Abstract
Classical cadherins are transmembrane proteins at the core of intercellular adhesion complexes in cohesive metazoan tissues. The extracellular domain of classical cadherins forms intercellular bonds with cadherins on neighboring cells, whereas the cytoplasmic domain recruits catenins, which in turn associate with additional cytoskeleton binding and regulatory proteins. Cadherin/catenin complexes are hypothesized to play a role in the transduction of mechanical forces that shape cells and tissues during development, regeneration, and disease. Whether mechanical forces are transduced directly through cadherins is unknown. To address this question, we used a Förster resonance energy transfer (FRET)-based molecular tension sensor to test the origin and magnitude of tensile forces transmitted through the cytoplasmic domain of E-cadherin in epithelial cells. We show that the actomyosin cytoskeleton exerts pN-tensile force on E-cadherin, and that this tension requires the catenin-binding domain of E-cadherin and αE-catenin. Surprisingly, the actomyosin cytoskeleton constitutively exerts tension on E-cadherin at the plasma membrane regardless of whether or not E-cadherin is recruited to cell–cell contacts, although tension is further increased at cell–cell contacts when adhering cells are stretched. Our findings thus point to a constitutive role of E-cadherin in transducing mechanical forces between the actomyosin cytoskeleton and the plasma membrane, not only at cell–cell junctions but throughout the cell surface.
Keywords: mechanotransduction, mechanobiology, signal transduction, mechanosensor, morphogenesis
Embryonic development is fundamentally a mechanical process (1), as are the morphogenetic events that occur during tissue regeneration and diseases such as cancer. As tissues change shape, they generate mechanical forces that are propagated throughout the organism (2, 3). Ultimately, these forces may regulate genetic programs by activating transcription factors (4), thereby providing a role for mechanotransduction in a feedback loop between phenotype and genotype (3).
Recent studies have focused on measuring forces transmitted between cells in cohesive tissues and identifying the causes of those forces in relation to cell–cell and cell–matrix contact shape and size, and to cell migration (5–9). In cohesive tissues, cells adhere directly to their neighbors through intercellular adhesion complexes, and intercellular adhesion is dependent on the activity of the actomyosin cytoskeleton (10, 11), which provides a significant source of mechanical force within cells.
The classical cadherin/catenin complex is the best understood intercellular adhesion complex and is found in most metazoan cohesive tissues (12). Classical cadherins are transmembrane proteins that form intercellular bonds through interactions between their extracellular domains on apposed cells. The classical cadherin cytoplasmic domain binds β-catenin, which in turn binds α-catenin. Although it is generally thought that cadherins are physically connected to the cytoskeleton through catenins (13), the cadherin/catenin complex appears to bind poorly to actin in vitro (14, 15). Therefore, the cadherin/catenin/actin linkage may be highly dynamic, or its components may require a specific conformation not recapitulated in vitro to bind actin. This linkage may also involve additional actin-binding proteins such as activated vinculin or epithelial protein lost in neoplasm (EPLIN), which bind to α-catenin (16–21). In addition to actin, the classical cadherin/γ-catenin (plakoglobin) complex can also associate with intermediate filaments in some instances (22, 23).
Mechanical coupling between the plasma membrane and the cortical cytoskeleton involves the cadherin cytoplasmic domain (24). In addition, mechanical stimulation of cells through the extracellular domain of cadherins results in vinculin-dependent cell stiffening (25), and membrane protrusion reorientation via a mechanism involving γ-catenin and intermediate filaments (26). Conversely, actomyosin activity is proposed to induce conformational changes in α-catenin and the recruitment of vinculin to cell–cell contacts (27), which provides resistance to cell–cell contact disruption (28, 29). Moreover, cadherins can transmit cell-generated forces to the extracellular environment in a stiffness-dependent manner (30–33).
Taken together, these studies suggest that tension exerted across cell–cell contacts is transduced between the cadherin extracellular domain and the cytoskeleton through the cadherin/catenin complex, thus positioning this protein complex upstream of mechanotransduction pathways that remain to be elucidated. A condition for this model is that the cytoplasmic domain of cadherin directly transmits force between the cytoskeleton and the cell environment and therefore experiences variable tension depending on the activity of the cytoskeleton and/or external mechanical stimuli. However, current data equally support an alternate model by which the cytoplasmic domain of cadherin may simply recruit cytoskeletal regulatory proteins, while other membrane–cytoskeleton linker proteins mediate mechanotransduction. Therefore, we sought to directly test these possibilities by using a Förster resonance energy transfer (FRET)-based molecular tension sensor strategy (34). We inserted a pN-sensitive force sensor derived from spider silk (35, 36) in the cytoplasmic domain of E-cadherin to measure the tension experienced by the E-cadherin cytoplasmic domain at cell–cell contacts and at contact-free regions of the plasma membrane in Madin–Darby canine kidney (MDCK) epithelial cells. We then tested whether disruption of actomyosin activity, αE-catenin depletion, and externally applied mechanical stimuli affected the tension experienced by the cytoplasmic domain of E-cadherin. Our results provide direct evidence that mechanical forces are transduced through the cytoplasmic domain of E-cadherin and support a mechanosensory role of the cadherin/catenin complex not only at cell–cell contacts but also at contact-free plasma membrane throughout the cell.
