Abstract
The type of metabolic compartmentalization that occurs in red blood cells differs from the types that exist in most eukaryotic cells, such as intracellular organelles. In red blood cells (ghosts), ATP is sequestered within the cytoskeletal–membrane complex. These pools of ATP are known to directly fuel both the Na+/K+ and Ca2+ pumps. ATP can be entrapped within these pools either by incubation with bulk ATP or by operation of the phosphoglycerate kinase and pyruvate kinase reactions to enzymatically generate ATP. When the pool is filled with nascent ATP, metabolic labeling of the Na+/K+ or Ca2+ pump phosphoproteins (ENa-P and ECa-P, respectively) from bulk [γ-32P]-ATP is prevented until the pool is emptied by various means. Importantly, the pool also can be filled with the fluorescent ATP analog trinitrophenol ATP, as well as with a photoactivatable ATP analog, 8-azido-ATP (N3-ATP). Using the fluorescent ATP, we show that ATP accumulates and then disappears from the membrane as the ATP pools are filled and subsequently emptied, respectively. By loading N3-ATP into the membrane pool, we demonstrate that membrane proteins that contribute to the pool’s architecture can be photolabeled. With the aid of an antibody to N3-ATP, we identify these labeled proteins by immunoblotting and characterize their derived peptides by mass spectrometry. These analyses show that the specific peptides that corral the entrapped ATP derive from sequences within β-spectrin, ankyrin, band 3, and GAPDH.
Keywords: confocal microscopy, Western blots, membrane peptides
The advent of techniques to isolate and characterize intracellular organelles has made it possible to establish that metabolic pathways and their associated intermediates are commonly localized to membrane-enclosed compartments (1, 2). Although mammalian red blood cells (RBCs) have no intracellular membranes or organelles, previous work has documented that the ATP that fuels the Na+/K+ (ATP1A1) and Ca2+ (ATP2B1) pumps resides within a structurally distinct compartment that constitutes the preferred source of ATP for both types of pumps (3–6). This membrane-associated ATP pool can be filled with exogenous ATP in either hemoglobin-free porous ghosts or inside-outside membrane vesicles (IOVs) in ways that allow control of the contents of the ATP pool. One method of filling this pool is to incubate the ghosts or IOVs with bulk ATP; another is to run reactions with the membrane-bound ATP synthesizing enzymes phosphoglycerate kinase (PGK) and pyruvate kinase (PK) in the forward direction (7–9). In all of these methods, the ghosts and IOVs are washed free of loading substrates, leaving only the ATP entrapped within the membrane-associated pools to energize the pumps. The ATP thus sequestered is unavailable for reaction with added hexokinase plus glucose (8, 9). Moreover, not only do the Na+ and Ca2+ pumps use the same pools of ATP, but the pools can be emptied either by running either pump forward or running the PGK reaction backward (7, 9). Pool ATP alone also has been shown to support a variety of Na+ and Ca2+ pump functions once thought to be energized by bulk ATP (9).
The hallmark test of whether or not ATP is in the confined pool is to evaluate the ability of either pump to form its respective phosphointermediate (E-P) on utilization of [γ-32P]-ATP in the presence of either Na+ or Ca2+ (7, 9, 10). If nonradioactive ATP is first entrapped within the pool, then neither of the pumps’ 32P-labeled E-P is seen until the unlabeled pools of ATP are emptied. In the case of IOVs, instead of E-Ps, ATP pool-dependent 22Na+ or 45Ca2+ uptake (transport) by their respective pumps can be measured (8, 9).
In recent studies reported in intact RBCs, the changes in nucleotide metabolism (i.e., concentrations of ATP, ADP, and AMP) were measured under conditions of marked stimulation of the Ca2+ pump (11). The patterns of changes in nucleotide concentrations were consistent with the requirement for nucleotide sequestration within the cytoskeletal–membrane complex, distinct from the nucleotides contained within the cytoplasm. Thus, these results complement the foregoing evidence for membrane pools of ATP. Importantly, that study was the first to identify ATP pools in intact cells, where previously their presence could only be inferred (12).
The present study not only confirms, by the use of different methods, the primary hallmarks for characterizing the membrane pool of ATP in ghosts as mentioned above, but also extends the analysis by identifying some of the cytoskeletal components that confine the pooled ATP. One method used to label the latter components involved an antibody raised against 8-azido-ATP (N3-ATP), a photoactivatable ATP analog (13). N3-ATP (obtained from Affinity Photoprobes) was entrapped within the ATP pool in the dark. On exposure to UV irradiation, the N3-ATP–labeled proteins were detected on Western blot analysis after solubilization and gel electrophoresis. Another method involved the use of 8-azido-[α-32P]-ATP with subsequent identification of the labeled proteins by phosphoimaging. In other experiments, N3-ATP–labeled pool proteins were identified by mass spectrometry (MS). Using these methodologies, we have unequivocally identified β-spectrin, ankyrin, glyceraldehyde-3-phosphate dehydrogenase, and band 3 as components that corral the ATP pools present in the cytoskeletal–membrane complex in RBC ghosts.
Results
Effects of Pool ATP on Pulse-Labeling of Na+ and Ca2+ Pump E-Ps.
