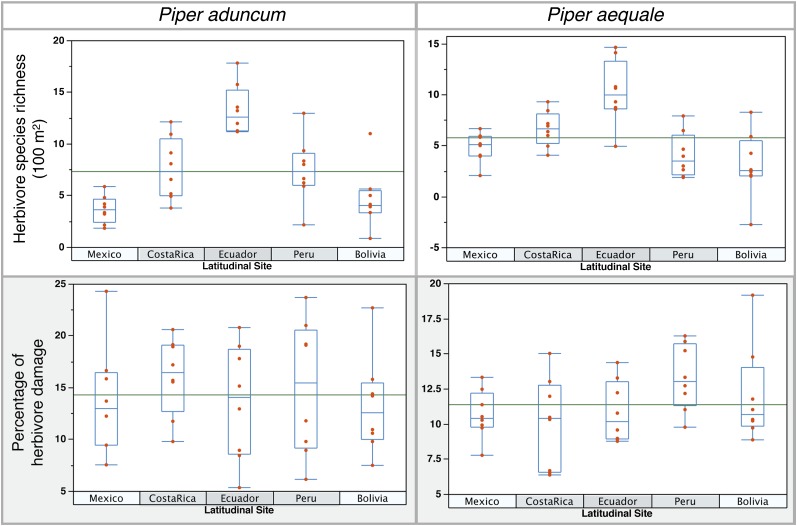
Fig. 1.
Total herbivore species richness and percentage of leaf herbivore damage per latitudinal site of Piper aduncum and P. aequale. Species richness was measured as the number of species of lepidopteran larvae present per 100-m2 leaf area. Leaf herbivory was measured as the percentage of leaf area removed by lepidopteran herbivores. Points in the graph represent each of the eight sampled populations per latitudinal site.
The three diet categories of herbivores showed different latitudinal patterns in species richness. Contrary to expectation, categories with a wider taxonomical diet breadth had a higher rate of increase toward the equator than did specialists (Fig. S1 and Table S1). True specialist herbivore species richness did not change with latitude. Only one true specialist herbivore species (Eois; Geometridae) was found per host plant and both of these herbivore species were found at all sites. Genus specialists increased with decreasing latitude; however, most differences in total species richness between sites were attributable to an increase in generalist herbivore diversity between the high-latitude sites (Mexico and Bolivia) and Ecuador. Notably, the gradients of herbivore richness held true for both sides of the equator (Fig. 2).
Fig. 2.
Herbivore species richness and abundance per square meter of leaf area at each latitudinal site. Herbivores are subdivided by diet breadth. Species richness was measured as the total number of species of lepidopteran herbivores. Herbivore abundance was measured as the total number individuals per 100-m2 leaf area at each latitudinal site.
Density of lepidopteran larvae per square meter of leaf area increased with decreasing latitude (F = 4.76 and P = 0.0046 for P. aduncum; F = 9.17 and P < 0.0001 for P. aequale). This latitudinal trend held true for all categories of herbivores except the P. aequale specialist, which showed a nonsignificant decrease in density at lower latitudes (Fig. 2, Fig. S2, and Table S1). Total herbivore density was 200% higher at the equator for P. aequale and 400% higher for P. aduncum compared with the high-latitude sites. Our data concur with a recent study of temperate saltmarsh plants that showed an increase of one to two species of herbivores with decreasing latitude, and this increase was also attributed to generalists (7).
With the exception of the specialist herbivores of P. aequale, the three diet categories of herbivores showed a pattern of increased species densities at lower latitudes. For both Piper species, genus specialists and generalist herbivores showed a significant increase in species densities at lower latitudes (Table S1). However, in contrast to the pattern found for species richness, genus specialists showed the greatest increase in species density with decreasing latitude (Fig. 2). All gradients of herbivore abundance also held true for both sides of the equator (Fig. 2). These strong latitudinal patterns of herbivore numbers, herbivore diversity, and changes in the average diet breadth of the herbivore community (specialist vs. generalists) are consistent with the existence of stronger qualitative herbivore pressure at lower latitudes within the tropical realm.
