Abstract
Mammalian pigmentation is driven by the intercellular transfer of pigment-containing melanosomes from the tips of melanocyte dendrites to surrounding keratinocytes. Tip accumulation of melanosomes requires myosin Va, because melanosomes concentrate in the center of melanocytes from myosin Va-null (dilute) mice. This distribution defect results in inefficient melanosome transfer and a dilution of coat color. Dilute mice that simultaneously lack melanoregulin, the product of the dilute suppressor locus, exhibit a nearly complete restoration of coat color, but, surprisingly, melanosomes remain concentrated in the center of their melanocytes. Here we show that dilute/dsu melanocytes, but not dilute melanocytes, readily transfer the melanosomes concentrated in their center to surrounding keratinocytes in situ. Using time-lapse imaging of WT melanocyte/keratinocyte cocultures in which the plasma membranes of the two cells are marked with different colors, we define an intercellular melanosome transfer pathway that involves the shedding by the melanocyte of melanosome-rich packages, which subsequently are phagocytosed by the keratinocyte. Shedding, which occurs primarily at dendritic tips but also from more central regions, involves adhesion to the keratinocyte, thinning behind the forming package, and apparent self-abscission. Finally, we show that shedding from the cell center is sixfold more frequent in cultured dilute/dsu melanocytes than in dilute melanocytes, consistent with the in situ data. Together, these results explain how dsu restores the coat color of dilute mice without restoring intracellular melanosome distribution, indicate that melanoregulin is a negative regulator of melanosome transfer, and provide insight into the mechanism of intercellular melanosome transfer.
The coloration of mammalian skin and hair requires the transfer of melanin pigment from melanocytes, which create the pigment, to keratinocytes, which make up the bulk of skin and hair (1). Although the exact mechanism of intercellular pigment transfer is unknown, efficient transfer is thought to require that melanosomes, the pigment-producing organelles generated within the melanocyte’s central cytoplasm, be accumulated at the tips of the melanocyte’s long dendritic extensions, the likely major site of transfer. The accumulation of melanosomes at dendritic tips is generated by a cooperative transport mechanism that couples long-range, bidirectional, microtubule-dependent transport within dendritic extensions with myosin Va-dependent capture of the organelles at tips (the Cooperative Capture model) (2). In melanocytes from dilute mice, which lack myosin Va (Myo5a), the connection between the melanosome and F-actin is broken, resulting in melanosomes redistributing according to microtubule distribution alone. The net result of this redistribution is the pronounced accumulation of the organelles in the central cytoplasm, in striking contrast to their normal accumulation in the cell periphery. In the mouse, this defect in intracellular melanosome distribution results in a striking reduction in the intensity of the animal’s coat color, most likely because of a significant reduction in the transfer of pigment from melanocytes to keratinocytes.
Non-agouti black mice that are homozygous for the dilute functional null allele dl20j appear gray (3). When these mice also are made homozygous for a functional null allele at the dilute suppressor (dsu) locus, their coat color is restored almost completely (i.e., from gray back to nearly black) (3, 4). In other words, the coat color defect resulting from the loss of expression of myosin Va is rescued by the loss of expression of melanoregulin, the product of the dsu locus. dsu is inherited in a semidominant fashion, because the presence of one mutant dsu allele partially suppresses the diluted coat color of dl20j /dl20j mice, whereas the presence of two mutant dsu alleles suppresses the coat color to nearly WT (i.e., black) (4). Studies performed using chimeric mice argue that melanoregulin functions cell autonomously within melanocytes to rescue coat color (5).
Positional cloning of the dsu locus revealed that melanoregulin is a vertebrate-specific, highly charged protein of 22 kDa with no obvious sequence similarity to any currently characterized protein (3). Northern blots suggest that the protein is highly expressed in melanocytes, heart, liver, testes, and thymus and at lower levels in other tissues. Moreover, Western blots indicate that melanoregulin is abundant in primary melanocytes. Consistent with the large deletion present in the sole extant mutant dsu allele, melanocytes from homozygous dsu mice were shown to be completely devoid of melanoregulin (3).
Very surprisingly, coat color rescue by dsu occurs without restoration of normal peripheral melanosome distribution within dilute melanocytes, based on images of melanocytes present in the Harderian glands of dl20j/dl20j, dsu/dsu mice (3). Specifically, Harderian gland melanocytes from these rescued black mice exhibit an accumulation of melanosomes in their central cytoplasm that is indistinguishable from that exhibited by Harderian gland melanocytes from gray dl20j/dl20j mice. This key observation, which we confirm and extend here in a variety of ways, flies directly in the face of the currently accepted mechanism thought to drive mammalian pigmentation, according to which the accumulation of melanosomes in peripheral regions of the melanocyte’s dendrites is a prerequisite for their effective transfer to keratinocytes and subsequent pigmentation. How then does dsu rescue the coat color of dilute mice without rescuing the underlying defect in intracellular melanosome distribution exhibited by dilute melanocytes? Although this conundrum remains completely unresolved, O’Sullivan et al. (3) reasonably could conclude that melanoregulin functions in a myosin Va-independent pathway to restore coat pigmentation. For example, melanoregulin might serve as a negative regulator of a myosin Va-independent intercellular melanosome transfer pathway. Here we show that this is indeed the case, identifying dsu as a mouse coat color mutant that specifically influences the intercellular transfer component of the pigmentation pathway.
Results
dsu Does Not Rescue the Defect in Intracellular Melanosome Distribution Exhibited by Dilute Melanocytes.
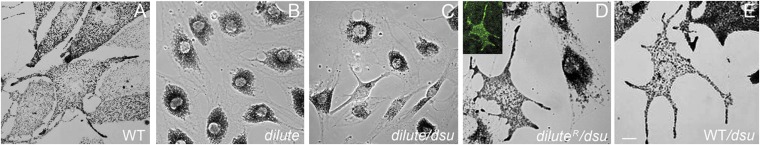
First, using primary melanocytes cultured from dl20j/dl20j, DSU/DSU mice (null at myosin Va, WT at dsu; referred to hereafter as “dilute”) (Fig. 1B) and from dl20j/dl20j, dsu/dsu mice (null at both myosin Va and dsu; referred to hereafter as “dilute/dsu”) (Fig. 1C), we confirmed that the loss of melanoregulin does not rescue the defect in intracellular melanosome distribution caused by the lack of myosin Va (compare these cells with the cells in Fig. 1A, which are from a D/D, DSU/DSU mouse, i.e., WT at both loci; referred to hereafter as “WT”). As expected, reintroduction of a full-length, GFP-tagged version of the melanocyte-spliced isoform of myosin Va into dilute/dsu melanocytes results in the complete restoration of peripheral melanosome distribution (Fig. 1D; diluteR/dsu). Similarly, primary melanocytes from D/D, dsu/dsu mice (WT at myosin Va, null at dsu; referred to hereafter as “WT/dsu”) exhibit normal melanosome distribution (Fig. 1E). Together, these results show that on both WT and null myosin Va backgrounds the presence or absence of melanoregulin does not influence the intracellular distribution of melanosomes within cultured primary melanocytes.
Fig. 1.
dsu does not rescue the defect in melanosome distribution exhibited by dilute melanocytes. Shown are bright-field images of WT (A), dilute (B), dilute/dsu (C), dilute/dsu transfected with GFP-tagged myosin Va (melanocyte-spliced isoform) i.e., diluteR/dsu (D) and WT/dsu (E) melanocytes in primary culture (see text for specifics on the genotypes). Inset in D shows the distribution of GFP-tagged myosin Va in the transfected, rescued diluteR/dsu melanocyte. (Scale bar: 10 μm.)
Melanosomes Readily Escape from the Center of dilute/dsu Melanocytes but Not from the Center of dilute Melanocytes.