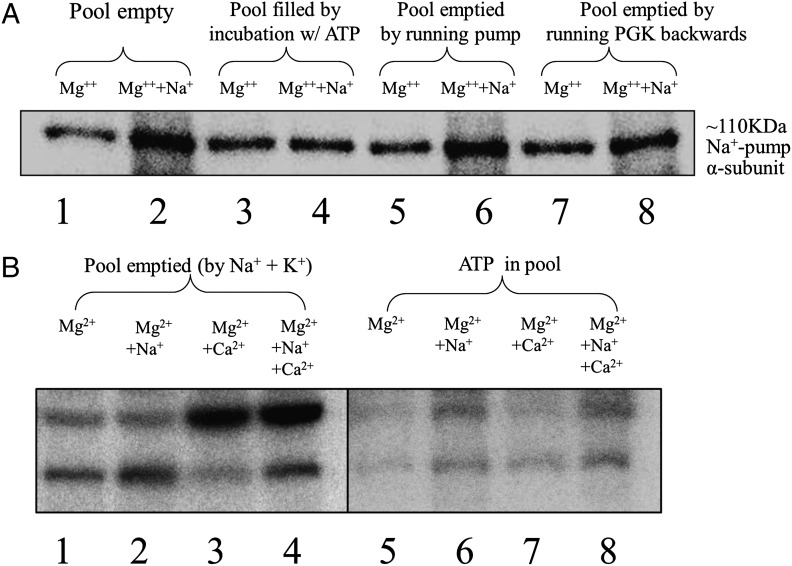
Central to the experiments described below, we felt it necessary to repeat the basic characteristics of loading and emptying the ghost pool of ATP as described above, because that work had been carried out in a different laboratory with different analytical methods. Hemoglobin-free porous (frozen-thawed) RBC ghosts were prepared from freshly drawn blood as described previously (9). These ghosts are permeable to all added constituents. Of essential importance, after all pool manipulations and incubations, the ghosts were thoroughly washed to remove all nonpool solutes (including ATP and its derivatives) from inside and outside of the ghosts. Assay for pulse-labeling of the ENa-P and ECa-P were carried out by incubation at 0 °C with ∼2 μM [γ-32P]-ATP for 20 s. All reactions were stopped by exposure to trichloroacetic acid (TCA) (Fig. 1). The precipitates were washed, solubilized in SDS, and then separated by SDS/PAGE electrophoresis. The relative radioactivity of the separated bands was assessed by phosphoimaging (Cyclone Plus Storage System; PerkinElmer). This method differs from previously used methods (9) in which total TCA precipitates were counted, thus including interference from other possible labeled constituents. The phosphoimages give precise resolution of the E-Ps of the Na+ and Ca2+ pumps (Fig. 1 A and B). Thus, it is clear that when the membrane pools contain unlabeled ATP, pulse-labeling of both the ENa-P and ECa-P with [γ-32P]-ATP is inhibited until the ATP pools have been emptied. The results presented in Fig. 1B confirm that the Na+ and Ca2+ pumps use the same pools of ATP (9).
Fig. 1.
Effect of loading the ATP pool in ghosts with nonradioactive ATP on the ability to detect [γ-32P]-labeled ENa-P and ECa-P by phosphoimaging. The ATP pool in ghosts was filled with nonradioactive ATP and then either emptied or left filled, as indicated. After addition of [γ-32P]-ATP and the desired cations, the ENa-P and ECa-P were detected by SDS/PAGE, followed by phosphoimaging. The relative intensities of these bands reflect the ability to label the ENa-P and ECa-P with a 20-s pulse of 2 μM [γ-32P]-ATP. (A) Lanes 1 and 2 show labeling of the EMg-P and ENa-P under conditions with all ATP in the pools removed by previous washing. Lanes 3 and 4 show the same experiment as in lanes 1 and 2, except with the membrane pools filled with unlabeled ATP before the addition of [γ-32P]-ATP. Lanes 5 and 6 and lanes 7 and 8 show the E-P values when the pools of ATP have been depleted either by running the Na+/K+ pump forward with Na+ and K+ (lanes 5 and 6) or by running the PGK reaction backward (lanes 7 and 8). Note that when the ATP pools are filled with unlabeled ATP (lanes 3 and 4), ENa-P and EMg-P are of similar intensity; however, if the ATP pools are emptied, thereby allowing [γ32P]-ATP to label the pumps, then the relative intensity of the band representing the ENa-P is greater than its Mg2+ counterpart. Quantitation ratios of the intensities of the bands for each pair of lanes were 2.9 for lanes 1 and 2, 0.88 for lanes 3 and 4, 2.2 for lanes 5 and 6, and 1.6 for lanes 7 and 8, with an SEM of ±3% for n = 2. (B) These lanes show the same band intensity relationships as in A for both the ENa-P (lower bands) and the ECa-P (upper bands), even when both types of E-Ps are labeled in the same incubation. Note that when the pools are filled with ATP (lanes 5–8), labeling of the E-Ps with [γ-32P]-ATP is inhibited. Moreover, when the ATP pool is emptied by running the Na+/K+ pump forward (lanes 1–4), the calcium pump also can be labeled with [γ-32P]-ATP, indicating that the Na+/K+ and Ca2+ pumps share the same ATP pools. The phosphoimages shown here are representative of multiple experiments.
Visualization of ATP Pool Filling and Emptying Using a Fluorescent ATP Analog.
The fluorescent analog used was 2′ (or 3′)-O-(2,4,6-trinitrophenyl) (TNP)-ATP or TNP-ADP. TNP-ATP is not a substrate for the Na+ pump (14, 15), but it does act like ATP, inhibiting the pumps by ∼76% when incorporated into the membrane pools (using the same protocol as with ATP). Evidence that TNP-ATP can fill the ATP pools derives from the fact that ghosts incubated with TNP-ATP cannot phosphorylate the Na+/K+ pump with [γ-32P]-ATP until the pool is emptied of TNP-ATP. Thus, the relative values of the 32P-labeled pump E-Ps measured on a 20-s exposure to [γ-32P]-ATP were 1.05 ± 0.03 pmol 32P/mg protein after preincubation without ATP, 0.13 ± 0.01 pmol 32P/mg protein after preincubation with ATP, and 0.35 ± 0.04 pmol 32P/mg protein after preincubation with TNP-ATP. Moreover, when TNP-ADP was preincubated with the ghosts and converted to TNP-ATP by running the PGK reaction forward, the level of the 32P-labeled pump phosphoimtermediate was 0.42 ± 0.01 pmol 32P/mg protein.