To test the general relationship between herbivore abundance and leaf damage, we first analyzed the data independently of latitude (pooling together all data from all populations). After a correlation analysis, we found that herbivore abundance significantly explained 37% of the variation in herbivore damage for P. aduncum (n = 40; P = 0.0001) and 14% for P. aequale (n = 40; P = 0.019). Furthermore, when analyzed independently, each one of our latitudinal sites also showed a significant positive relationship between herbivore density and herbivore damage. Nevertheless, sites at lower latitudes had smaller regression slopes than sites at higher latitudes (Fig. S3 and Table S2). An identical pattern was found for herbivore diversity (Fig. S3 and Table S2). However, we did not find significant differences in herbivore damage per site for either Piper species hosts, despite the changes in herbivore richness and density across the latitudinal gradient (F = 0.33 and P = 0.85 for P. aduncum; F = 1.48 and P = 0.23 for P. aequale; Figs. 1 and 3 and Table S1). This lack of latitudinal trend in damage, coupled with greater density of herbivores at lower latitudes, suggests that the contribution each individual herbivore made to total leaf area damage was smaller at lower latitudes.
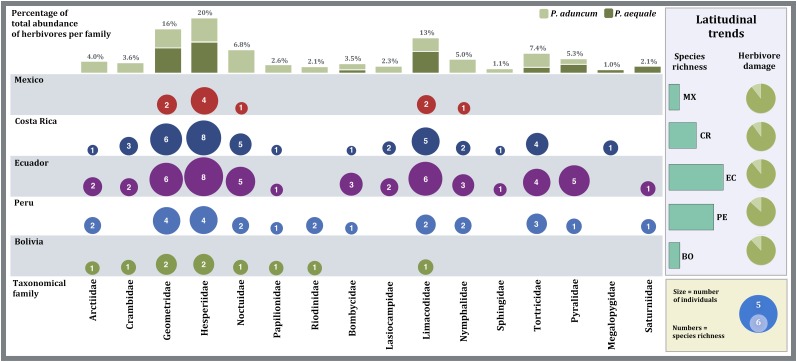
Fig. 3.
Visualization of the latitudinal changes in species diversity of the most common lepidopteran herbivores found feeding on P. aduncum and P. aequale. Bubble size represents the abundance of herbivores of a particular family at each latitudinal site (hebivore individuals per 100 m2 of leaf area). Numbers inside bubbles are the number of species of a particular family at each site. Vertical bars above represent the percentage of the total abundance of herbivores accounted for by each taxonomic family per plant host (light green: P. aduncum; dark green: P. aequale). In the “latitudinal trends” box, horizontal bars represent changes in total herbivore species richness across the latitudinal gradient. The pie charts show the mean percentage of herbivore damage at each latitudinal site (dark green: undamaged leaf area; light green: leaf area removed by lepidopteran herbivore).
Although several studies have shown differences in herbivore leaf damage between high and low latitudes (6), most report small differences, between 0.5 and 7 percentage points. These previous comparisons have been made without controlling for herbivore or plant taxa, leaving uncertain the relative importance of site (latitude) vs. phylogeny for damage estimates. In addition, a recent metaanalysis (20) found no significant relationship between latitude and herbivore damage for 38 latitudinal comparisons. When we controlled for phylogeny (by using the same widespread species of host plants), we found no differences in damage levels either north or south of the equator (Fig. 1).
Despite the fact that our data show no significant changes in quantitative herbivore pressure (leaf damage), it is likely that the higher herbivore species richness and the additional number of taxonomic families of lepidopteran herbivores found at lower latitudes (Fig. 3 and Tables S1 and S3) translate into greater qualitative herbivore pressure closer to the equator. The distinct evolutionary makeup of each additional herbivore lineage is likely to influence the response of that particular taxon to the mechanical and chemical defenses of its host (11). Piper aequale in Ecuador was attacked by caterpillars from four families not found on that plant species in either Mexico or Bolivia, and P. aduncum by seven additional families (Fig. 3 and Table S3). Furthermore, the increment in the abundance and diversity of generalist species suggests that the nature and evolutionary direction of the herbivore pressure at lower latitudes is significantly different from that at higher latitudes. Within this context, it is possible that the additional herbivore pressure imposed by generalists could increase the relative abundance, diversity, and efficiency of qualitative defense compound types such as piperamides (23) at lower latitudes. If this holds true, this could help explain the reduction of the relative effect that herbivores have on leaf damage at lower latitudes found in this study.