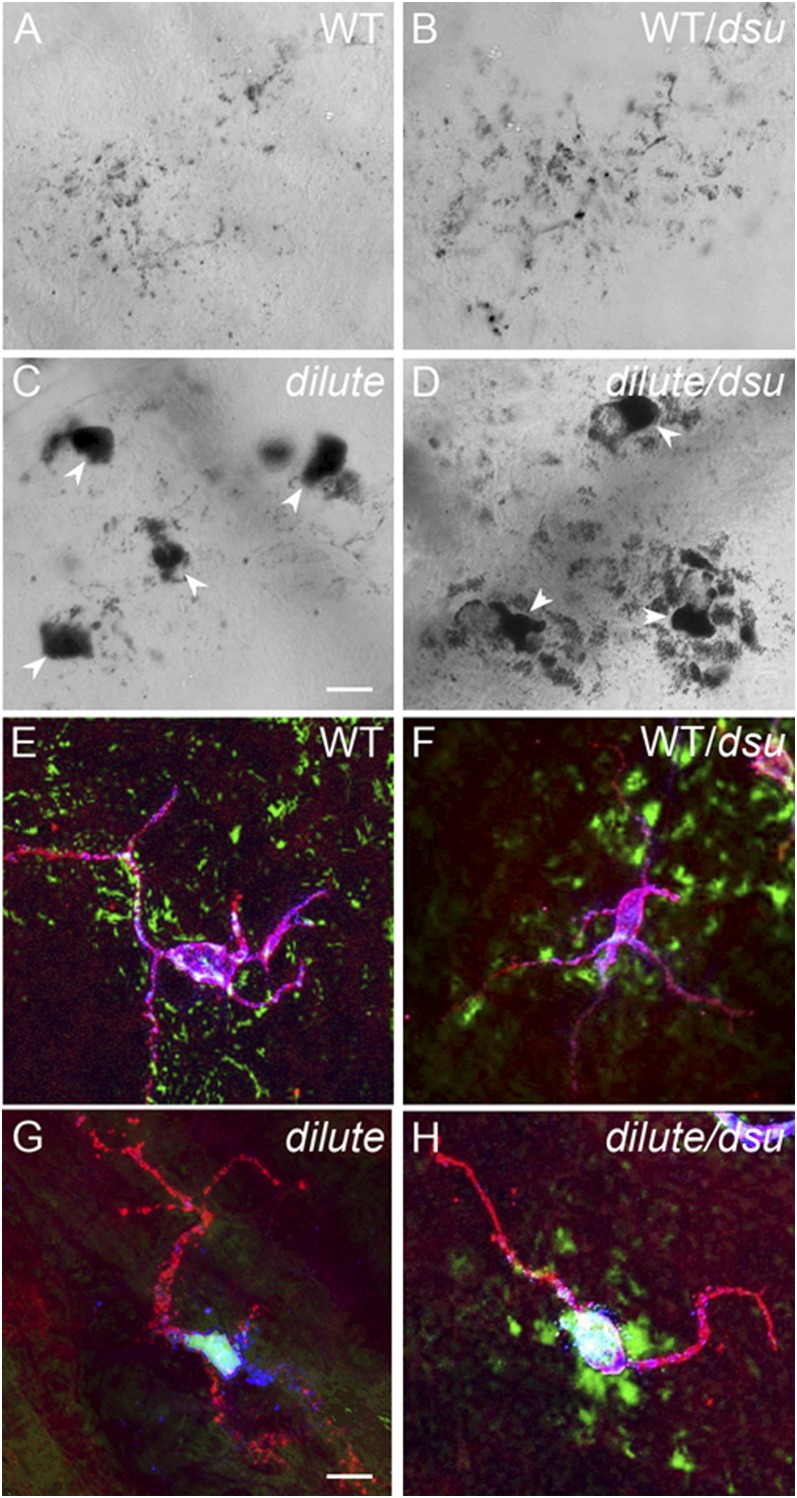
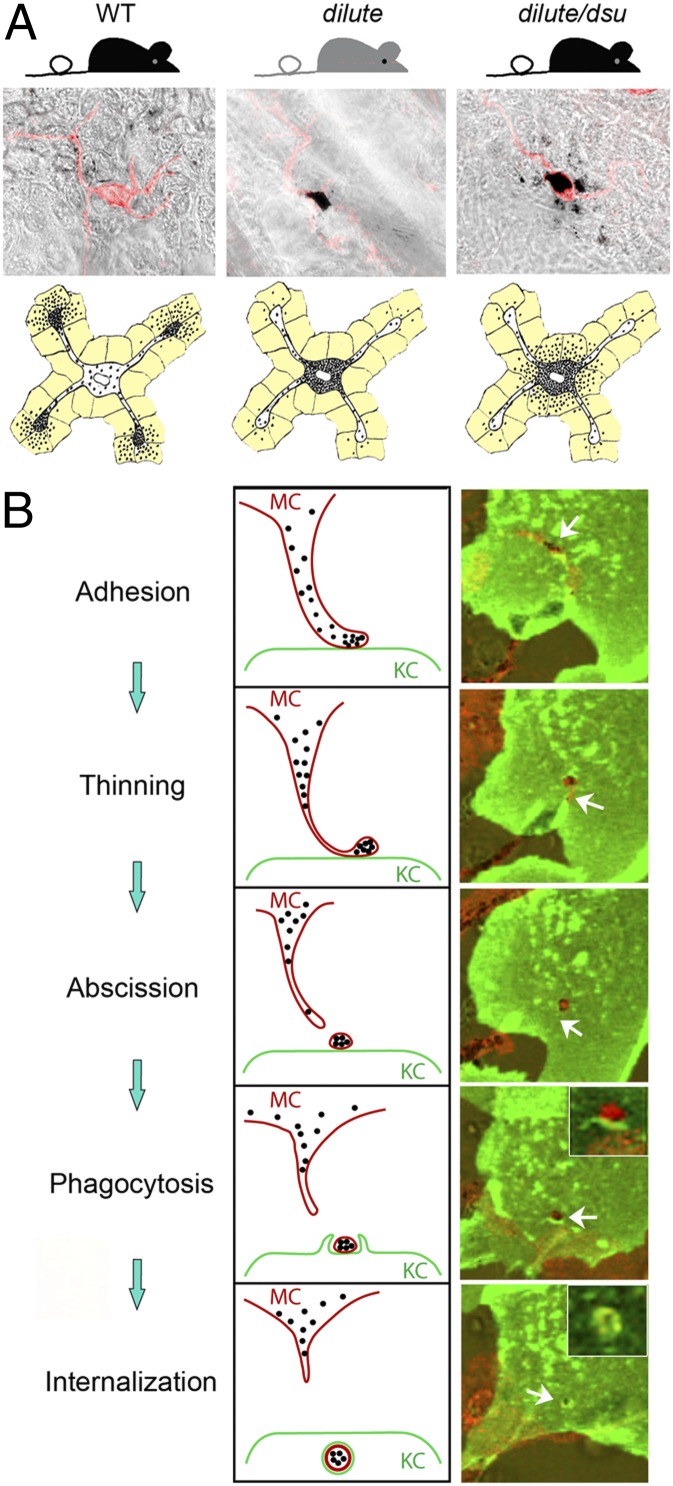
To address the central conundrum of how the coat color of dilute/dsu mice is rescued when the cellular defect is not, we switched to studying pigment distribution in situ. Specifically we used staining and imaging methods that can reveal the shapes of melanocytes and keratinocytes, as well as the distribution of pigment within them, in the ear skin of WT, WT/dsu, dilute, and dilute/dsu mice. Initial imaging of ear skin using only bright-field imaging to visualize the distribution of pigment (Fig. 2 A–D) hinted at the eventual outcome. First, pigment is spread very evenly in ear skin from both WT (Fig. 2A) and WT/dsu (Fig. 2B) mice, as expected. Also as expected, pigment is very highly concentrated in the cell bodies of melanocytes in ear skin from both dilute (arrowheads in Fig. 2C ) and dilute/dsu (arrowheads in Fig. 2D) mice. Unexpectedly, however, pigment appears to accumulate in tissue immediately surrounding the cell bodies of dilute/dsu melanocytes (Fig. 2D) but not in tissue surrounding the cell bodies of dilute melanocytes (Fig. 2C). This conclusion was confirmed using an antibody to the plasma membrane receptor for Kit to delineate the shape of melanocytes (Fig. 2 E–H). Note that in these images the melanocyte’s shape is shown in red, the distribution of black pigment is shown in green (by pseudo coloring), and the distribution of melanosomes inside of melanocytes is shown in blue using an antibody to the melanosomal membrane protein TRP1 (yielding a blue/white color when superimposed on red and green). Comparison of the images for ear skins from dilute (Fig. 2G) and dilute/dsu (Fig. 2H) mice indicates clearly that pigment has escaped readily from the center of dilute/dsu melanocytes but not from the center of dilute melanocytes.