Fig. 2A shows a fluorescent image of porous ghosts when the pools are filled with TNP-ATP. The TNP-ATP incorporated into membrane pools has a punctate appearance, localized on the membrane, with the interior of the ghosts empty. When the pool of TNP-ATP is emptied by running the PGK reaction backward (Materials and Methods), the relative fluorescence is markedly decreased (Fig. 2B). Fig. 2 C and D shows control experiments in which the PGK reaction is dependent on its full complement of substrates, including NADH and 3-phosphoglycerate (PGA), to remove the pool of TNP-ATP. In Fig. 2C, one of the substrates (PGA) needed to run the reaction backward is omitted, and in Fig. 2D, both substrates (PGA and NADH) required in the reaction are omitted. In these two controls, membrane-associated fluorescence is similar to that shown in Fig. 2A. These results demonstrate the dynamic nature of the filling and emptying of the ATP pools.
Fig. 2.
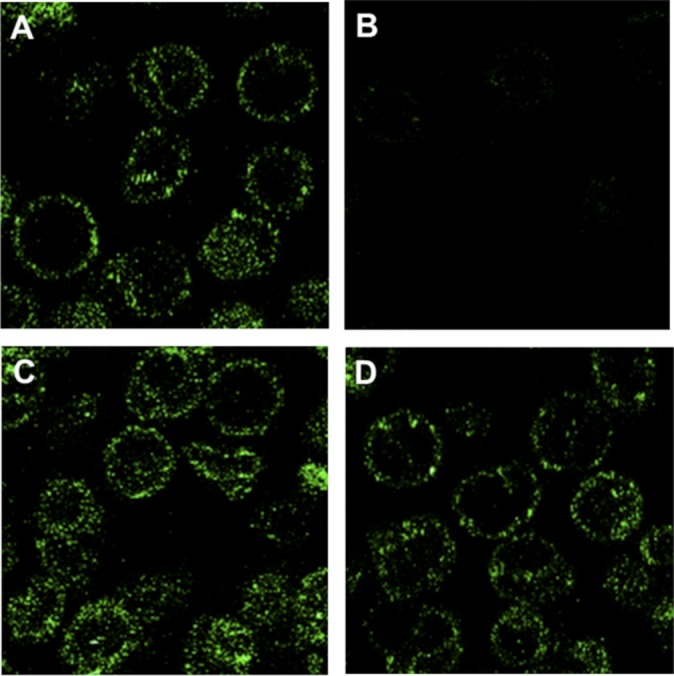
Dynamic imaging by confocal microscopy of the loading and emptying of membrane pools of ATP using the fluorescent ATP analog TNP-ATP. ATP pools in porous ghosts were either loaded or loaded and then emptied of TNP-ATP by running the PGK reaction backward. Ghosts were then observed by confocal microscopy. (A) Ghosts with their ATP pools filled with TNP-ATP. Because a large ensemble of ghosts is shown, the plane of focus passes through the center of most ghosts, showing the fluorescent ATP only on the cell periphery where the plane of focus coincides with the membrane. However, in a few ghosts, the plane of focus passes through part of the membrane, revealing pool ATP in the mid region of the cell. The fact that ghosts exhibit only punctate fluorescence in a ring on the cell periphery indicates that the TNP-ATP–loaded pools are localized to the membrane (12). (B) The entrapped TNP-ATP is labile and susceptible to removal by running the PGK reaction backward. (C and D) Controls, with one of the substrates (PGA) needed to run the PGK reaction backward omitted (C) and both substrates (PGA and NADH) omitted (D). In D, the fluorescent images of the loaded ghosts are stable even after continued incubation in the absence of both substrates. These images are similar to ones as studied by Hoffman et al. (12).
Identification of Pool Corral Components by Photolabeling with N3-ATP.
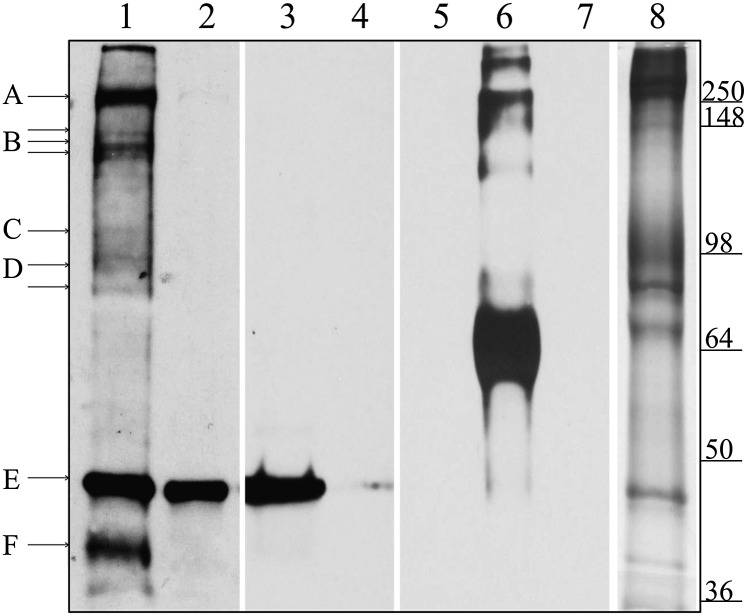
To identify the membrane proteins that form the compartment containing the entrapped ATP, the ATP pool was filled with N3-ATP, and photolabeling of pool corral components was initiated by UV illumination. To identify each photolabeled component, an antibody was raised against N3-ATP, as described in Materials and Methods, and used in immunoblots to stain those proteins photolabeled with N3-ATP. As shown in Fig. 3, the anti–N3-ATP antibody readily detected BSA photolyzed in the presence of N3-ATP (lane 6), but not unlabeled BSA (lane 5) or unlabeled RBC ghosts (lane 7).
Fig. 3.