Results
We hypothesized that if tension is exerted by the actomyosin cytoskeleton on E-cadherin, then it should be transmitted through the cytoplasmic domain (Fig. 1A). To directly measure tension, we generated a variant E-cadherin (EcadTSMod) that contained a tension sensor module (TSMod) (36) inserted in the cytoplasmic domain between the transmembrane domain and the catenin-binding domain (Fig. 1B; see SI Materials and Methods). This sensor allowed us to measure pN-range forces transmitted between the transmembrane domain and the catenin-binding domain by FRET microscopy (see SI Materials and Methods).
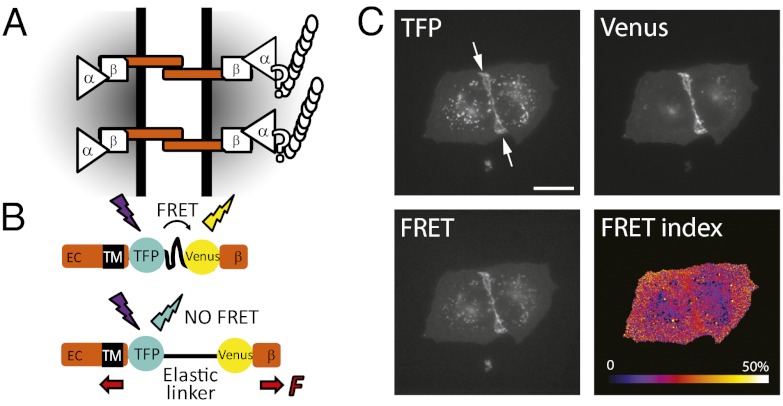
Fig. 1.
(A) Working model for mechanotransduction through the E-cadherin/catenin complex. E-cadherin transmits mechanical tension between cells via transinteracting extracellular (EC) domains and to the actin cytoskeleton through β-catenin, αΕ-catenin, and possibly other proteins. (B) The tension sensitive module (TSMod) consists of the mTFP/Venus FRET pair separated by an elastic linker (GPGGA)8 derived from spider silk. TSMod was inserted into the cytoplasmic domain of E-cadherin, where it can sense forces transmitted between the transmembrane domain (TM) and the β-catenin-binding domain (β). High and low FRET indices correspond to low and high tension, respectively. (C) Fluorescence imaging of two adherent MDCK cells expressing the EcadTSMod construct in the mTFP, Venus, and FRET (mTFP excitation; Venus emission) channels, and the corresponding map of FRET index = IFRET/(IFRET + ImTFP), where I is the fluorescence intensity of the subscript channel corrected for background and spectral bleed-through. (Scale bar: 20 μm.)
EcadTSMod localized prominently to the plasma membrane of MDCK cells and was recruited to cell–cell contacts, similar to the endogenous protein (Fig. 1C); EcadTSMod also labeled some endo-/exocytic vesicles and intracellular compartments, as already shown for E-cadherin-GFP (37). Importantly, EcadTSMod rescued cell–cell adhesion and plasma membrane recruitment of β-catenin and α-catenin in L fibroblasts (Fig. S1A) that normally lack cadherin expression (38). EcadTSModΔcyto, a control construct in which the β-catenin–binding domain was deleted, was also recruited to cell–cell contacts but did not recruit the catenins, as expected (Fig. S1B). Therefore, EcadTSMod recapitulated wild-type E-cadherin association with catenins and cell–cell adhesion function.
Next, we sought to assess whether EcadTSMod was under tension in MDCK cells. The intensities of the signals emitted from the TSMod fluorophores monomeric teal fluorescent protein (mTFP) and Venus were used to compute the FRET index, a measure of TSMod stretching and tension transmitted through the cytoplasmic domain of E-cadherin: the lower the FRET index, the higher the tension (Fig. 1 B and C; and SI Materials and Methods). We calibrated the FRET index to FRET efficiency by measuring the FRET index of the 229aa tumor necrosis factor receptor associated factor flanked with mTFP and Venus (mTFP-TRAF-Venus) and mTFP-5aa-Venus expressed in MDCK cells (Fig. S2 and SI Materials and Methods), two constructs with known FRET efficiencies (39). We used a previously published FRET efficiency–force calibration to infer molecular tension (36).