We argue that the lack of concordance between the latitudinal changes in herbivore density and diversity and the resulting herbivore damage supports the long-standing hypothesis of better plant antiherbivore defenses at lower latitudes (1, 6, 8, 24). These changes in palatability could be the result of latitudinal variations in leaf quality (6), or specifically, local plant host adaptation in terms of (i) higher concentration of secondary compounds, (ii) locally endemic secondary chemicals, (iii) increased indirect defenses (e.g., ant-plant mutualisms), or (iv) greater structural defenses. Average leaf toughness also did not differ among sites, suggesting that possible differences in leaf palatability are likely associated with leaf chemistry (P. aduncum: F = 1.1417, P = 0.3531; P. aequale: F = 0.2247, P = 0.92; Fig. S4). Nevertheless, changes in leaf palatability alone do not successfully explain the apparent paradox of higher herbivore abundances without higher leaf damage.
A potential explanation for this paradox is a decrease in the herbivore-feeding performance (25, 26) across the latitudinal gradient attributable to higher parasitism rates. Previous attempts to find latitudinal gradients in herbivore parasitism have met with mixed results (2). In our study, percentage of parasitism of reared caterpillars increased from 3.20% in Mexico to 8.05% in Costa Rica, and then again to 9.93% in Ecuador (n = 125, 298, and 292, respectively), lending support to this hypothesis. Another possible explanation is a latitudinal increase in host-plant leaf turnover. With a higher per plant leaf turnover at lower latitudes, the observed estimates of herbivore damage would underestimate the actual amount of leaf area removed per unit time. However, recent studies have shown that when leaf longevity is taken in account when comparing herbivory rates between low and high latitudes, leaf damage is equal or more intense at higher latitudes (27–29). Finally, this pattern could be the result of reduced body size and, therefore, reduced lifetime leaf tissue consumption per herbivore, at lower latitudes (e.g., Bergmann’s rule) (30, 31).
Contrary to expectations, our findings do not support the hypothesis that a particular host-plant species will have a more abundant or a more diverse array of specialist herbivores at lower latitudes. Instead, we found that increases in herbivore species richness at lower latitudes were attributable to the addition of generalist herbivore species. It is likely that as plant species diversity increases toward the equator, the relative abundance of any given host plant decreases. This scenario will likely make it difficult for specialist herbivores to find their specific host, while giving an ecological and evolutionary advantage to an herbivorous insect with a generalized diet (32). Nevertheless, we recognize the existence of an evolutionary conundrum for herbivores at lower latitudes. Although increasing host-plant diversity likely makes a generalist diet beneficial, the increasing diversity and abundance of herbivores will generate strong selection for narrower diet breadths to reduce intraguild competition.
Although we found that generalist and not specialist herbivores contributed to higher herbivore species richness at lower latitudes, we cannot draw any clear conclusions about possible patterns of herbivore specialization across the latitudinal gradient (3, 5). Our study was not designed to exhaustively measure herbivore diet breadth. Thus, it is possible that generalist herbivores are more “specialized” at lower latitudes in terms of the number of host plant species on which they feed (3). We did find a significant correlation between richness of genus specialist species and the number of local Piper species (r2 = 0.7; P < 0.002), lending support to the hypothesis that latitudinal changes in herbivore species richness mirror changes in plant host diversity (5).
We acknowledge that any changes in plant–herbivore interactions across the latitudinal gradient are likely attributable to multiple evolutionary and ecological factors, including changes in predator abundance (33) and in the surrounding plant community structure (32, 34). However, our analysis is based on herbivores found feeding on their host. Thus, processes that could change the encounter rate between herbivore and host will not have a major effect on our results.
Conclusions
Overall, we found support for the hypothesis that plants suffer greater herbivore pressure at lower latitudes. Despite the lack of significant differences in quantitative herbivore pressure across latitudinal sites, the increase in herbivore diversity and abundance are likely to generate a comparable increase in the selective pressure that herbivores inflict on their host plants. Furthermore, changes in the relative abundance of generalist vs. specialist herbivores suggest that the selective pressure imposed by insect herbivores on plants is likely to be significantly different between higher and lower latitudes.