Fig. 2.
Melanosomes escape readily from the center of dilute/dsu ear skin melanocytes but not from the center of dilute ear skin melanocytes. Shown are bright-field images of ear skin from WT (A), WT/dsu (B), dilute (C), and dilute/dsu (D) mice. Also shown are images of ear skin from WT (E), WT/dsu (F), dilute (G), and dilute/dsu (H) mice that had been stained with an antibody to the plasma membrane receptor for Kit to define the shape of melanocytes and with an antibody to the melanosomal membrane protein TRP1. The anti-Kit signal is in red, black pigment is pseudo colored green, and the anti-TRP1 signal is in blue (yielding a blue-white color inside melanocytes because of the overlay with the red and green signals). (Scale bars: 10 μm in A–D; 7 μm in E–H.)
Pigment that Escapes the Center of dilute/dsu Melanocytes Is Inside Adjacent Keratinocytes.
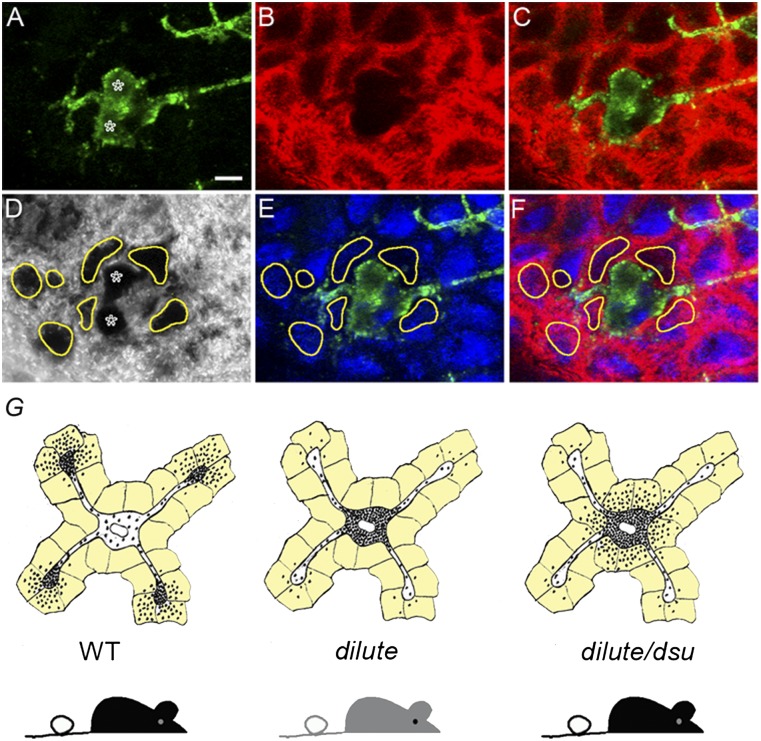
To demonstrate that this escaped pigment actually is inside keratinocytes immediately surrounding the cell body of dilute/dsu melanocytes, we stained dilute/dsu ear skin with anti-Kit to visualize the shape of melanocytes (green signals in Fig. 3 A, C, E, and F), with anti-keratin 14 to see the distribution of keratin filaments within keratinocytes (red signals in Fig. 3 B, C, and F), and with DAPI to identify the positions of nuclei (blue signals in Fig 3 E and F). In the corresponding transmitted-light image in Fig. 3D, we circled in yellow the dramatic accumulations of pigment that surround the two dilute/dsu melanocyte cell bodies present in this field (which are marked with asterisks in Fig. 3 A and D). Importantly, overlay of this transmitted-light image (including the yellow circles) with the DAPI and Kit signals in Fig. 3E shows that the accumulations of pigment outside these two dilute/dsu melanocytes fall almost entirely on top of the DAPI signals marking the nuclei of surrounding keratinocytes (compare the yellow circles with the blue DAPI signals in Fig. 3E; note that the DAPI signals are attenuated to some extent by the presence of the pigment). Even more importantly, overlay of the transmitted-light image (including the yellow circles) with the DAPI, Kit, and keratin 14 signals in Fig. 3F shows that the accumulations of pigment outside these two dilute/dsu melanocytes fall almost entirely inside the red oval keratin 14 signals that surround nuclei and that mark the peripheral cytoplasm of the keratinocytes (compare the yellow circles with the red keratin and blue DAPI signals in Fig. 3F). Together, these images demonstrate that essentially all the pigment that has escaped the cell bodies of dilute/dsu melanocytes is inside keratinocytes that immediately surround the melanocyte’s cell body. These results, which are summarized in cartoon form in Fig. 3G, show that the loss of melanoregulin in dilute melanocytes allows the melanosomes accumulated in their central cytoplasm to be transferred readily to adjacent keratinocytes. Importantly, this critical observation can explain how dsu largely restores the coat color of dilute mice without at all restoring the defect in intracellular melanosome distribution caused by the lack of myosin Va. Moreover, this observation is consistent with the increase in the density of pigment seen in the central shaft and tip of hair from dilute/dsu mice relative to dilute mice (3). These experiments also support the emerging idea that melanoregulin functions as a negative regulator of melanosome transfer.
Fig. 3.
Pigment that escapes the center of dilute/dsu melanocytes is inside adjacent keratinocytes. Shown are images of ear skin from a dilute/dsu mouse that had been stained for Kit to reveal the shape of melanocytes (green signal in A, C, E, and F), keratin 14 to reveal the presence of keratin filaments within keratinocytes (red signal in B, C, and E), and DAPI to reveal the positions of nuclei (blue signal in E and F) and the distribution of pigment (black in the transmitted-light image in D). (A–C) Two adjacent melanocyte cell bodies in green (asterisks in A) surrounded by keratinocytes, which appear as red ovals because of the keratin filaments present in their peripheral cytoplasm (D) In the transmitted-light image, the dramatic accumulations of pigment that surround the two melanocyte cell bodies (marked with asterisks) are circled in yellow. (E) Overlay of this transmitted-light image (including the yellow circles) with the DAPI and Kit signals. (F) Overlay of this transmitted-light image (including the yellow circles) with the DAPI, cKit, and keratin 14 signals. (Scale bar: 7 μm.) (G) Cartoon depicting the basic concept supported by the images in Figs. 2 and 3, i.e., that dsu rescues the coat color of dilute mice without rescuing the defect in intracellular melanosome distribution exhibited by dilute melanocytes by allowing the melanosomes accumulated in their central cytoplasm to be transferred readily to the keratinocytes that immediately surround the melanocyte’s cell body (the keratinocytes in the cartoon are colored light yellow).
Importantly, melanoregulin also appears to function as a negative regulator of melanosome transfer when myosin Va is present, because the density of pigment in the central shaft of hair from WT/dsu mice was shown previously to be greater than that in WT mice (an effect of dsu on a WT background was missed in prior visual examinations of such mice, presumably because it is hard to appreciate such an increase in pigment density, given the blackness of the animal’s coat) (3). Moreover, using dilute mice that previously were engineered via BAC transgenic methods to carry four to eight copies of the WT DSU structural gene (on a WT DSU background) and that were visibly lighter than dilute mice that also are WT at DSU (3), we show that there is significantly less pigment in the central shaft of the hair from these transgenic animals than in the hair from the dilute animals (Fig. S1). Therefore, the likely overexpression of this negative regulator of melanosome transfer appears to suppress further the already reduced level of melanosome transfer exhibited by dilute melanocytes. Together, these results argue from both sides of the expression variable (reduced/loss of expression and likely overexpression) that melanoregulin acts as a negative regulator of melanosome transfer.