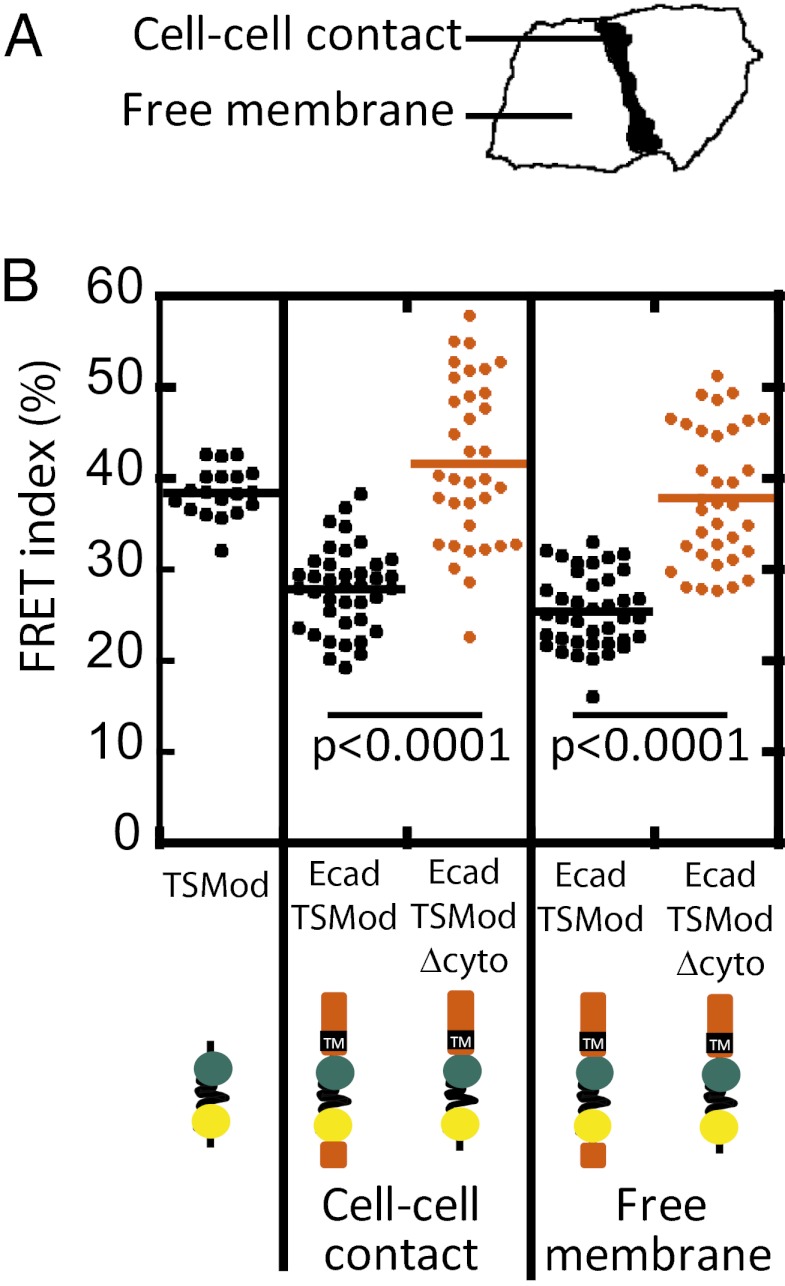
We measured FRET indices for TSMod (expressed in the cytoplasm), EcadTSMod, and EcadTSModΔcyto at cell–cell contacts and EcadTSMod and EcadTSModΔcyto at the contact-free plasma membrane (Fig. 2A). Within cell–cell contacts, the EcadTSMod FRET index was ∼30%, compared with ∼40% for either EcadTSModΔcyto or cytoplasmic TSMod (Fig. 2B). Therefore, EcadTSMod appeared to be under tension, and this tension required the catenin-binding domain of E-cadherin. Surprisingly, the EcadTSMod FRET index was lower than that of EcadTSModΔcyto at plasma membrane not in contact with other cells (Fig. 2B). Thus, E-cadherin appears to be under constitutive tension at the plasma membrane whether or not it is localized to cell–cell contacts.
Fig. 2.
(A) FRET index measured at cell–cell contacts and at the free membrane of expressing cells revealed that the EcadTSMod is under constitutive tension. (B) FRET index for TSMod in the cytoplasm, EcadTSMod and EcadTSModΔcyto at cell–cell contacts, and EcadTSMod and EcadTSModΔcyto at free membrane in MDCK cells. EcadTSMod had a lower FRET index than either cytoplasmic TSMod or EcadTSModΔcyto regardless of their subcellular localization, indicating that it was under tension. P values are calculated using a two-tailed Mann–Whitney test.