Identification of ghost proteins labeled with pool-associated N3-ATP. Here the ghosts were filled or emptied of N3-ATP, as described in Materials and Methods. After washing, the membranes were photolyzed and separated by SDS/PAGE. Equal amounts of ghost proteins (30 μg) were loaded in all lanes. Lanes 1–7 were transferred to nitrocellulose and immunoblotted with an antibody to N3-ATP, whereas lane 8 was stained with Coomassie blue. Lane 1 shows pools filled with N3-ATP; lanes 2 and 3, pools first filled with N3-ATP and then emptied by either running the Na+ pump forward or running PGK reaction backward, respectively. In lane 4, pools are filled with N3-ATP in the presence of excess unlabeled ATP to block any specific ATP-binding sites. Lanes 5–7 show the specificity of the N3-ATP antibody, demonstrating that it labels only BSA photolyzed with N3-ATP (lane 6), not unlabeled BSA (lane 5) or unlabeled RBC ghosts (lane 7). Lane 8 shows a Coomassie blue stain of ghost proteins after separation by SDS/PAGE. The proteins tentatively identified are indicated with lettered lines. Possible protein candidates for the labeled bands are A, β-spectrin and/or ankyrin; B, Ca2+ pump (Mr ∼140 kDa) or fragments of spectrin/ankyrin; C, α-subunit of the Na+/K+ pump (Mr ∼110 kDa); D, band 3; E, actin; and F, GAPDH.
Fig. 3, lane 1, shows Western blots of N3-ATP–labeled proteins after the pools were filled with N3-ATP and the entrapped N3-ATP was photoactivated. As shown in lane 1, proteins that migrate near β-spectrin and/or ankyrin (Mr ∼250 kDa), the Ca2+ pump (Mr ∼140 kDa), or fragments of spectrin/ankyrin, the α-subunit of Na+/K+ pump (Mr ∼110 kDa), band 3, actin, and GAPDH are labeled by this protocol. Lane 2 shows a control experiment in which the pool N3-ATP was removed before exposure to UV irradiation by running the Na+ pump forward in the dark (with Na+ and K+). Importantly, labeling of all membrane proteins except actin (Mr ∼42 kDa) was prevented by this procedure, suggesting that all of the labeled proteins except possibly actin participate in fencing of the ATP pools. Lane 3 is a similar control to that shown lane 2, but with the pool N3-ATP emptied by running the PGK reaction backward in the dark (with NADH and PGA) before exposure to UV irradiation. Lane 4 shows a very different control, in which both nonspecific and pool-specific ATP binding sites were blocked by coincubation with a 20-fold excess of unlabeled ATP. In this latter case, photolabeling of the actin band was prevented as well. Inhibition of actin labeling in this latter result was obviously expected, given that actin contains an ATP-binding site that will bind ATP even in the absence of a membrane-associated pool (16, 17). Based on a comparison with the staining pattern of RBC membrane proteins (lane 8), the foregoing data collectively suggest that membrane/cytoskeletal-associated ATP pool components might include β-spectrin, ankyrin, the Ca2+ pump, the Na+/K+ pump, band 3, and GAPDH.
Analysis of ATP-Associated Membrane and Cytoskeletal Proteins Labeled with [α-32P]-N3-ATP.
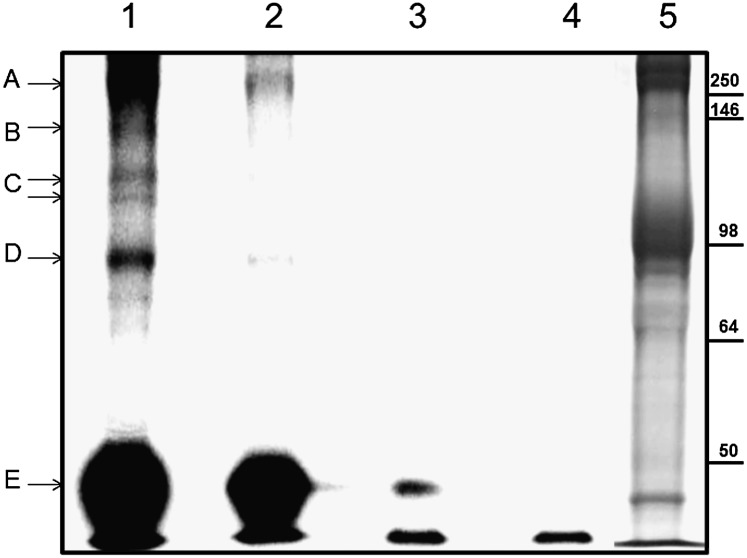
To validate the foregoing antibody staining of ATP pool components photolabeled with N3-ATP, we turned to analysis of ghost proteins photolabeled with [α-32P]-N3-ATP after using, following an analogous protocol, the radiolabeled form of the ATP analog to either fill or empty the ATP pools of ATP. Thus, autoradiographs were analyzed instead of Western blots. Fig. 4, lanes 1–4, shows autoradiographs of the labeled proteins after separation by SDS/PAGE, and lane 5 shows a Coomassie blue-stained control. Examination of lane 1 reveals that proteins that migrate near spectrin, ankyrin, the Ca2+ pump, the α-subunit of Na+/K+ pump, band 3, and actin are all radiolabeled by [α-32P]-N3-ATP. The ghosts in lane 2 were emptied in the dark of entrapped [α-32P]-N3-ATP by running the Na+/K+ pump forward with Na+ plus K+ before exposure UV irradiation. Lanes 3 and 4 are controls in which the pool was filled (lane 3) with or emptied of (lane 4) the radiolabeled ATP analog, but with neither lane exposed to UV irradiation. It is evident that, except for actin, the only membrane proteins labeled are those exposed to the conditions described for lane 1.
Fig. 4.
Labeling of ATP pool-associated membrane proteins with [α-32P]-N3-ATP. This was accomplished following the same protocol used for N3-ATP. Labeled proteins were separated by SDS/PAGE and analyzed with a Cyclone phosphoimager. Lanes 1–4 show phosphoimages of ghost proteins after separation. Lane 5 is the Coomassie blue-stained counterpart. The proteins tentatively identified are indicated with arrows. Possible protein candidates for the labeled bands are: A, β-spectrin and/or ankyrin; B, Ca2+ pump (Mr ∼140 kDa) or fragments of spectrin/ankyrin; C, α-subunit of the Na+/K+ pump (Mr ∼110 kDa); D, band 3; and E, actin.
MS Identification of Specific Peptides of ATP Pool-Associated Membrane Proteins Labeled with N3-ATP.