Methods
Data Collection.
At each site, we selected eight populations of each Piper species, at least 5 km apart. (See SI Text for description of the field sites and target Piper species; Fig. S5.) At each population, we randomly choose 20 adult plants (reproductive), all of similar size (no smaller than 1.5 m tall). Every leaf and every branch of each plant were carefully searched for lepidopteron larvae. When a larva was found, it was collected, identified, and cataloged (SI Text). All herbivore specimens were photographed to compare the species between populations and sites (photographs of herbivores are available upon request). Additionally, all other Piper and most common non-Piper species at each site were also explored for herbivores to assist in the determination of diet breadth of the herbivores feeding on P. aduncum and P. aequale. To standardize herbivore abundances and species diversities, we counted the total number of leaves present on each Piper plant of the herbivore census. Finally, we also assessed the average leaf area of each Piper population.
Herbivore Data.
All herbivores collected during the census were placed in plastic bags (30 × 30 cm) with fresh plant material from either P. aduncum or P. aequale. The bags were placed in improvised rearing facilities at ambient temperature and protected from direct sunlight. Bags were checked daily to remove frass and to add new leaf material as needed. All bags with pupated larvae were marked and followed. Once emerged, butterflies and moths were photographed for later identification.
Herbivore diet breadth was assessed using three main strategies. First, if specimens of herbivore species were abundant at the site, no-choice feeding trials were implemented in the field. Herbivores were placed in plastic bags with the two most abundant Piper species (excluding the two target species) and the two most abundant non-Piper plant species at the site. Successful feeding was recorded when herbivores fed on the new host as larvae completed pupation. Secondly, general herbivore censuses were carried out with 30-m transects along trails at each latitudinal site. No less than seven transects were performed at each site. At each transect, all plants within 3 m of the trail were searched and all caterpillars found were collected and compared with the one found feeding from the two target species. Finally, to confirm the results of the two previous strategies and to assess the diet breadth of the abundant caterpillar herbivores, we carried out an extensive literature and database review (SI Text).
Plant Data.
Herbivore damage on the two Piper species was assessed visually. To reduce possible error on the assessment of herbivory, a training period was carried out before the first herbivore census. Visual assessments of herbivory followed by actual measurements of leaf area (using image processing and analysis software, ImageJ; http://rsbweb.nih.gov/ij/) allowed us to compare, correct, and standardize visual assessments. Additionally, extensive research has been done on the patterns of herbivore damage on Piper species (35). This research allowed us to discriminate most of the herbivore leaf damage caused by lepidopteran herbivores from the damage caused by nonlepidopteran herbivores and leaf pathogens. All herbivory estimates on this study are limited to damage caused by lepidopteran larvae, as much as possible (Fig. S6).
To assess changes in leaf toughness between Piper populations and latitudinal sites, we randomly collected 30 fully expanded leaves of both Piper species from every population. Only fully expanded and the most distal leaves on a growing branch were assessed. To estimate toughness, we used a Wagner Force Dial (FDK 32; Wagner Instruments) to measure the grams of force needed to pierce a 0.5-cm-diameter hole at the center of the leaf but never through a primary or secondary vein. Parasitism levels of caterpillars at the different latitudinal sites were estimated by calculating the percentage of parasitism found across all larval rearing at each improvised rearing laboratory. Parasitism was confirmed by the presence of at least one larva, pupa, or adult parasitoid inside the rearing bag. Dead caterpillars without signs of parasitism were left in their rearing bags for 8 d to allow possible parasitoids to emerge. If no parasitoid emerged before the eighth day, the caterpillar was considered to be free of parasites.
Statistical Analysis.
We used one-way ANOVAs to compare herbivore species abundances, species richness, herbivore damage, and leaf toughness among all sites. To contrast the effect of herbivore density and herbivore species richness on leaf herbivory among the latitudinal sites we used linear regression through the origin.
Full methods and any associated references are available in SI Text.