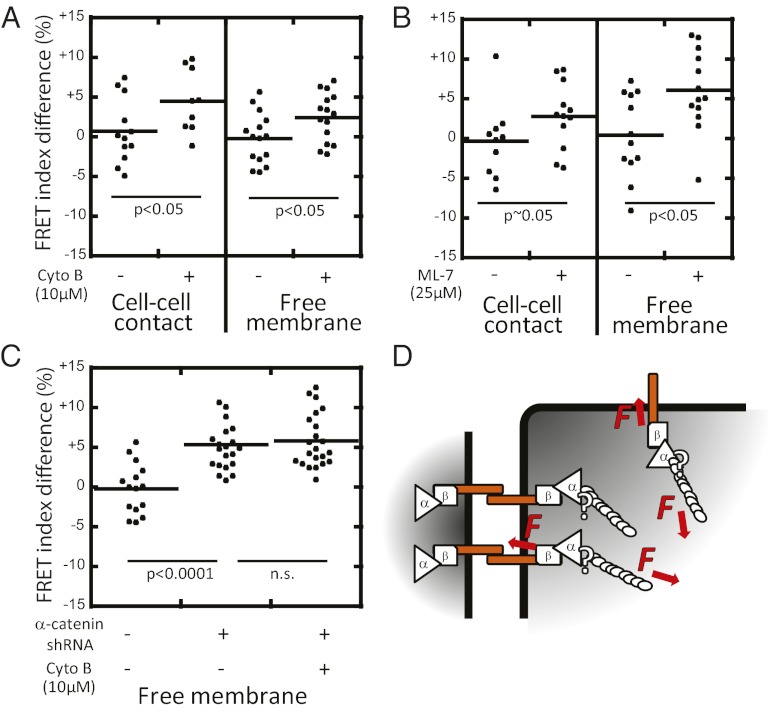
We sought to test whether the tension exerted on E-cadherin was generated by filamentous actin and myosin II activity. To test whether filamentous actin was required, cells were incubated in cytochalasin B, an inhibitor of actin polymerization (40). This treatment resulted in an increase in the EcadTSMod FRET index at both cell–cell contacts and the contact-free plasma membrane (Fig. 3A). We tested whether myosin II activity was required by incubating cells with ML-7, an inhibitor of myosin light chain kinase (41), which also resulted in an increase in the EcadTSMod FRET index at both cell–cell contacts and the contact-free plasma membrane (Fig. 3B).
Fig. 3.
Tension on EcadTSMod requires actomyosin activity and αΕ-catenin. FRET index difference before and after perturbation with: actin polymerization inhibitor cytochalasin B (A); myosin II activity inhibitor ML-7 (B); and αΕ-catenin depletion (C). Measurements were performed at cell–cell contacts (A and B) and at contact-free plasma membrane (A–C). Lack of E-cadherin recruitment to cell–cell contacts upon αE-catenin depletion prevented the measurement of a cell–cell contact FRET index in C. All three disruptions resulted in increased FRET index. Combined αE-catenin depletion and cytochalasin B addition did not increase the FRET index further, suggesting that either disruption interrupts force transmission through E-cadherin. (D) Proposed model: the cytoplasmic domain of E-cadherin is under constitutive tension generated by the actomyosin cytoskeleton and mediated by αE-catenin at cell–cell contacts and at contact-free membranes. P values are calculated using a two-tailed Mann–Whitney test.
Actomyosin perturbation induced a smaller increase of the FRET index than deletion of the cytoplasmic domain (Fig. 2B). We tested whether this could result from increased intermolecular FRET rather than tension relaxation. Intermolecular FRET may arise between proximal fluorescent E-cadherins, especially if they cluster at the plasma membrane. To directly quantify the contribution of intermolecular FRET, we measured the FRET index of EcadTSModΔcyto, which is not under tension, as a function of its expression level. We found a weak dependence of the FRET index on fluorescent protein expression level, consistent with small differences in the chance of encounters between fluorescent proteins over large variations in surface density (Fig. S3A). Perturbation of cells with ML-7 or cytochalasin B, however, did not induce significant or quantitative changes in EcadTSMod surface density (Fig. S3B) despite significant increases in EcadTSMod FRET index (Fig. 3 A and B). Thus, intermolecular FRET is unlikely to account for the increase in the FRET index observed upon actomyosin perturbation.
We next tested whether αE-catenin was required to transmit actomyosin-generated tension to E-cadherin. EcadTSMod was cotransfected with a shRNA against αE-catenin (42–44). Cells expressing EcadTSMod and depleted of αE-catenin did not recruit EcadTSMod or β-catenin between closely apposed neighboring cells (Fig. S4). Nevertheless, depletion of αE-catenin caused an increase in the EcadTSMod FRET index at the contact-free membrane (Fig. 3C). Significantly, addition of cytochalasin B to αE-catenin–depleted cells did not induce a further increase in the EcadTSMod FRET index (Fig. 3C). Together, these results show that actin filaments and myosin II activity require αE-catenin to exert constitutive tension on the cytoplasmic domain of E-cadherin (Fig. 3D).
