Abstract
Genetic variation in the IL-7 receptor-α (IL-7R) gene is associated with susceptibility to human type 1 diabetes (T1D). Here we investigate the therapeutic efficacy and mechanism of IL-7Rα antibody in a mouse model of T1D. IL-7Rα antibody induces durable, complete remission in newly onset diabetic mice after only two to three injections. IL-7 increases, whereas IL-7Rα antibody therapy reduces, the IFN-γ–producing CD4+ (TH1) and IFN-γ–producing CD8+ T cells. Conversely, IL-7 decreases and IL-7Rα antibody enhances the inhibitory receptor Programmed Death 1 (PD-1) expression in the effector T cells. Programmed Death 1 blockade reversed the immune tolerance mediated by the IL-7Rα antibody therapy. Furthermore, IL-7Rα antibody therapy increases the frequency of regulatory T cells without affecting their suppressor activity. The durable efficacy and the multipronged tolerogenic mechanisms of IL-7Rα antibody therapy suggest a unique disease-modifying approach to T1D.
Keywords: T cell depletion, programmed death ligand 1, biologics, adoptive transfer
Type I diabetes (T1D) in both humans and animal models, such as nonobese diabetic (NOD) mice, is a complex, multifactorial autoimmune disease in which the islet-specific T-cell immune response destroys insulin-producing β-cells in the islets of Langerhans (1–4). At the clinical onset of diabetes, some residual β-cells still produce insulin, offering a potential window for therapeutic intervention to stop the autoimmune destruction and preserve β-cell function (5).
MHC class II genes are the major genetic loci determining the susceptibility of T1D in human and NOD mice, although MHC class II genes alone cannot fully account for genetic predisposition to T1D (6, 7). Recently, a SNP in the IL-7 receptor (IL-7Rα) gene was identified as one of the non-MHC–linked loci associated with risk of multiple sclerosis (8–10) and T1D (11, 12). IL-7 is a major survival factor implicated in mouse and human immune homeostasis and disorders (13). IL-7 is essential for the homeostatic proliferation of naïve T cells and also contributes to that of CD8+ memory T cells in mice (14, 15). Provision of exogenous IL-7 or lymphopenia-induced production of IL-7 can promote the expansion of self-reactive T-cell clones (16). The IL-7 receptor is composed of two subunits: the common γ-chain and the IL-7Rα chain. In humans, IL-7Rα deficiency results in the absence of T cells but B-cell counts remain normal (17), whereas the IL-7Rα KO mice are essentially devoid of T and B cells (18), suggesting that the effect of IL-7/IL-7Rα signaling in T-cell development is shared between humans and mice.
The islet autoreactive CD4+ helper T (TH) cells and CD8+ cytotoxic T (TC) cells are involved in the immune pathogenesis of human T1D and NOD mice (19–25). Recently we showed that IL-7 can promote the development of IFN-γ–producing TH1 cells, but not IL-17–producing TH17 cells, from the naïve T cells of humans and of the C57BL/6 mice (26). This finding raises an intriguing possibility that IL-7/IL-7Rα pathway may be linked to T1D risk at least in part through the regulation of TH1 development. In addition, it was not known whether IL-7 could influence the development of the IFN-γ–expressing CD8+ TC1 cells.
The activities of CD4+ and CD8+ T effector cells (Teffs) are normally under stringent control by a number of negative regulators expressed on their surface. One major negative regulator, Programmed Death 1 (PD-1) interacts with its ligand (PD-L1) (27) to maintain the robust long-term tolerance of Teffs in the inflamed tissue (28–30). IL-7 was recently shown to down-regulate PD-1 in a murine viral infection model (31). It is thus pertinent to ask whether IL-7 may contribute to the PD-1 down-regulation in the NOD mice.
In this study we investigate how the IL-7/IL-7Rα pathway contributes to the development of T1D in the NOD mouse model by using IL-7Rα blocking antibodies. We also elucidate the cellular and molecular mechanisms that underlie the promising therapeutic efficacy of IL-7Rα antibody therapy.
Results
IL-7Rα Antibody Treatment Showed Efficacy in the Prevention of Diabetes.
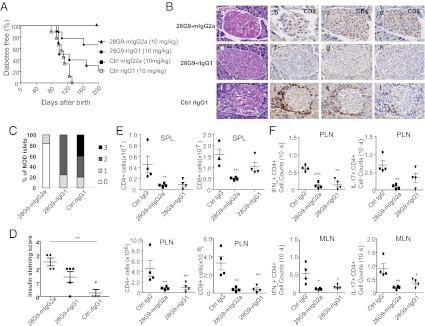
To study the role of IL-7/IL-7Rα pathway in T1D, female NOD mice were given either 10 mg/kg of control IgG (mIgG2a or rat IgG1) or an anti–IL-7Rα, 28G9 (of rat IgG1 isotype or rIgG1), or 28G9-mIgG2a in which the Fc portion of the original rIgG1 clone is replaced with that of mouse IgG2a (Table S1). In the prophylactic treatment paradigm, we administered these different antibodies to NOD mice once weekly from 9 wk of age till the end of the study. We found that 100% of NOD mice were prevented from diabetes by 28G9-mIgG2a, 67% (six of nine) by 28G9-rIgG1 compared with 0–20% of mice treated with rat IgG1 isotype or mouse IgG2a isotype controls, respectively (Fig. 1A). We also observed a pronounced dose-dependent effect of 28G9-rIgG1 in the prophylactic paradigm (Fig. S1 A–C).
Fig. 1.