Supplementary Material
Acknowledgments
We thank K. Boege, P. Diaz, A. Fuentes, Diego Santiago-Alarcon, Hidalgo-Miño, Estación de Biología Los Tuxtlas (Universidad Nacional Autónoma de México), Tirimbina, Jatun Sacha, Chalalan, MEXU (UNAM), LPB (Universidad Mayor de San Andrés), QCNE, and USJ (Universidad de Costa Rica) for all their help; and the R.J.M. laboratory, John T. Lill, and two anonymous reviewers for helpful comments on earlier versions. Financial support was provided by the Jane and Stanley Birge Tropical Research Scholarship, the Stephen Doyle Memorial Scholarship, the Christensen Fund Graduate Fellowship for Plant Conservation, the Whitney R. Harris World Ecology Center, National Science Foundation Grant DEB0743457 (to P. M. Jørgensen), the Missouri Research Board (E. A. Kellogg), and the Organization for Tropical Studies for a pilot data grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202907109/-/DCSupplemental.
References
- 1.Dobzhansky T. Evolution in the tropics. Am Sci. 1950;38:209–221. [Google Scholar]
- 2.Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. Is there a latitudinal gradient in the importance of biotic interactions? Annu Rev Ecol Evol Syst. 2009;40:245–269. [Google Scholar]
- 3.Dyer LA, et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature. 2007;448:696–699. doi: 10.1038/nature05884. [DOI] [PubMed] [Google Scholar]
- 4.Hillebrand H. On the generality of the latitudinal diversity gradient. Am Nat. 2004;163:192–211. doi: 10.1086/381004. [DOI] [PubMed] [Google Scholar]
- 5.Novotny V, et al. Why are there so many species of herbivorous insects in tropical rainforests? Science. 2006;313:1115–1118. doi: 10.1126/science.1129237. [DOI] [PubMed] [Google Scholar]
- 6.Coley PD, Barone JA. Herbivory and plant defenses in tropical forests. Annu Rev Ecol Syst. 1996;27:305–335. [Google Scholar]
- 7.Pennings SC, et al. Latitudinal variation in herbivore pressure in Atlantic Coast salt marshes. Ecology. 2009;90:183–195. doi: 10.1890/08-0222.1. [DOI] [PubMed] [Google Scholar]
- 8.Pennings SC, Silliman BR. Linking biogeography and community ecology: Latitudinal variation in plant-herbivore interaction strength. Ecology. 2005;86:2310–2319. [Google Scholar]
- 9.Marquis RJ. Leaf herbivores decrease fitness of a tropical plant. Science. 1984;226:537–539. doi: 10.1126/science.226.4674.537. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal AA. Induced responses to herbivory in wild radish: Effects on several herbivores and plant fitness. Ecology. 1999;80:1713–1723. [Google Scholar]
- 11.Opitz SEW, Muller C. Plant chemistry and insect sequestration. Chemoecology. 2009;19:117–154. [Google Scholar]
- 12.Karban R, Agrawal AA. Herbivore offense. Annu Rev Ecol Syst. 2002;33:641–664. [Google Scholar]
- 13.Muola A, et al. Associations of plant fitness, leaf chemistry, and damage suggest selection mosaic in plant-herbivore interactions. Ecology. 2010;91:2650–2659. doi: 10.1890/09-0589.1. [DOI] [PubMed] [Google Scholar]
- 14.Orians CM, Ward D. Evolution of plant defenses in nonindigenous environments. Annu Rev Entomol. 2010;55:439–459. doi: 10.1146/annurev-ento-112408-085333. [DOI] [PubMed] [Google Scholar]
- 15.Leimu R, Riipi M, Stærk D. Food preference and performance of the larvae of a specialist herbivore: Variation among and within host-plant populations. Acta Oecol. 2005;28:325–330. [Google Scholar]
- 16.Mathur V, et al. Temporal dynamics of herbivore-induced responses in Brassica juncea and their effect on generalist and specialist herbivores. Entomol Exp Appl. 2011;139:215–225. [Google Scholar]
- 17.Marquis RJ. Herbivore fauna of Piper (Piperaceae) in a Costa Rican wet forest: diversity, specificity and impact. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW, editors. Plant-Animal Interactions, Evolutionary Ecology in Tropical and Temperate Regions. New York: J. Wiley and Sons; 1991. pp. 179–208. [Google Scholar]