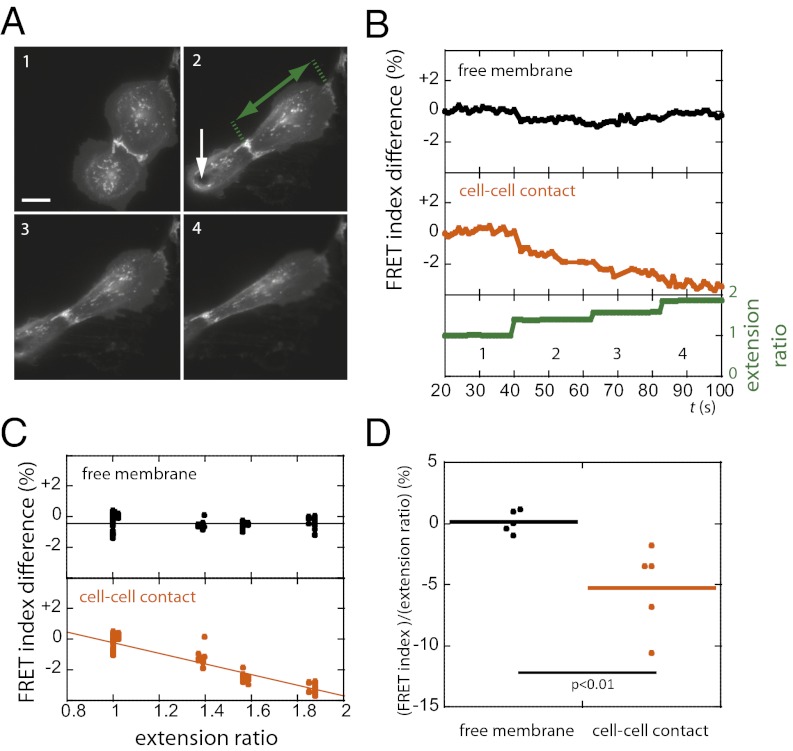
Finally, we sought to assess whether stretching pairs of cells increased tension in the cytoplasmic domain of E-cadherin. We used micromanipulation to apply increasing steps of uniaxial stretch across cell–cell contacts between adhering cells (Fig. 4A and SI Materials and Methods) and measured the EcadTSMod FRET index over time (Fig. 4B). We found that the FRET index at the cell–cell contact decreased as a function of cell extension (Fig. 4C). In contrast, the FRET index at contact-free regions of the plasma membrane exhibited no correlation with cell extension (Fig. 4C). On average, the change in the FRET index per unit change in extension ratio at cell–cell contacts was significantly different from that at contact-free plasma membrane (Fig. 4D). Thus, the cytoplasmic domain of E-cadherin in stretched cells transmits increased tension within cell–cell contacts, but not at contact-free regions of the plasma membrane.
Fig. 4.
Externally applied uniaxial stretch between pairs of cells decreased the FRET index of EcadTSMod at cell–cell junctions but not at the contact-free plasma membrane. (A) Fluorescence images from a sequence of increasing steps (1, 2, 3, 4; ∼20-s intervals) of stretch applied to a cell doublet using a microneedle. (In image 2, the white arrow shows the point of contact between the needle and the cell, and the green arrow shows the cell extension.) (B) Extension ratio (cell length in the direction of the stretch normalized by cell length at rest) and EcadTSMod FRET index at the cell–cell contact and at the free membrane for the cell that is not directly in contact with the needle in A. FRET decreased at the junction but not at the free membrane. (C) EcadTSMod FRET index vs. extension ratio from cell in A. Solid lines show linear fits. At the cell–cell contact: R2 = 0.88 and Δy/Δx = −3.5% ± 0.1% (SEM); at contact-free membrane: R2 = 8.10−5 and Δy/Δx = 0% ± 0.1% (SEM). Slopes are significantly different with P < 0.001, t test. (D) Change in FRET index per unit change in extension ratio (n = 6 cells). FRET index decreases with increasing extension ratio at the cell–cell contact but not at the free membrane. P value is calculated using two-tailed Mann–Whitney test. (Scale bar: 20 μm.)
Discussion
Mechanotransduction is ubiquitous during embryonic development, tissue regeneration, and disease (1, 4). Cell adhesion proteins are structural and functional hubs between the cytoskeleton and the extracellular environment and hence are strategically positioned to transduce mechanical signals (45). Mechanotransduction through integrin adhesion proteins to the extracellular matrix is well studied (46). Protein components in these adhesion complexes respond directly to mechanical forces by changing conformation, which exposes cryptic binding sites to putative binding partners and phosphorylation (47, 48). Recently, a FRET-based tension sensor, which we used here, revealed that vinculin experiences pN forces at integrin-based focal adhesions in cultured cells (36).