Melanocytes Present in Primary Skin Cultures Appear to Shed Melanosome-Rich Packages from the Tips of Their Dendrites.
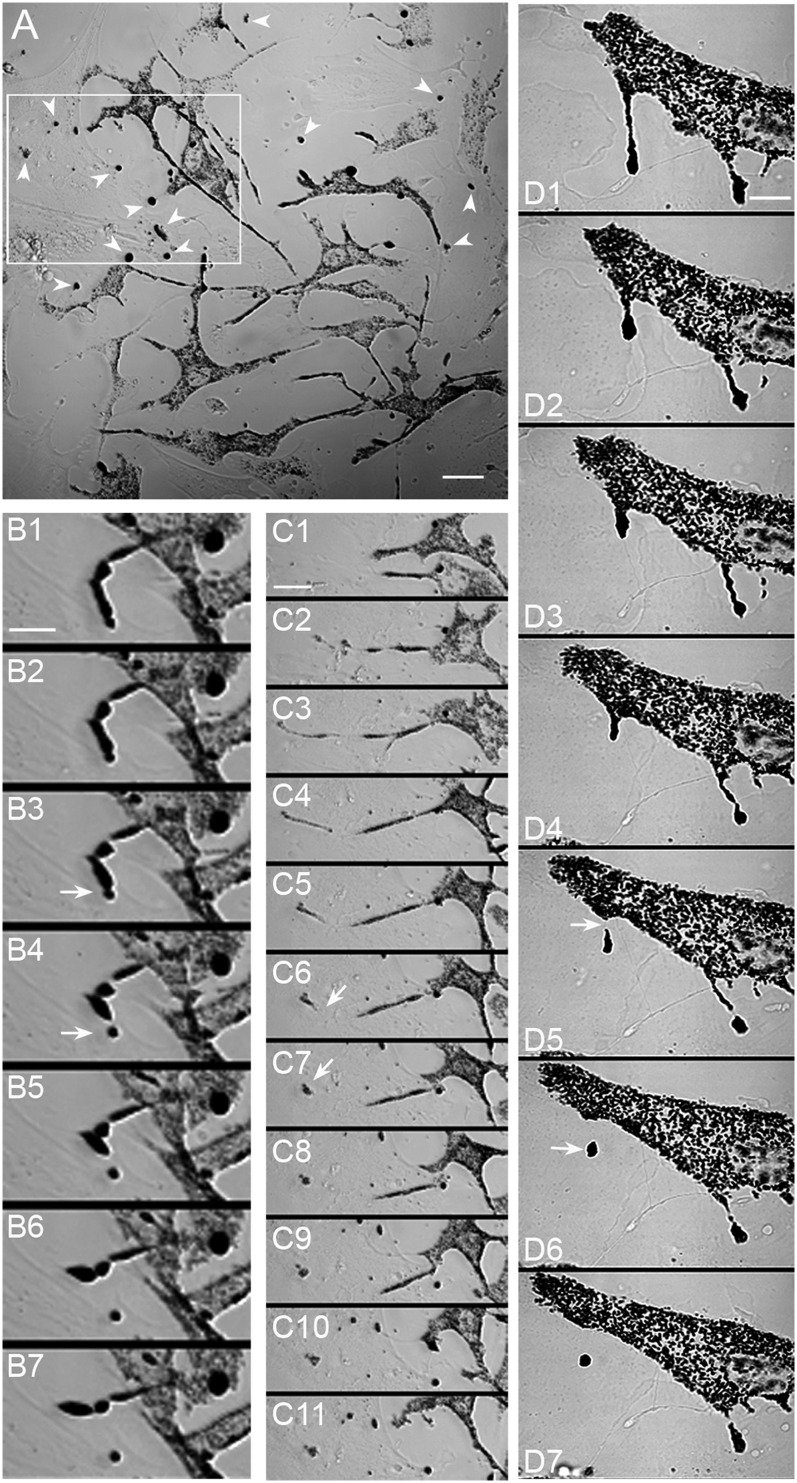
Given the above observations, it is clear that the mechanism by which dsu rescues coat color cannot be resolved until the mechanism by which melanosomes normally are transferred from the melanocyte to the keratinocyte is defined. To begin to address this question, we performed long-term (i.e., ∼18 h), time-lapse, transmitted-light imaging of early (day 2–7) primary skin cultures from WT mice. Importantly, these early cultures contain both melanocytes (visible in transmitted-light images by virtue of their pigment) and keratinocytes (barely visible in transmitted-light images but very obvious in fixed samples stained for keratin 14) (Fig. S2). Fig. 4A shows a still image of a typical field from an early culture. In addition to the black melanosomes that are clearly present within melanocytes, there are numerous round, black “balls” that appear to be outside the melanocytes (see arrows). Critically, time-lapse imaging suggests that these black balls are created via some sort of shedding mechanism occurring commonly at the tips of the melanocyte’s dendrites (Movie S1). Two apparent shedding events that occurred within the boxed region in Fig. 4A are shown more clearly in Movies S2 and S3 and in the corresponding still images in Fig. 4 B and C, 1–8. These images, as well as the higher-magnification images of the shedding event shown in Fig. 4D and Movie S4, are consistent with the shedding by the melanocyte of its melanosome-rich dendritic tips, resulting in the release of plasma membrane-enclosed “packages” containing multiple melanosomes (marked in the still images by a white arrow in the frame most likely immediately preceding and in the frame most likely immediately following their release). Although most shedding events occurred at the tips or sides of dendrites, some occurred from much more central regions of the melanocyte (see Movie S5 and the corresponding still images in Fig. S3). Measurement of the frequency of shedding yielded an average value of 3.0 ± 2.6 packages per melanocyte per day (24 cells), although rates as high as 15 packages per melanocyte per day were observed. Finally, although most events resulted in the creation of packages containing multiple melanosomes, in a few instances the packages appeared to contain only a single melanosome (see below).
Fig. 4.
Melanocytes present in primary skin cultures appear to shed melanosome-rich packages from the tips of their dendrites. (A) Low-magnification bright-field image of a primary skin culture. In addition to melanocytes (evident because of their pigmentation) and keratinocytes (barely visible but evident in cultures stained for keratin; see Fig. S1), these cultures contain numerous round black “balls” (arrowheads) that appear to be outside melanocytes. The creation of these packages via some sort of shedding mechanism can be seen at low magnification in Movie S1. The still images in B and in C 1–8 show at higher magnification two apparent shedding events that occurred from the tips of a melanocyte’s dendrite within the boxed region in A (see also Movies S2 and S3, from which these stills were taken). The still images in D (and Movie S4, from which these stills were taken) show another tip shedding event that occurred in a different culture. The white arrows in these sequential still images mark the position of the forming package in the frame most likely immediately preceding (B 3, C 6, and D 5) and the frame most likely immediately following (B 4, C 7, and D 6) the release of the package. The still images in B and D are 2 and 4 min apart, respectively. The still images in C 4–11 are 4 min apart; the still images in C 1 3 are 30 min apart. (Scale bars: 18 μm in A; 8 μm in B; 15 μm in C; 9.5 μm in D.)
Labeling Melanocytes and Keratinocytes with Different Colors Shows That Package Shedding Drives Intercellular Melanosome Transfer via a Process Involving Adhesion, Dendrite Thinning, Abscission, and Phagocytosis.