- 18.Marquis RJ. A bite is a bite is a bite? Constraints on response to folivory in Piper arieianum (Piperaceae) Ecology. 1992;73:143–152. [Google Scholar]
- 19.Manzaneda AJ, Prasad KVSK, Mitchell-Olds T. Variation and fitness costs for tolerance to different types of herbivore damage in Boechera stricta genotypes with contrasting glucosinolate structures. New Phytol. 2010;188:464–477. doi: 10.1111/j.1469-8137.2010.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moles AT, Bonser SP, Poore AG, Wallis IR, Foley WJ. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Funct Ecol. 2011;25:380–388. [Google Scholar]
- 21.Jaramillo MA, et al. A phylogeny of the tropical genus Piper using ITS and the chloroplast intron psbJ-petA. Syst Bot. 2008;33:647–660. [Google Scholar]
- 22.Marquis RJ. Biogeography of Neotropical Piper. In: Dyer LA, Palmer DN, editors. Piper: A Model Genus for Studies of Phytochemistry, Ecology, and Evolution. New York, USA: Kluwer Academic/Plenum Publishers; 2004. pp. 78–96. [Google Scholar]
- 23.Dyer LA, et al. Ecological causes and consequences of variation in defensive chemistry of a Neotropical shrub. Ecology. 2004;85:2795–2803. [Google Scholar]
- 24.Rasmann S, Agrawal AA. Latitudinal patterns in plant defense: Evolution of cardenolides, their toxicity and induction following herbivory. Ecol Lett. 2011;14:476–483. doi: 10.1111/j.1461-0248.2011.01609.x. [DOI] [PubMed] [Google Scholar]
- 25.Ammunét T, Klemola N, Heisswolf A, Klemola T. Larval parasitism of the autumnal moth reduces feeding intensity on the mountain birch. Oecologia. 2009;159:539–547. doi: 10.1007/s00442-008-1240-6. [DOI] [PubMed] [Google Scholar]
- 26.Duodu YA, Antoh FF. Effects of parasitism by Apanteles sagax [Hym.: Braconidae] on growth, food consumption and food utilization in Sylepta derogata larvae. Entomophaga. 1984;29:63–71. [Google Scholar]
- 27.Adams JM, Zhang Y. Is there more insect folivory in warmer temperate climates? A latitudinal comparison of insect folivory in eastern North America. J Ecol. 2009;97:933–940. [Google Scholar]
- 28.Adams JM, Zhang Y, Basri M, Shukor N. Do tropical forest leaves suffer more insect herbivory? A comparison of tropical versus temperate herbivory, estimated from leaf litter. Ecol Res. 2009;24:1381–1392. [Google Scholar]
- 29.Andrew NR, Hughes L. Herbivore damage along a latitudinal gradient: Relative impacts of different feeding guilds. Oikos. 2005;108:176–182. [Google Scholar]
- 30.Chown SL, Gaston KJ. Body size variation in insects: A macroecological perspective. Biol Rev Camb Philos Soc. 2010;85:139–169. doi: 10.1111/j.1469-185X.2009.00097.x. [DOI] [PubMed] [Google Scholar]
- 31.Kivelä SM, Välimäki P, Carrasco D, Mäenpää MI, Oksanen J. Latitudinal insect body size clines revisited: A critical evaluation of the saw-tooth model. J Anim Ecol. 2011;80:1184–1195. doi: 10.1111/j.1365-2656.2011.01864.x. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal AA, Lau JA, Hambäck PA. Community heterogeneity and the evolution of interactions between plants and insect herbivores. Q Rev Biol. 2006;81:349–376. doi: 10.1086/511529. [DOI] [PubMed] [Google Scholar]
- 33.Björkman C, Berggren A, Bylund H. Causes behind insect folivory patterns in latitudinal gradients. J Ecol. 2011;99:367–369. doi: 10.1111/j.1365-2745.2010.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Root RB. Organization of a Plant-Arthropod Association in Simple and Diverse Habitats - Fauna of Collards (Brassica oleracea) Ecol Monogr. 1973;43:95–120. [Google Scholar]
- 35.Dyer LA, Letourneau DK, Chavarria GV, Amoretti DS. Herbivores on a dominant understory shrub increase local plant diversity in rain forest communities. Ecology. 2010;91:3707–3718. doi: 10.1890/08-1634.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.