In contrast to studies of mechanotransduction at integrin-based adhesions, relatively little is known about the mechanical features of the cadherin/catenin adhesion complex at the molecular level. Recent studies have reported that cadherins transmit actomyosin-generated forces to the extracellular environment (32, 33). External forces applied to cells through cadherins reveal a mechanical coupling between the membrane and the cytoskeleton that requires the cadherin cytoplasmic domain (24) and may cause changes in cellular mechanical properties or protrusive activity (25, 26). Conformational changes in α-catenin and/or the recruitment of cytoskeleton-binding proteins to sites of cadherin-mediated adhesion may be involved (17, 18, 27–29). However, there is no direct evidence at the molecular level that cadherins are under actomyosin-dependent tension or that cadherins transmit extracellular forces to the cytoskeleton through the catenin-binding cytoplasmic domain. Here, we addressed this problem directly by using variants of E-cadherin (Fig. S5) that contain a tension sensor module (TSMod), and showed that: (i) the E-cadherin cytoplasmic domain is under tension that is dependent on an intact actin cytoskeleton, αE-catenin and myosin II activity; (ii) tension generated by the actomyosin cytoskeleton on E-cadherin is constitutive, regardless of whether E-cadherin is localized within or outside of cell–cell contacts at the plasma membrane; and (iii) tension in the cytoplasmic domain of E-cadherin is increased specifically at cell–cell contacts upon externally applied uniaxial stretch.
The force sensitivity of the flagelliform linker in the TSMod construct was determined previously by single-molecule stretching using optical tweezers (36). Based on those measurements and FRET index calibration (Fig. S2), we estimate that a 1-pN increase in force corresponds to a ∼6% decrease in FRET index on our experimental setup (SI Materials and Methods). The force sensor TSMod was inserted into a region of the E-cadherin juxtamembrane domain that is unstructured even when E-cadherin binds to β-catenin (49, 50). Previous results indicate that E-cadherin at the plasma membrane is constitutively bound to β-catenin (51, 52), whether or not the E-cadherin/β-catenin complex is associated with αE-catenin or actin (15). Thus, the observed changes in FRET index are unlikely to reflect conformational changes in the E-cadherin juxtamembrane region. Moreover, we showed directly that changes in intermolecular FRET between fluorescent E-cadherins are unlikely to account for the FRET index differences observed upon actomyosin perturbation (Fig. S3). Taken together, these data indicate that the E-cadherin cytoplasmic domain is under 1- to 2-pN constitutive load from the cytoskeleton (Figs. 2 and 3). This value is similar to that observed for vinculin in focal adhesions between integrins and the extracellular matrix (36), indicating that the forces exerted across proteins of both adhesion complexes are of the same order of magnitude. It is noteworthy, however, that this value likely masks a range of forces that are averaged out over many molecules and over time and space. This may explain why tension on E-cadherin was only partially reduced upon pharmacological perturbation of actin polymerization, myosin II activity, and shRNA-mediated depletion of αE-catenin (Fig. 3). It is possible that low levels of αE-catenin and actomyosin activity suffice to exert some tension on E-cadherin after drug treatment and/or protein depletion. In addition, E-cadherin may interact with intermediate filaments through desmosomal linker proteins (22, 23, 26, 53). Intermediate filaments might constitutively exert tension on E-cadherin or compensate for reduced αE-catenin–mediated actomyosin tension following drug treatment or αE-catenin depletion.
We found that E-cadherin is not only under actomyosin-dependent tension within cell–cell contacts but also in regions of plasma membrane where there was no cell–cell adhesion. Thus, the cadherin/catenin complex may be a constitutive membrane anchor for the cortical actomyosin cytoskeleton. In this context, it is noteworthy that cadherin lateral mobility in the plasma membrane depends on the catenin-binding region of the cytoplasmic domain even outside of cell–cell contacts and is regulated by the underlying cortical actin cytoskeleton (54, 55). That the cadherin/catenin complex functions in membrane-to-cortex attachment may explain why this complex is important for regulating macropinocytosis in contact-free protruding membranes (56), single-cell wound closure in Drosophila (57), and plasma membrane blebbing during early embryogenesis in zebrafish (58). Plasma membrane blebbing involves functional crosstalk between the cadherin/catenin complex and ezrin–radixin–moesin (ERM) proteins (58), which also mediate membrane-to-cortex attachment (59). Although the molecular events directly downstream of E-cadherin tension remain unclear, E-cadherin, and likely αE-catenin, may be involved in a ubiquitous tension-sensing mechanism that regulates cortical cytoskeleton activity as a function of cell shape, size, or membrane activity.
Externally applied stretch on cell pairs increases tension in the cytoplasmic domain of E-cadherin at cell–cell contacts by ∼1 pN (Fig. 4). Importantly, tension across the E-cadherin cytoplasmic domain appears to increase in proportion to the applied stretch, which may allow a graded signaling output from the adhesion complex. However, tension does not appear to be propagated to E-cadherin in the plasma membrane outside the cell–cell contact, suggesting a lack of lateral mechanical coupling. Additionally, within the timescale of our experiments, the increased tension across E-cadherin induced by stretching cells did not relax to its original constitutive value, which may enable localized and sustained adhesion-specific signaling. Force-induced conformation changes within the cadherin/catenin complex could elicit downstream signaling, for example, by modulating binding affinity to direct or indirect binding partners such as EPLIN or vinculin (17–21, 25, 27–29). Protein accumulation at adhesion sites could thus promote changes in cell and tissue mechanical properties and cell adhesive and migratory behavior (18, 25, 26, 28, 29). Over longer timescales, this may also regulate gene expression and likewise have important roles during development and disease.