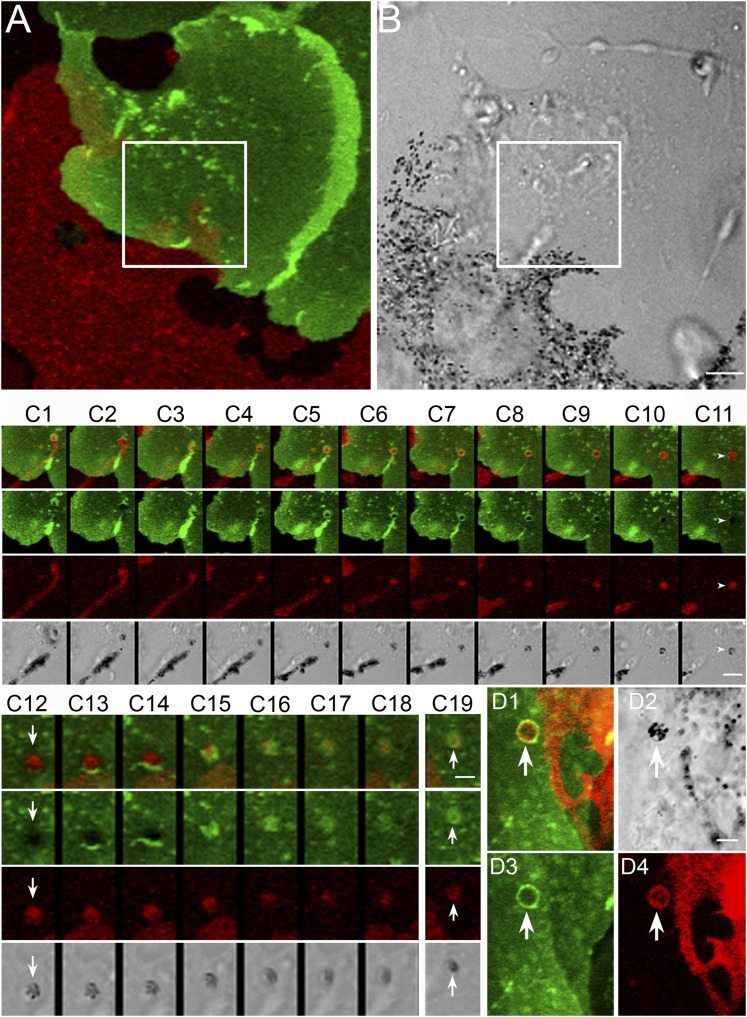
Identifying the role played by keratinocytes in the shedding process was hampered greatly by their being essentially invisible in transmitted-light images. Moreover, they are indistinguishable in such images from the fibroblasts that also are present in the cultures. Perhaps most importantly, transmitted-light images never can resolve unequivocally whether melanosomes are inside melanocytes, inside keratinocytes, or extracellular. To overcome these major hurdles to defining the shedding mechanism, as well as the likely transfer (either simultaneously or subsequently) of the shed melanosome packages to keratinocytes, we developed a system in which the plasma membranes of these two cell types could be distinguished readily. Specifically, we prepared primary skin cultures from mice generated by crossing a mouse in which the plasma membranes of all cells are red because of the transgenic expression of farnesylated td-Tomato with a mouse expressing cre recombinase under the direction of a keratin-14 promoter to drive the conversion of farnesylated td-Tomato to farnesylated GFP in keratinocytes. The net result is that primary skin cultures from these mice, which we refer to as “Holly Skin” mice, contain melanocytes and keratinocytes with red and green plasma membranes, respectively (Fig. 5 A and B). Thus we were able to determine unequivocally the roles played by both cell types in the shedding process as well as in potential intercellular transfer events.
Fig. 5.
Package shedding drives the intercellular transfer of melanosomes via a process involving adhesion, dendrite thinning, and abscission to create the package, followed by phagocytosis of the package by the keratinocyte. (A and B) A melanocyte/keratinocyte pair from a Holly Skin mouse in which a shedding/transfer event occurs within the boxed region from the tip of the melanocyte’s dendrite (see also Movie S6, from which these two images were taken). The still images in C 1–11, which were taken from the “Thinning/Abscission” portion of Movie S7, demonstrate the thinning of the melanocyte’s dendrite behind the tip to be shed and the subsequent abscission step, which together result in the shedding of a melanosome package that initially sits on the surface of the keratinocyte (arrowheads in C 11 and arrows in C 12). The still images in C 12–18, which were taken from the “Phagocytosis” portion of Movie S7, demonstrate the subsequent phagocytosis of the extracellular melanosome package by the keratinocyte. C 19 shows this package shortly after it had been engulfed by the keratinocyte (arrows). The still images in C 1–11 are 4 min apart; the still images in C 12–18 are 2 min apart; and the image in C 19 is 16 min after C 18. (D) An engulfed package from a separate transfer event (arrows). (Scale bars: 5 μm in A and B; 3.8 μm in C 1–11; 2 μm in C12–19; 2.9 μm in D.)
Fig. 5 C, 1–18 and the movies from which these still images were taken (Movie S6 at low magnification and Movie S7 at high magnification) show that the interaction between the two cells in Fig. 5A resulted not only in a typical shedding event but also in the subsequent uptake of the melanosome package by the keratinocyte, i.e., intercellular transfer. The shedding process occurs in three steps, the first being apparent adhesion between the melanocyte’s dendritic extension and the keratinocyte (which for this cell pair was initiated before the start of the Movie S6; see the “Adhesion” header in Movie S6). The second step, the actual shedding event, involves two substeps (see the “Thinning/Abscission” header in Movie S6). The first substep is the thinning of the dendrite behind the tip destined to be shed. Although this thinning appears to be an invariable aspect of the shedding mechanism, it takes several guises (see below and Discussion). In this melanocyte/keratinocyte pair, a significant portion of the melanocyte’s dendrite can be seen to thin over time (Movie S6). This thinning substep is clearer in the higher-magnification movie (Movie S7; see the “Thinning/Abscission” header) and in the still images (Fig. 5 C, 1–11) taken from this movie (see in particular the red still images in Fig. 5 C, 1–8 for the thinning substep). Then, in the second substep, the connection between the dendrite and the tip breaks within the thinnest region of the dendrite, leaving behind the remnants of the tip (see the “Thinning/Abscission” header in Movie S6). This abscission substep also is clearer in the higher-magnification movie, Movie S7 (see the “Thinning/Abscission” header) and in the still images (Fig. 5 C, 1–11) taken from this movie (see in particular the red still images in Fig. 5 C, 9–11 for the abscission substep). In the transmitted-light image (Fig. 5 C, 11), this tip remnant (indicated by a white arrowhead) looks exactly like the black balls seen in our initial bright-field time-lapse images (Fig. 3). Moreover, at higher magnification the remnant appears as a group of black melanosomes completely surrounded by red plasma membrane derived from the melanocyte and sitting on top of the green plasma membrane of the keratinocyte (white arrows in Fig. 5C). In other words, the package initially is extracellular and is in contact with the surface of the keratinocyte. This important aspect of the shedding and transfer mechanisms was evident in all events where there was a significant time delay between package formation and the subsequent phagocytosis of the package by the keratinocyte.
Finally, in the third step, after a significant delay during which time the extracellular package remains in place on the surface of the keratinocyte (see the “Extracellular Package” header in Movie S6), the extracellular package is phagocytosed by the keratinocyte (see the “Phagocytosis” header in Movie S6). This phagocytosis step, which is clearer in the higher-magnification movie (Movie S7; see the “Phagocytosis” header) and in the still images (Fig. 5 C, 12–18) taken from this movie, is evidenced in this cell pair by the flash of green that advances and closes over the black and red ball (see, in particular, the green and red still images in Fig. 5 C, 13–17). This flash of green corresponds to the extension of the keratinocyte’s green plasma membrane as it engulfs the package by phagocytosis. As expected, engulfed packages appear inside the keratinocyte as a tight cluster of black melanosomes encircled by a red membrane that in turn is encircled by a green phagosomal membrane (arrows in Fig. 5 C, 19). Fig. 5D shows another such engulfed package (indicated by arrows).