We first evaluated whether the liquid chromatography–tandem MS (LC-MS/MS) protocol used (Materials and Methods) would provide the necessary sensitivity to detect the peptides of ghost proteins. For this purpose, a crude trypsin digest of ghost proteins was loaded onto a C18 column of an Eksigent Ultra2D NanoLC system, which was coupled inline to a hybrid dual-cell linear trap-orbitrap mass spectrometer (LTQ-Orbitrap Velos; Thermo Fisher) for peptide sequencing. Peptides from all ghost membrane/cytoskeletal proteins were detected (Table S1).
To determine which ghost proteins might be involved in corralling the ATP entrapped within the membrane pools, we first photolabeled the pool-associated elements with N3-ATP as described earlier (Fig. 3). We then isolated the ATP-derivitized peptides by affinity chromatography with anti-ATP antibody-coated beads and/or a polymer-based metal ion capturing (PolyMAC) reagent, as described in Materials and Methods. To detect N3-ATP labeled peptides by MS, we found it necessary to first treat the samples with alkaline phosphatase to remove negatively charged phosphates from the labeled peptides. This step gave us the ability to identify specific peptides labeled with N3-ATP when the ATP pool was first filled with the photoactivatable ATP.
Peptides of β-spectrin, ankyrin, band 3, and actin (Table 1) were found to contain the extra mass attributed to N3-ATP labeling. The ATP labeling on β-spectrin is at the extreme COOH terminus, near the tetramerization site and just beyond the ankyrin- binding site (18, 19). Ankyrin is labeled in its ZU-5 domain, within the spectrin-binding sequence of ankyrin (20, 21). Band 3 is labeled specifically at a glycolytic enzyme-binding site that can associate with GAPDH, aldolase, phosphofructokinase, and lactate dehydrogenase. Actin is labeled on amino acids proximal to its known N3-ATP–binding site (17). GAPDH seen on the immunoblots of labeled proteins (Fig. 3) was not detected in our MS/MS analyses. However, for unknown reasons, only 9% of GAPDH peptides in fresh preparations of trypsin-digested ghosts could be detected by this procedure (Table S1), suggesting that GAPDH peptides may be photolabeled, as indicated in Fig. 3, but not readily detected by MS. Although proteins corresponding to the molecular weights of the catalytic subunits of both the Na+/K+ and Ca2+ pumps were seen in the immunoblots of N3-ATP–labeled proteins, neither of these polypeptides was identified by MS.
Table 1.
N3-ATP–labeled peptides of proteins that entrap the membrane-associated pool of ATP
| Protein description | Accession no. | Peptide sequence |
| Spectrin β chain | P11277 | 2124-SSWESLQPEPSHPY-2137 |
| Ankyrin | P16157 | 961-TPPPLAEEEGLSDR-974 |
| Ankyrin | P16157 | 1069-LCQDYDTIGPEGGSLK-1084 |
| Band 3 | P02730 | 361-GLDLNGGPDDPLQQTGQL-378 |
| Actin | P68133 | 121-QIMFETFNVPAMYVAIQAVLSLYASGR-147 |
The letters in bold type in each sequence represent the amino acids directly labeled with N3-ATP.
Discussion
Previous work has established that ATP is sequestered within compartments residing in the membrane–cytoskeleton complex in human RBC ghosts. The ATP so entrapped serves as the preferential and proximal substrate for both the Na+/K+ and Ca2+ pumps. The main results reported in this paper identify several of the membrane and cytoskeletal components that serve to corral the local pools of ATP, including β-spectrin, ankyrin, GAPDH, and band 3 (Figs. 3 and 4 and Table 1). The methods used to identify these proteins involve first filling the pool with N3-ATP, then after photolysis, identifying the proteins in SDS polyacrylamide gels or isolating the N3-ATP–labeled peptides and determining their identities by MS. Clearly, we have not identified all of the elements involved in the corral, including some unknown protein bands labeled in Figs. 3 and 4, but not detected by MS. Moreover, we detected no peptides from either the Na+/K+ or Ca2+ pump on our MS analyses, even though polypeptides corresponding to their respective molecular weights (∼110 kDa for the Na+/K+ pump and ∼140 kDa for the Ca2+ pump) are labeled with the N3-ATP in Figs. 3 and 4. We presume that this inability to detect the two pumps by MS derives from (i) the poor capacity of their peptides to ionize in the mass spectrometer, as demonstrated by our inability to detect any peptides from either pump in MS analyses of unmodified membranes (Table S1); (ii) their relatively low copy numbers in the ATP pool complex; or (iii) the stringent threshold that we set for inclusion of an N3-ATP–labeled peptide in our MS dataset.
Several aspects of our results merit further comment. First, it is clear that the entrapped ATP cannot be tightly bound, because strongly immobilized ATP cannot serve as a substrate for either the glycolytic enzymes, PGK and PK, or the Na+/K+ or Ca2+ pump. Nevertheless, it is likely that the entrapped ATP, estimated as 100–600 molecules per pool (7), resides in a hydrophobic environment, given that the fluorescence of TNP-ATP is largely quenched in aqueous solution, but becomes more intense as solvent polarity declines (14). The strong fluorescence of TNP-ATP entrapped in the ghost membrane pool (Fig. 2) is consistent with a hydrophobic ATP compartment.