Conclusion
Recent studies suggested a role of the cadherin/catenin complex in mechanotransduction at cell–cell contacts. In this study, we provide direct evidence that mechanical tension is transmitted through the E-cadherin cytoplasmic domain as a consequence of actomyosin activity and involving αE-catenin and as a response to external mechanical stimulus through neighboring cells. These results therefore validate a critical condition for cadherins as bona fide mechanosensors at cell–cell contacts. Surprisingly, our results also show that the cytoplasmic domain of E-cadherin is subject to constitutive, actomyosin-generated pN-scale tension at the contact-free plasma membrane, revealing a constitutive function of E-cadherin as a link between the cell membrane and the actomyosin cytoskeleton. Although the signaling pathways downstream of mechanically stimulated cadherins remain to be characterized, our results suggest that cadherins function as adhesion-dependent mechanosensors during morphogenesis of multicellular assemblies and as adhesion-independent mechanosensors that adapt their signaling output in response to changes in cell size, shape, or membrane activity.
Materials and Methods
MDCK type II G cells stably or transiently expressing fluorescently tagged proteins were monitored on a wide-field epifluorescence inverted microscope 2–3 d after shRNA transfection or within an hour after drug treatment, depending on experiment. Image analysis was performed with Image J and publicly available plugins and macros.
Full materials and methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the members of the W.J.N., A.R.D., and B.L.P. laboratories for insightful discussions; the M. B. Goodman laboratory (Stanford University, Stanford, CA) for sharing materials and equipment; and R. N. Day (Indiana University) and I. G. Macara (University of Virginia) for the gifts of constructs. This material is based in part upon work supported by a Stanford Bio-X IIP award and the National Science Foundation under Emerging Frontiers in Research and Innovation (EFRI) Grant 1136790 (to B.L.P., W.I.W., W.J.N., and A.R.D.), National Institutes of Health (NIH) New Innovator Award 1DP2OD007078-01 (to A.R.D.), NIH Grant GM35527 (to W.J.N.), and a Burroughs-Wellcome Career Award at the Scientific Interface (to A.R.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204390109/-/DCSupplemental.
References
- 1.Keller R, Shook D, Skoglund P. The forces that shape embryos: Physical aspects of convergent extension by cell intercalation. Phys Biol. 2008;5:015007. doi: 10.1088/1478-3975/5/1/015007. [DOI] [PubMed] [Google Scholar]
- 2.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- 3.Beloussov LV, Dorfman JG, Cherdantzev VG. Mechanical stresses and morphological patterns in amphibian embryos. J Embryol Exp Morphol. 1975;34:559–574. [PubMed] [Google Scholar]
- 4.Farge E. Mechanotransduction in development. Curr Top Dev Biol. 2011;95:243–265. doi: 10.1016/B978-0-12-385065-2.00008-6. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, et al. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci USA. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci USA. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trepat X, et al. Physical forces during collective cell migration. Nat Phys. 2009;5:426–430. [Google Scholar]
- 8.Tambe DT, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng Q, et al. Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proc Natl Acad Sci USA. 2012;109:1506–1511. doi: 10.1073/pnas.1106377109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghi N, James Nelson W. Intercellular adhesion in morphogenesis: Molecular and biophysical considerations. Curr Top Dev Biol. 2009;89:1–32. doi: 10.1016/S0070-2153(09)89001-7. [DOI] [PubMed] [Google Scholar]
- 11.Chu Y-S, et al. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halbleib JM, Nelson WJ. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 13.Alberts B. Molecular Biology of the Cell. New York: Garland Science; 2002. [Google Scholar]
- 14.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci USA. 2008;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taguchi K, Ishiuchi T, Takeichi M. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J Cell Biol. 2011;194:643–656. doi: 10.1083/jcb.201104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chervin-Pétinot A, et al. Epithelial protein lost in neoplasm (EPLIN) interacts with α-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J Biol Chem. 2012;287:7556–7572. doi: 10.1074/jbc.M111.328682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rangarajan ES, Izard T. The cytoskeletal protein α-catenin unfurls upon binding to vinculin. J Biol Chem. 2012;287:18492–18499. doi: 10.1074/jbc.M112.351023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng X, Maiers JL, Choudhury D, Craig SW, DeMali KA. α-Catenin uses a novel mechanism to activate vinculin. J Biol Chem. 2012;287:7728–7737. doi: 10.1074/jbc.M111.297481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi HJ, et al. αE-catenin is an autoinhibited molecule that co-activates vinculin. Proc Natl Acad Sci USA. 2012;109:8576–8581. doi: 10.1073/pnas.1203906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalczyk AP, et al. VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: A pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions. J Cell Sci. 1998;111:3045–3057. doi: 10.1242/jcs.111.20.3045. [DOI] [PubMed] [Google Scholar]