Movie S8 and the corresponding still mages in Fig. S4 show a similar transfer event that is noteworthy in that the single dendrite of the melanocyte in this cell pair yields three melanosome packages in rapid succession. Like the melanocyte in Fig. 5, these three events involve thinning of the dendrite in areas between the forming packages (Fig. 5 C, 2–6), the generation of extracellular melanosome packages sitting on the surface of the keratinocyte (arrowheads in Fig. 5 C, 7 and 8), and their eventual uptake by the keratinocyte via phagocytosis (seen for one of the three packages in Movie S8 and indicated by arrows in Fig. 5 C, 11 and 12). Finally, although close inspection of our dynamic images shows clearly that packages routinely contain multiple melanosomes, we did observe several shedding events in which the package appeared to contain only a single melanosome (see, for example, Movie S9 and the corresponding still images in Fig. S5).
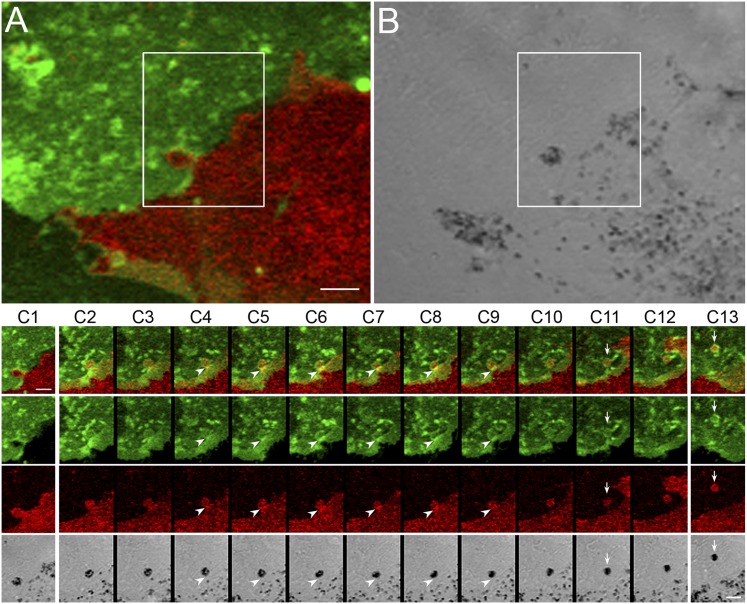
Melanosome Transfer also Occurs from More Central Regions of Melanocytes.
Although most transfer events occur from the tips of dendrites, some (∼10%) occur from more central regions of the melanocyte (e.g., the base of dendrites and the cell body). Fig. 6 A and B and Movie S10 show a melanocyte/keratinocyte cell pair that exhibited such a central transfer event. This event is somewhat distinct from the events just described, in that the thinning step is not obvious (this event looks much more like a “budding” event), and the processes of abscission and phagocytosis are compressed both spatially and temporally. The still images in Fig. 6 C, 2–12 demonstrate this compression. Interestingly, the generation and engulfment of this package was accompanied by transient increases in the plasma membrane signals from both the melanocyte and the keratinocyte at the base of the forming package (arrowheads in Fig. 6 C, 4–9); this increase occurred just before the abscission step (arrows in Fig. 6 C, 11). Conclusion of this transfer event resulted once again in a black and red package surrounded by a green phagosomal membrane that is present inside the keratinocyte (arrows in Fig. 6 C, 13). Moreover, this internalized package can be seen to move toward the center of the keratinocyte in subsequent video frames (Movie S10).
Fig. 6.
Melanosome transfer also occurs from central regions of melanocytes. (A and B) A melanocyte/keratinocyte pair in which a shedding/transfer event occurs within the boxed region at the side of the melanocyte rather than at a dendritic tip (see also Movie S10, from which these two images were taken). The still images in C, which also were taken from Movie S10, demonstrate this side-shedding/transfer event in more detail. Arrowheads in C 4–9 point to transient increases at the base of the forming package of the plasma membrane signals from both the melanocyte and the keratinocyte, which occurred just before the abscission step (arrows in C 11). C 13 shows the package (arrows) 30 min after its engulfment by the keratinocyte. This internalized package can be seen to move toward the center of the keratinocyte in subsequent video frames (see Movie S10). The still images in C 2–12 are 2 min apart. The image in C 1 precedes the image in C 2 by 20 min. (Scale bars: 5 μm in A and B; 3 μm in C).
The transfer event depicted in Fig. 6 also is noteworthy in that package formation appears to occur without any obvious external stretching force being applied. Package formation without any apparent application of external force also is very clear in the shedding event shown in Movie S11 and the corresponding still images in Fig. S6. Indeed, this shedding event looks very much like the formation and subsequent separation of a “hanging droplet.” These observations suggest some sort of self-abscission mechanism functions within the melanocyte to facilitate shedding (Discussion).
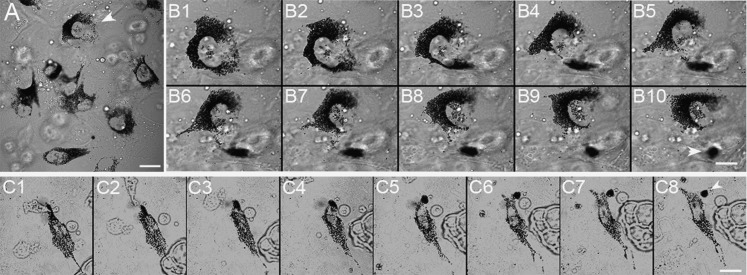
Cultured dilute/dsu Melanocytes Shed Packages from Their Cell Center at Six Times the Frequency of dilute Melanocytes.
Given the above observations and the results from the ear-staining studies showing that dsu enhances the intercellular transfer of melanosomes accumulated in the center of dilute melanocytes to keratinocytes immediately surrounding the cell body, we asked whether primary dilute/dsu melanocytes in culture shed melanosome packages from their cell center and, if so, whether the frequency of such shedding events is significantly higher than that seen for primary dilute melanocytes. The answer to both questions was yes. Specifically, Fig. 7B and Movie S12 (see Example 1), from which these stills were taken, show that dilute/dsu melanocytes shed melanosome packages from their cell center. Fig. 7C and Movie S12 ( Example 2) show a second example of this central shedding. Very importantly, the frequency with which these melanocytes shed packages from their central cytoplasm (1.25 ± 0.73 packages per melanocyte per day; 39 melanocytes isolated from five separate pups) was found to be ∼six times higher than the frequency with which age-controlled dilute melanocytes shed packages from their central cytoplasm (0.22 ± 0.16 packages per melanocyte per day; 67 melanocytes isolated from eight separate pups; P < 0.02). Therefore we conclude that the dsu mutation suppresses the coat color defect of dilute mice without restoring melanosome distribution within dilute melanocytes by up-regulating an intercellular melanosome transfer pathway involving the shedding of melanosome-rich packages by the melanocyte for subsequent engulfment by the keratinocyte.
Fig. 7.
Cultured dilute/dsu melanocytes shed packages from their cell center. A shows a field of cultured dilute/dsu melanocytes. Still images in B show at higher magnification a shedding event that occurred from the center of the cell marked with an arrowhead in A (see also Movie S12, Example 1, from which these stills were taken). The still images in C show a second example of a shedding event from the center of a dilute/dsu melanocyte (see also Movie S12, Example 2, from which these stills were taken). The white arrowheads in B 10 and C 8 indicate the shed packages. The still images in B are 8 min apart. The still images in C are 10 min apart. (Scale bars: 12 μm in A; 8 μm in B; 15 μm in C.)
Discussion
This study focused on identifying the pathway by which dsu rescues the coat color of dilute mice without restoring the normal peripheral distribution of melanosomes within the animal’s melanocytes. Examination of the distributions of melanocytes, keratinocytes, and pigment in situ within ear skin indicated that dsu greatly up-regulates the transfer of the melanosomes accumulated in the center of dilute melanocytes to surrounding keratinocytes. Because dsu is a loss-of-function suppressor, this core observation argues that melanoregulin serves as a negative regulator of melanosome transfer. This role also is evident on a WT myosin Va background as an increase in pigment content in the hair (3). Importantly, these and other results indicate that melanoregulin does not influence the site of melanosome transfer but only how efficiently the melanosomes are transferred from wherever they accumulate (i.e., the cell center in dilute and dendritic tips in WT mice).