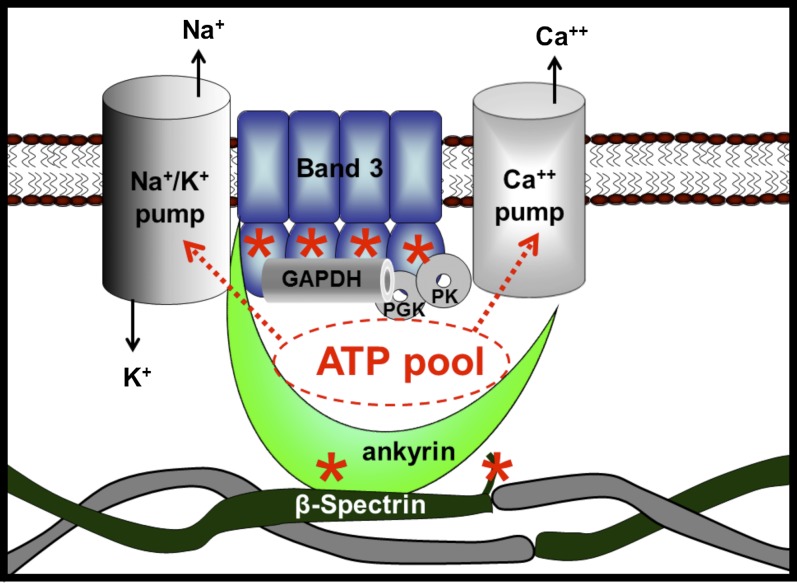
Our results also raise the question of how the corrals that harbor the ATP pool relate to the known structures of the membrane–cytoskeletal complexes (22–24). The major RBC membrane complexes are of two types, a junctional complex and an ankyrin complex, with some overlap in the constituents of the two (25, 26). The prime distinctions between the two complexes lie in the fact that the former contains actin, adducin, and protein 4.1, whereas the latter contains ankyrin. Our results clearly demonstrate that N3-ATP labels ankyrin in its ZU5 domain, which contains the binding site for β-spectrin (20) and lies adjacent to the ankyrin-binding site for band 3 (27, 28). N3-ATP also labels β-spectrin in a flexible region near the site responsible for association of αβ-spectrin dimers to tetramers and not too distant from the ankyrin-binding site on β-spectrin (18–20). In addition, N3-ATP labels band 3 near the junction of its cytoplasmic and membrane-spanning domains within a peptide recently found to include a second binding site for GAPDH. Because this peptide resides close to the docking site of ankyrin on band 3 (27, 28), and because ankyrin is known to bind the cytoplasmic domain of the Na+ pump (29), it can be argued that all of the prominent N3-ATP–labeling sites on the membrane except actin appear to reside within or near the ankyrin–band 3 complex, as depicted in Fig. 5.
Fig. 5.
Possible arrangement of membrane components that form the ATP pools that fuel the cation pumps. Membrane components that were photolabeled with N3-ATP and identified by MS are shown in color, and other membrane components implicated in the pool’s architecture but not identified by MS are shown in gray. The locations of the major N3-ATP–labeled peptides in ankyrin (residues 961–974), band 3 (residues 361–378), and β-spectrin (residues 2124–2137) are marked with an asterisk.
The fact that N3-ATP labels actin, which resides at the junctional complex at least ∼30 nm from the ankyrin complex, warrants further discussion. Although an actin-associated ATP pool cannot be unequivocally excluded by our data, the fact that labeling of actin was not prevented by emptying N3-ATP from the pool by running either the Na+ pump forward or the PGK reaction backward (Fig. 3, lanes 2 and 3) suggests that actin labeling is not mediated by N3-ATP within the pool. We suggest instead that N3-ATP bound to the ATP-binding site on actin is responsible for this prominent labeling. This conclusion is supported by the observation that addition of excess unlabeled ATP competitively blocks N3-ATP labeling of actin in porous ghosts (Fig. 3, lane 4).
Another problem concerns the locations of the two enzymes (PGK and PK) that provide ATP for the pool. Although recent data from our laboratory suggest that PK, like many other glycolytic enzymes, binds to band 3, no information exists on the location of PGK on the membrane. Nevertheless, association of PGK with the membrane appears likely, given the report that an antibody to PGK inhibits the activity of purified PGK, but not PGK bound to the RBC membrane (30), indicating that PGK could be tightly associated with pool ATP in the RBC membrane.
Finally, it is not known what other functions might be associated with the RBC membrane ATP pool. Possible candidates include providing fuel for an ATP-dependent glucose transporter (31), energizing a flippase (32), or supplying the ATP for the deoxygenation-promoted ATP release pathway (33, 34). With regard to the latter, we have preliminary evidence that TNP-ATP entrapped in the ATP pool of resealed ghosts is released to the outside of the ghosts on deoxygenation.
Materials and Methods
Loading and Emptying the Membrane Pools of ATP in Porous Ghosts.
Two incubations, where indicated, were needed, the first to fill the pools and the second to empty them of entrapped ATP. Thus, whether or not the pools were empty could be determined by a pulse chase experiment with 2 μM [γ32-P]-ATP for 20 s at 0 °C, as described above. All incubations and ATP type pool manipulations were carried out using the methods and solutions described previously (7, 9).
The first incubation was carried out for 30 min at 37 °C in solution (A), containing 10 mM Tris (pH 7.5), 40 mM NaCl, 2 mM MgCl2, and 0.25 mM EDTA. In addition, for those ghosts in which the pools were to be loaded with ATP, solution (A) also contained 1.5 mM ATP. The ghosts were washed (always with 17 mM Tris, pH 7.5) and then exposed to a second incubation for another 15 min at 37 °C in solution (A), but this time the buffer also contained either 10 mM KCl (to run the Na+/K+ pump forward) or solution (B) (5 mM MgSO4, 17.5 mM NaHCO3, 20 mM cysteine, 50 mM glycine, 5 mM 3-phosphoglyceric acid, and 0.25 mM NADH; pH 7.0), to run the phosphoglycerate (PGK) reaction backward. In other experiments, the ATP pools also could be filled by running the PGK reaction forward. This was done using solution (C), containing 0.83 mM Triose-P, 0.415 mM NAD, 0.25 mM ADP, 5.0 mM MgSO4, 50.0 mM Na2HPO4/NaH2PO4, and 132 mM glycine. The ghosts were always washed to remove all bulk substrates before attempts to pulse-label the Na+/K+ pump or Ca2+ pump with [γ-32P]-ATP. After pulse-labeling, the reaction was stopped by adding a solution containing 2.5% TCA, 1 mM ATP, and 1 mM K3PO4. The precipitate was washed with this solution before preparation for analysis by phosphoimaging.