- 23.Leonard M, Chan Y, Menko AS. Identification of a novel intermediate filament-linked N-cadherin/gamma-catenin complex involved in the establishment of the cytoarchitecture of differentiated lens fiber cells. Dev Biol. 2008;319:298–308. doi: 10.1016/j.ydbio.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabdanov E, Borghi N, Brochard-Wyart F, Dufour S, Thiery J-P. Role of E-cadherin in membrane-cortex interaction probed by nanotube extrusion. Biophys J. 2009;96:2457–2465. doi: 10.1016/j.bpj.2008.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.le Duc Q, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber GF, Bjerke MA, DeSimone DW. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell. 2012;22:104–115. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 28.Huveneers S, et al. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumida GM, Tomita TM, Shih W, Yamada S. Myosin II activity dependent and independent vinculin recruitment to the sites of E-cadherin-mediated cell-cell adhesion. BMC Cell Biol. 2011;12:48. doi: 10.1186/1471-2121-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopra A, Tabdanov E, Patel H, Janmey PA, Kresh JY. Cardiac myocyte remodeling mediated by N-cadherin-dependent mechanosensing. Am J Physiol Heart Circ Physiol. 2011;300:H1252–H1266. doi: 10.1152/ajpheart.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai J, Kam L. Rigidity-dependent cross talk between integrin and cadherin signaling. Biophys J. 2009;96:L39–L41. doi: 10.1016/j.bpj.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganz A, et al. Traction forces exerted through N-cadherin contacts. Biol Cell. 2006;98:721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- 33.Ladoux B, et al. Strength dependence of cadherin-mediated adhesions. Biophys J. 2010;98:534–542. doi: 10.1016/j.bpj.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Meng F, Sachs F. Genetically encoded force sensors for measuring mechanical forces in proteins. Commun Integr Biol. 2011;4:385–390. doi: 10.4161/cib.4.4.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker N, et al. Molecular nanosprings in spider capture-silk threads. Nat Mater. 2003;2:278–283. doi: 10.1038/nmat858. [DOI] [PubMed] [Google Scholar]
- 36.Grashoff C, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams CL, Chen Y-T, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- 39.Day RN, Booker CF, Periasamy A. Characterization of an improved donor fluorescent protein for Forster resonance energy transfer microscopy. J Biomed Opt. 2008;13:031203. doi: 10.1117/1.2939094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- 42.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borghi N, Lowndes M, Maruthamuthu V, Gardel ML, Nelson WJ. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc Natl Acad Sci USA. 2010;107:13324–13329. doi: 10.1073/pnas.1002662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamin JM, et al. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J Cell Biol. 2010;189:339–352. doi: 10.1083/jcb.200910041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.del Rio A, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem. 2001;276:12301–12309. doi: 10.1074/jbc.M010377200. [DOI] [PubMed] [Google Scholar]
- 50.Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 51.Hinck L, Näthke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: Novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/β-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-Cadherin in polarized MDCK cells. Cell. 1999;144:687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calkins CC, et al. The Armadillo family protein p0071 is a VE-cadherin- and desmoplakin-binding protein. J Biol Chem. 2003;278:1774–1783. doi: 10.1074/jbc.M205693200. [DOI] [PubMed] [Google Scholar]
- 54.Sako Y, Nagafuchi A, Tsukita S, Takeichi M, Kusumi A. Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: Corralling and tethering by the membrane skeleton. J Cell Biol. 1998;140:1227–1240. doi: 10.1083/jcb.140.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bard L, et al. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J Neurosci. 2008;28:5879–5890. doi: 10.1523/JNEUROSCI.5331-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabatini PJB, Zhang M, Silverman-Gavrila RV, Bendeck MP. Cadherins at cell-autonomous membrane contacts control macropinocytosis. J Cell Sci. 2011;124:2013–2020. doi: 10.1242/jcs.076901. [DOI] [PubMed] [Google Scholar]
- 57.Abreu-Blanco MT, Verboon JM, Parkhurst SM. Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling. J Cell Biol. 2011;193:455–464. doi: 10.1083/jcb.201011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schepis A, Sepich D, Nelson WJ. αE-catenin regulates cell-cell adhesion and membrane blebbing during zebrafish epiboly. Development. 2012;139:537–546. doi: 10.1242/dev.073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arpin M, Chirivino D, Naba A, Zwaenepoel I. Emerging role for ERM proteins in cell adhesion and migration. Cell Adhes Migr. 2011;5:199–206. doi: 10.4161/cam.5.2.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.