In an effort to address the cellular mechanism by which the loss of melanoregulin rescues the coat color of a dilute mouse, we sought to define the mechanism of melanosome transfer. Importantly, visible pigmentation in mammals requires this intercellular transfer of pigment, because skin and hair are populated almost entirely by keratinocytes (1). As a result, only the transfer of pigment from melanocytes into keratinocytes (each melanocyte is thought to serve upwards of 40 keratinocytes in skin) yields visible pigmentation of the animal. Despite the essential role played by melanosome transfer in mammalian pigmentation, the exact mechanism of transfer has long evaded identification (6). Using early primary cocultures of WT melanocytes and keratinocytes in which the plasma membranes of these two cell types had been marked with different colors, we identified a transfer mechanism that appears to involve several steps. These steps include, in sequential order, (i) the attachment of the melanocyte’s dendrite to the surface of the keratinocyte; (ii) the deposition on the surface of the keratinocyte of a plasma membrane-enclosed package of melanosomes via shedding of a portion of the melanocyte’s dendrite; and (iii) the subsequent phagocytic uptake of the package by the keratinocyte. Finally, bringing these observations back to dsu, we show that dilute/dsu melanocytes shed similar melanosome packages from their cell center and that the frequency of this central shedding is ∼sixfold higher that that seen in age-matched cocultures from dilute mice. This last observation provides a clear functional explanation for the difference in pigment distribution seen in ear skin from dilute/dsu versus dilute mice as well as the difference in the coat colors of these two animals.
The shedding step is both the most fascinating and the most enigmatic aspect of the transfer mechanism we visualized. It invariably involves the thinning of the melanocyte’s cytoplasm just behind the region to be shed. At least three forces could contribute to this thinning. The first, which is pictured most easily for the shedding of dendritic tips, could be external tension on the dendrite that results from adhesion between the tip of the dendrite and the plasma membrane of the keratinocyte, coupled with motions away from the point of contact by the dendrite (or keratinocyte) that serve to stretch the dendrite, leading eventually to its physical tearing. Such forces certainly are possible and in principal are consistent with some shedding events. For example, in the shedding event depicted in Fig. 4, the melanocyte’s dendritic extension appears to retreat following package formation, suggesting that a build up of tension within the dendrite is released upon shedding. That said, many thinning/shedding events occur without any obvious contribution of a “stretching and tearing” force (for examples, see Fig. 6 and Fig. S6). Moreover, the fidelity of the shedding process appears to be quite high, because transfer events never were seen to result in the release of free melanosomes into the medium. A stretching and tearing mechanism does not seem compatible with such a high-fidelity mechanism.
The second force driving thinning and shedding could derive from efforts by the keratinocyte to phagocytose the forming package. Indeed, the most widely discussed idea over the last three decades regarding the mechanism of intercellular melanosome transfer has been that the keratinocyte phagocytoses the entire melanosome-rich dendritic tip of the melanocyte (6). Although the extension of a phagocytic cup around the forming package certainly could contribute to thinning and subsequent shedding, it clearly is not required for successful shedding, because we routinely observed complete thinning/shedding events that precede phagocytosis of the package by the keratinocyte. This critical observation also fits with our long-held reservation regarding the idea that transfer is driven solely by keratinocyte-based phagocytosis, because phagocytosis is not capable, to our knowledge, of “biting off” what it seeks to engulf. Rather, phagocytic extensions are capable only of extending around the object until they eventually meet up. Indeed, this limitation is the reason why efforts by one cell to phagocytose part of another cell lead to the engulfment of the entire cell (7)). That said, forces associated with efforts by the keratinocyte to phagocytose the forming package might act in close temporal coordination with thinning and self-abscission to contribute to shedding events in vivo.
The third force driving shedding could derive from a mechanism functioning within the melanocyte’s dendrite to drive thinning and generation of the package. Although this mechanism is certainly the most theoretical of the three putative forces, it is supported indirectly by the fact that thinning and abscission events like the one in Fig. 6 and Fig. S6 can occur without any obvious external force. Moreover, it is consistent with the fact that melanoregulin acts cell autonomously (i.e., within the melanocyte) to regulate melanosome transfer negatively (5). How could the melanocyte rearrange its plasma membrane to drive such self-abscission? Importantly, such a rearrangement would be consistent topologically with the endosomal sorting complex required for transport (ESCRT)-dependent membrane rearrangements that drive multivesicular body formation, the shedding of HIV, and abscission at the end of cytokinesis (8, 9). Indeed, a melanocyte-based self-abscission mechanism could well be very similar to the recently characterized mechanism of abscission that occurs at the end of cytokinesis (10). This rapidly evolving story has revealed that abscission is driven by an internal mechanism involving the ESRCT complex, which appears to drive both the thinning of the bridge between the two daughter cells and its eventual abscission. By providing a self-enclosed, extracellular package that is an ideal substrate for phagocytosis by the keratinocyte, an ESCRT-dependent self-abscission mechanism driving melanosome shedding would avoid the problem that phagocytosis by itself probably cannot generate the package. Therefore future studies should examine the role of the ESCRT machinery in melanosome transfer. It also will be important to define the relationship between the shedding phenomena identified here and the shedding of ectosomes via ectocytosis (11). Finally, in terms of the site of action of melanoregulin, exogenous GFP-tagged melanoregulin recently has been shown to target to the surface of the melanosome (12). Therefore, future efforts to define the exact molecular mechanism by which melanoregulin negatively regulates shedding must take this site of action into consideration.
Although we think our demonstration via dynamic imaging of the mechanism of melanosome transfer in primary melanocyte/keratinocyte cocultures is compelling, the final conclusion as to the mechanism must await the visualization of the transfer mechanism in living skin. Relevant to this point, we observed thinning, shedding, and subsequent transfer events in our cultures that occurred from both the tip/sides of the melanocyte’s dendrites and, to a lesser extent, at the cell body. Although it is easy to imagine this latter type of transfer occurring readily in the close confines of tissue, we think that the former type of event could occur readily as well. For example, the thinning and abscission of the ends of dendrites could occur in dendrites residing between adjacent keratinocytes in the skin and in dendrites extending outward from the center of the hair bulb to contact keratinocytes as they pass by on their way into the hair shaft. Finally, we do not completely exclude the possibility that other mechanisms of transfer (e.g., the exocytosis of naked melanin and its uptake by the keratinocyte) also might occur, to some extent, in parallel with the shedding pathway we observed here.
Mouse myosin Va has design features that should make it ideally suited to transport vesicular cargo in vivo (13). Clear evidence of such point-to-point organelle transport has been slow to accumulate, however (13). Because of this lack of clear evidence, and for other reasons, current thinking is that in many cases this myosin may serve primarily as a dynamic tether to capture organelles in the actin cortex following their long-range transport on microtubules. Indeed, the Cooperative Capture model of melanosome transport and distribution (2) is often cited as a clear example of such a tethering role for myosin Va. Importantly, the mechanism of intercellular melanosome transfer identified here adds further weight to this argument, because it appears that effective transfer via shedding requires only that melanosomes be captured at the sides and tips of dendrites by myosin Va. Finally, we note that our results, although not consistent with filopodia being the conduit for melanosome transfer (14), do not rigorously exclude this possibility.