For the results shown in Fig. 1B, the experiments were performed by loading the pool with ATP as before, by first incubating at 37 °C for 30 min in modified solution (A) containing 1.5 mM ATP, 15 mM Tris (pH 7.5), 12 μM MgCl2, 50 mM NaCl, or 50 mM NMDG. The second incubation, to unload the pooled ATP, was carried out as described above for running the Na+ pump forward with Na+ plus K+. The ghosts were then washed before pulse-labeling with [γ-32P]-ATP to measure Na+ as well as Ca2+ phosphoproteins, as described previously.
Fluorescence Microscopy.
The ghost’s ATP pools were either loaded with TNP-ATP or loaded, then emptied of TNP-ATP following the same protocol as described for the loading manipulations with ATP. The ghosts were washed and imaged with an Olympus FluoView FV1000 inverted confocal microscope with a 60× oil-immersion objective.
Production and Use of a Polyclonal Antibody to N3-ATP.
Keyhole limpet hemocyanin photolabeled with N3-ATP (13) was used as an antigen. UV photolysis was carried out in PBS buffer (pH 7.4) at room temperature. The antigen suspended in Gold Adjuvant Reagent (Sigma-Aldrich) was injected into rabbits at regular intervals over a 4-mo period. The separated serum was stored at −80 °C until use. Specificity of the antibody was determined by Western blot analysis (Fig. 3).
Photolabeling and Detection of Pool-Related Protein Components by N3-ATP.
The ghost’s ATP pools were loaded with N3-ATP in the dark following the same protocol as for ATP. The ghosts were washed before exposure to UV irradiation, which resulted in photolabeling of the proteins residing adjacent to the entrapped N3-ATP. The ghosts were solubilized with 2% SDS, and protein concentration was determined using the Micro BCA Assay Kit (Thermo Scientific). Ghost proteins were separated by SDS/PAGE, with the N3-ATP–labeled proteins detected by Western blot analysis using the anti–N3-ATP antibody and the Thermo Scientific Enhanced Chemiluminescence Kit.
Radiolabeling and Detection of ATP Pool-Associated Protein Components.
These experiments were carried out using the same protocol described above for N3-ATP–labeled protein components. After separation by SDS/PAGE, the [32P]-labeled proteins were detected by phosphoimaging.
Purification of N3-ATP–Photolabeled Peptides.
ATP pool components of ghosts were photolabeled with N3-ATP, as described above, with 2% C12E8 (Affymetrix) in 100 mM Tris-HCl (pH 7.5) used to solubilize the ghosts. The samples were then diluted 20 times with 100 mM Tris-HCl and precleared with rabbit preimmune IgG immobilized on Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) following the manufacturer’s protocol. N3-ATP–labeled proteins were purified by immobilized anti–N3-ATP antibody on Affi-Gel Hz hydrazide gel (Bio-Rad), then trypsin-digested to prepare a mixture of labeled and unlabeled peptides. PolyMAC reagent functionalized with Ti (IV) ions (Tymora Analytical) was used to separate N3-ATP–labeled peptides from unlabeled peptides as described previously (35). Alkaline phosphatase (New England BioLabs) was added to remove the 5′-phosphate from N3-ATP, and then removed from the peptide mixture using a 10-kDa cutoff protein concentrator (Millipore). Peptide samples were desalted using a Sep-Pak C18 desalting column (Waters) before MS analysis. In some studies, total membrane protein extracts were first digested with trypsin (35), and then N3-ATP–labeled peptides were isolated as described above.
LC-MS/MS Analysis.
Tryptic peptides were analyzed by LC-MS/MS on a high-resolution hybrid duel-cell linear ion trap orbitrap mass spectrometer (LTQ-Orbitrap Velos; Thermo Fisher) interfaced with an Eksigent Ultra 2D NanoLC HPLC system. Peptide samples were dissolved in 8 μL of 0.1% formic acid and injected into a capillary column (75 μm i.d. and 12 cm bed length) packed with 5 μm of C18 Magic beads resin (Michrom). Peptides were then eluted at 300 nL/min using a 0–100% acetonitrile gradient over 90 min. The electrospray ionization emitter tip was generated on the column with a laser puller (model P-2000; Sutter Instruments). The mass spectrometer was operated in the data-dependent mode in which a full-scan MS (from m/z 300–1,700 with a resolution of 60,000 at m/z 400) was followed by 20 MS/MS scans of the most abundant ions. Ions with a charge state of +1 were excluded. The mass exclusion time was 90 s.
Peptide Identification.
The LTQ-Orbitrap raw files were searched directly against a Homo sapiens database with no redundant entries (67,250 entries; human International Protein Index v.3.64) using the SEQUEST algorithm on Proteome Discoverer version 1.2 (Thermo Fisher) or Sorcerer IDA server version 2.5.6 (Sage-N Research). Proteome Discoverer created DTA files from the raw data with a minimum ion threshold of 15 and absolute intensity threshold of 50. Peptide precursor mass tolerance was set at 10 ppm, and MS/MS tolerance was set at 0.8 Da. Search criteria included a static modification of cysteine residues of +57.0214 Da, a variable modification of +15.9949 Da to include potential oxidation of methionine residues. N3-ATP–photolabeled peptides were identified based on the additional mass associated with the N-adenosine adduct (+280.092 Da) or the N-adenine adduct (+148.050 Da). False discovery rates were set for 1%.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 GM24417 (to P.S.L.) and R01 GM088317 (to W.A.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209014109/-/DCSupplemental.