Relevant to our findings, Ando et al. (15) recently reported that black “globules” containing multiple melanosomes accumulate over time in the media of cultured human melanocytes and that these packages, when purified and added to keratinocyte cultures, are taken up by the keratinocytes. Moreover, based solely on still images at the light and electron microscopy levels, they argue that these melanosome-rich packages are shed from the melanocyte’s surface (16). Importantly, our dynamic imaging proves, in a way that their still images cannot, that this shedding is an active process involving contact between the melanocyte and the keratinocyte, thinning of the melanocyte’s dendrite behind the region to be shed, and apparent self-abscission. Moreover, in contrast to the static imaging performed by Ando et al. (16) on pure melanocyte cultures, our union of both dynamic imaging and melanocyte/keratinocyte coculture provides important temporal and spatial information about the roles played by both cell types in the sequential steps driving transfer, including adhesion, dendrite thinning, abscission, and phagocytosis. Finally, the fact that Ando et al. (16) used pure melanocyte cultures argues that the shedding of melanosome packages is (or at least can be) a purely melanocyte cell autonomous phenomena. That said, we never witnessed a single shedding event in Holly Skin mouse cocultures that did not involve intimate contact between the melanocyte and a keratinocyte. Moreover, using the same source of human melanocytes and the same culture conditions as Ando et al. (16), we did not observe a single shedding event in more than 70 h of viewing (147 cells; 7- to 18-h recordings), although some melanosome “packages” were present in the medium. Further work is required to resolve this issue.
Recently, Ohbayashi et al. (12) published that melanoregulin, rather than Rab7 as previously reported (17), serves as the anchor on the melanosome surface for the complex of RILP, dynactin, and dynein, which together drive the movements of the organelle directed towards the minus end of the microtubule. Moreover, they report that the knock down of melanoregulin in ashen melanocytes, which are in essence myosin Va-deficient because they are Rab27a-deficient, restores the peripheral distribution of melanosomes, presumably by downregulating the transport of the organelles directed towards the minus end of the microtubule. This observation is completely at odds with our characterization here of cells, hair, and tissue from mice harboring a null mutation in myosin Va with or without a null mutation in dsu, which shows that the loss of melanoregulin does not restore even slightly the defect in melanosome distribution caused by the lack of myosin Va. Ohbayashi et al. (12) attempt to explain this major discrepancy by suggesting that the dsu mouse has compensated for the lack of melanoregulin by up-regulating a secondary dynein receptor on the surface of the melanosome. Even if this scenario were true, it would not explain the central conundrum of the dilute/dsu mouse, which is that the coat color is restored without restoration of normal melanosome distribution within the melanocytes. Most importantly, the mechanism identified here by which dsu rescues coat color fully explains this conundrum. Moreover, our efforts to define this mechanism have led to insights into the long-standing question of how melanosomes are transferred from melanocytes to keratinocytes.
Methods
The preparation of primary mouse skin cultures containing melanocytes and keratinocytes from individual postnatal day 1 (P1) to P3 pups, the breeding of all mouse strains containing mutant alleles at dilute and/or dsu, and the genotyping to determine which cultures were from homozygous dl20J/dl20J mice were performed as described previously (2). Mouse care was performed according to institutional guidelines. The cultures were used for imaging within 7 d, during which time they contained both keratinocytes and melanocytes (Fig. S1). (By 10 d, keratinocytes differentiate to the point that they begin to detach). To create mice for the preparation of Holly Skin cultures, a C57BL/6 strain that ubiquitously expresses plasma membrane-targeted td-Tomato off a floxed cassette (18) were purchased from the Jackson Laboratory and bred as homozygous. Into this strain was back-crossed (for >8 generations) as heterozygous a strain expressing Cre recombinase under the keratin-14 promoter (gift of S. Gutkind, National Institute of Dental and Craniofacial Research, Bethesda), which drives Cre expression preferentially in keratinocytes that populate the basal layer of skin. Cre expression leads to deletion of the td-Tomato cassette, allowing expression of plasma membrane-targeted EGFP instead. Long-term, time-lapse imaging was performed using a Zeiss LSM 510 confocal microscope equipped with a 40×, 1.4 NA objective and a humidified environmental chamber providing 5% CO2 and 37 °C. Imaging duration ranged from 2 to 24 h. The time interval between images was 2 min. Movies play at either 3,600× (Movies S1, S3–S5, and S8) or 1,800× (Movies S2, S6, S7, and S9–S12) real time. Holly Skin primary cultures were imaged using 488 and 543 nm laser lines. The preparation and staining of whole-mount mouse ear skin with mouse anti-KIT, rabbit anti–TRP-1 (gift of V. Hearing, National Cancer Institute, Bethesda), and the mouse anti-TRP1 antibody Mel-5 was performed as described previously (19). The mouse anti–keratin-14 antibody was purchased from Covance (PRB-155P) and was used at a dilution of 1:100. Mouse hair was prepared as described previously (3). Dilute/dsu melanocytes were transfected with GFP-tagged myosin Va as described previously (2). Human melanocytes from darkly pigmented newborn foreskin (Invitrogen) were obtained and cultured as described by Ando et al. (16). To stain hair, dorsal skin from P5 WT pups was embedded in OCT, frozen in liquid nitrogen, sectioned (6- to 10-μm sections), and stained with DAPI.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 12276 (volume 109, number 31).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209397109/-/DCSupplemental.
References
- 1.Kondo T, Hearing VJ. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev Dermatol. 2011;6(1):97–108. doi: 10.1586/edm.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu X, Bowers B, Rao K, Wei Q, Hammer JA., 3rd Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan TN, et al. dsu functions in a MYO5A-independent pathway to suppress the coat color of dilute mice. Proc Natl Acad Sci USA. 2004;101:16831–16836. doi: 10.1073/pnas.0407339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore KJ, et al. Dilute suppressor dsu acts semidominantly to suppress the coat color phenotype of a deletion mutation, dl20J, of the murine dilute locus. Proc Natl Acad Sci USA. 1988;85:8131–8135. doi: 10.1073/pnas.85.21.8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore KJ, Swing DA, Copeland NG, Jenkins NA. The murine dilute suppressor gene encodes a cell autonomous suppressor. Genetics. 1994;138:491–497. doi: 10.1093/genetics/138.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Den Bossche K, Naeyaert JM, Lambert J. The quest for the mechanism of melanin transfer. Traffic. 2006;7:769–778. doi: 10.1111/j.1600-0854.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 7.Han CZ, Ravichandran KS. Metabolic connections during apoptotic cell engulfment. Cell. 2011;147:1442–1445. doi: 10.1016/j.cell.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: It’s all in the neck. Nat Rev Mol Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guizetti J, et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331:1616–1620. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- 10.Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci USA. 2011;108:4846–4851. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: Artifacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Ohbayashi N, et al. Melanoregulin regulates retrograde melanosome transport through interaction with the RILP{middle dot}p150Glued complex in melanocytes. J Cell Sci. 2012;125(6):1508–1518. doi: 10.1242/jcs.094185. [DOI] [PubMed] [Google Scholar]
- 13.Hammer JA, 3rd, Sellers JR. Walking to work: Roles for class V myosins as cargo transporters. Nat Rev Mol Cell Biol. 2012;13:13–26. doi: 10.1038/nrm3248. [DOI] [PubMed] [Google Scholar]
- 14.Singh SK, et al. Melanin transfer in human skin cells is mediated by filopodia—a model for homotypic and heterotypic lysosome-related organelle transfer. FASEB J. 2010;24:3756–3769. doi: 10.1096/fj.10-159046. [DOI] [PubMed] [Google Scholar]
- 15.Ando H, et al. Involvement of pigment globules containing multiple melanosomes in the transfer of melanosomes from melanocytes to keratinocytes. Cell Logist. 2011;1:12–20. doi: 10.4161/cl.1.1.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ando H, et al. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol. 2012;132:1222–1229. doi: 10.1038/jid.2011.413. [DOI] [PubMed] [Google Scholar]
- 17.Jordens I, et al. Rab7 and Rab27a control two motor protein activities involved in melanosomal transport. Pigment Cell Res. 2006;19:412–423. doi: 10.1111/j.1600-0749.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- 18.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 19.Wei Q, Wu X, Hammer JA., 3rd The predominant defect in dilute melanocytes is in melanosome distribution and not cell shape, supporting a role for myosin V in melanosome transport. J Muscle Res Cell Motil. 1997;18:517–527. doi: 10.1023/a:1018659117569. [DOI] [PubMed] [Google Scholar]