References
- 1.Alberts B, et al. In: Molecular Biology of the Cell. 4th Ed. Alberts B, et al., editors. New York: Garland Science; 2002. pp. 659–669. [Google Scholar]
- 2.Srere PA. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- 3.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- 4.Schatzmann HJ. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966;22:364–365. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- 5.Knauf PA, Proverbio F, Hoffman JF. Electrophoretic separation of different phophosproteins associated with Ca-ATPase and Na, K-ATPase in human red cell ghosts. J Gen Physiol. 1974;63:324–336. doi: 10.1085/jgp.63.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman JF. The link between metabolism and active transport of sodium in human red cell ghosts. J Membr Biol. 1980;57:143–161. doi: 10.1007/BF01869000. [DOI] [PubMed] [Google Scholar]
- 7.Proverbio F, Hoffman JF. Membrane compartmentalized ATP and its preferential use by the Na,K-ATPase of human red cell ghosts. J Gen Physiol. 1977;69:605–632. doi: 10.1085/jgp.69.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercer RW, Dunham PB. Membrane-bound ATP fuels the Na/K pump: Studies on membrane-bound glycolytic enzymes on inside-out vesicles from human red cell membranes. J Gen Physiol. 1981;78:547–568. doi: 10.1085/jgp.78.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman JF, Dodson A, Proverbio F. On the functional use of the membrane compartmentalized pool of ATP by the Na+ and Ca++ pumps in human red blood cell ghosts. J Gen Physiol. 2009;134:351–361. doi: 10.1085/jgp.200910270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blostein R. Relationships between erythrocyte membrane phosphorylation and adenosine triphosphate hydrolysis. J Biol Chem. 1968;243:1957–1965. [PubMed] [Google Scholar]
- 11.Tiffert T, Lew VL. Elevated intracellular Ca2+ reveals a functional membrane nucleotide pool in intact human red blood cells. J Gen Physiol. 2011;138:381–391. doi: 10.1085/jgp.201110660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman JF. ATP compartmentation in human erythrocytes. Curr Opin Hematol. 1997;4:112–115. doi: 10.1097/00062752-199704020-00006. [DOI] [PubMed] [Google Scholar]
- 13.Haley BE, Hoffman JF. Interactions of a photo-affinity ATP analog with cation-stimulated adenosine triphosphatases of human red cell membranes. Proc Natl Acad Sci USA. 1974;71:3367–3371. doi: 10.1073/pnas.71.9.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moczydlowski EG, Fortes PA. Characterization of 2′,3′-O-(2,4,6-trinitrocyclohexadienylidine)adenosine 5′-triphosphate as a fluorescent probe of the ATP site of sodium and potassium transport adenosine triphosphatase: Determination of nucleotide binding stoichiometry and ion-induced changes in affinity for ATP. J Biol Chem. 1981;256:2346–2356. [PubMed] [Google Scholar]
- 15.Moczydlowski EG, Fortes PA. Inhibition of sodium and potassium adenosine triphosphatase by 2′,3′-O-(2,4,6-trinitrocyclohexadienylidene) adenine nucleotides: Implications for the structure and mechanism of the Na:K pump. J Biol Chem. 1981;256:2357–2366. [PubMed] [Google Scholar]
- 16.Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin:DNase I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- 17.Hegyi G, Szilagyi L, Elzinga M. Photoaffinity labeling of the nucleotide binding site of actin. Biochemistry. 1986;25:5793–5798. doi: 10.1021/bi00367a067. [DOI] [PubMed] [Google Scholar]
- 18.Ipsaro JJ, et al. Crystal structure and functional interpretation of the erythrocyte spectrin tetramerization domain complex. Blood. 2010;115:4843–4852. doi: 10.1182/blood-2010-01-261396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ipsaro JJ, Mondragón A. Structural basis for spectrin recognition by ankyrin. Blood. 2010;115:4093–4101. doi: 10.1182/blood-2009-11-255604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ipsaro JJ, Huang L, Gutierrez L, MacDonald RI. Molecular epitopes of the ankyrin-spectrin interaction. Biochemistry. 2008;47:7452–7464. doi: 10.1021/bi702525z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ipsaro JJ, Huang L, Mondragón A. Structures of the spectrin-ankyrin interaction binding domains. Blood. 2009;113:5385–5393. doi: 10.1182/blood-2008-10-184358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salomao M, et al. Protein 4.1R-dependent multiprotein complex: New insights into the structural organization of the red blood cell membrane. Proc Natl Acad Sci USA. 2008;105:8026–8031. doi: 10.1073/pnas.0803225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campanella ME, et al. Characterization of glycolytic enzyme interactions with murine erythrocyte membranes in wild-type and membrane protein knockout mice. Blood. 2008;112:3900–3906. doi: 10.1182/blood-2008-03-146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci USA. 2005;102:2402–2407. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anong WA, et al. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood. 2009;114:1904–1912. doi: 10.1182/blood-2009-02-203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baines AJ. The spectrin-ankyrin-4.1-adducin membrane skeleton: Adapting eukaryotic cells to the demands of animal life. Protoplasma. 2010;244:99–131. doi: 10.1007/s00709-010-0181-1. [DOI] [PubMed] [Google Scholar]
- 27.Chang SH, Low PS. Identification of a critical ankyrin-binding loop on the cytoplasmic domain of erythrocyte membrane band 3 by crystal structure analysis and site-directed mutagenesis. J Biol Chem. 2003;278:6879–6884. doi: 10.1074/jbc.M211137200. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96:2925–2933. [PubMed] [Google Scholar]
- 29.Zhang Z, Devarajan P, Dorfman AL, Morrow JS. Structure of the ankyrin-binding domain of alpha-Na,K-ATPase. J Biol Chem. 1998;273:18681–18684. doi: 10.1074/jbc.273.30.18681. [DOI] [PubMed] [Google Scholar]
- 30.Okonkwo PO, Longenecker G, Askari A. Studies on the mechanism of inhibition of the red cell metabolism by cardiac glycosides. J Pharmacol Exp Ther. 1975;194:244–254. [PubMed] [Google Scholar]
- 31.Hebert DN, Carruthers A. Direct evidence for ATP modulation of sugar transport in human erythrocyte ghosts. J Biol Chem. 1986;261:10093–10099. [PubMed] [Google Scholar]
- 32.Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: Relation to shape changes. Proc Natl Acad Sci USA. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis IA, Campanella ME, Markley JL, Low PS. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci USA. 2009;106:18515–18520. doi: 10.1073/pnas.0905999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 35.Iliuk AB, Martin VA, Alicie BM, Geahlen RL, Tao WA. In-depth analyses of kinase-dependent tyrosine phosphoproteomes based on metal ion-functionalized soluble nanopolymers. Mol Cell Proteomics. 2010;9:2162–2172. doi: 10.1074/mcp.M110.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.