IL-7Rα antibody show antidiabetic efficacy in the prophylactic treatment. (A) Diabetes incidence in NOD mice treated with 10 mg/kg of 28G9-mIgG2a (n = 10), 28G9 (rat IgG1), mIgG2a (n = 10), or rat IgG1 (n = 9), starting at 9 wk of age until 29 wk of age, at which time the tissues were analyzed. Data are from one representative experiment of two independent experiments: 28G9-mIgG2a vs. mIgG2a P = 0.001; 28G9-mIgG2a vs. rat IgG1 P < 0.001; 28G9-rIgG1 vs. rat IgG1 P = 0.001; 28G9-rIgG1 vs. mIgG2a P = 0.037; and with P > 0.05 for all of the rest of pair comparison (Log-rank test). (B) Histological and immunohistochemical analysis of insulitis in mice treated with (a–d) 28G9-mIgG2a, (e–h) 28G9-rIgG1, (i–l) control IgG; (a, e, and i) H&E staining. Immunostaining of distinct cell subsets was performed using mAbs against CD3 (b, f, j), CD4 (c, g, k), and CD8 (d, h, l) at 29 wk of age. (Magnification: 20×.) (C) Intraislet infiltration in 28G9-mIgG2a, 28G9-rIgG1, or isotype control-treated mice was quantified histologically at 20–30 wk of age. The graph shows the fraction of islets with no infiltration (0), peri-insulitis (1), moderate insulitis with <50% islet area infiltrated (2), or severe insulitis with >50% islet area infiltrated (3), respectively. Data are from six to nine mice per group. (D) Insulin-staining score from treated animals, n = 4–5 from two experiments. Data in C and D are from one representative experiment of two independent experiments including four animals per group. (E) Absolute cell counts of CD4+, CD8+ from spleen (SPL) and PLN of 28G9-mIgG2a, 28G9-rIgG1, or IgG control-treated mice at 12 wk of age. (F) Absolute cell counts of IFN-γ+CD4+, IL-17+CD4+ from mesenteric lymph node (MLN) and PLN of 28G9-mIgG2a, 28G9-rIgG1, or isotype control at 12-wk of age. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA with post tests relative to the control IgG group).
Histological examination of mice at the end of the prophylactic experiment revealed that pancreatic islets were heavily infiltrated by T cells in control IgG-treated mice, but less in those treated with 28G9-mIgG2a or with 28G9-rIgG1 (Fig. 1 B and C). Moreover, insulin staining in the islets of the 28G9-mIgG2a–treated mice was significantly higher than that in the control IgG-treated group (Fig. 1D).
In the spleen of NOD mice, CD4+ and CD8+ T cells expressed high levels of IL-7Rα, B cells expressed low levels of IL-7Rα, but regulatory T cells (Tregs), NK, and CD11b+ cells did not express IL-7Rα (Fig. S2A). We also confirmed that CD4+ and CD8+ naïve, effector memory, and central memory T cells from NOD mice all expressed IL-7Rα compared with the isotype IgG staining (Fig. S2B). In consonance with the cellular expression pattern of IL-7Rα, 28G9-mIgG2a, and 28G9-rIgG1 reduced CD4+ and CD8+ T cells in most of the lymphoid tissues (Fig. 1E, and Figs. S1D and S2C). Similarly, the pathogenic TH1 and TH17 cells were significantly reduced in the mesenteric lymph nodes and pancreatic lymph nodes (PLNs) by 28G9-mIgG2a and by 28G9-rIgG1 (Fig. 1F). B and NK cell populations remained unaffected by any antibody tested in most peripheral tissues (Fig. S2 D and E). These initial data suggested that the IL-7Rα antibody primarily targets CD4+ and CD8+ T cells in the NOD mice. In later studies described below, however, we found that T-cell depletion was not absolutely required for the therapeutic efficacy.
IL-7Rα Antibody Treatment Induces Durable Disease Remission in Newly Established Diabetes.
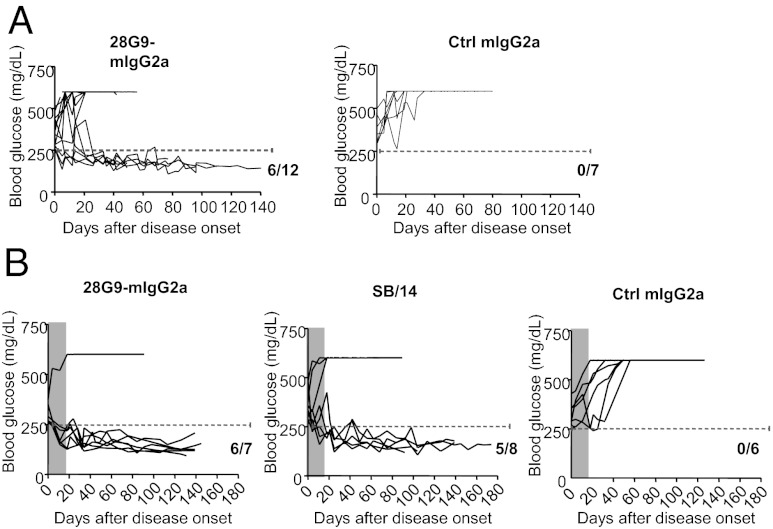
Next, we assessed whether IL-7Rα antibody can reverse established diabetes in NOD mice. In one experiment we found that weekly administration of 28G9-mIgG2a led to remission in 50% (6 of 12) of newly onset diabetic mice (Fig. 2A). These 28G9-mIgG2a–treated mice remained normoglycemic over a long period compared with the control IgG-treated mice (n = 7), none of which could maintain normoglycemia (Fig. 2A).
Fig. 2.
Diabetes remission induced by IL-7Rα antibody therapy after disease onset. (A) Newly onset diabetic NOD mice (based on two consecutive blood-glucose concentrations over 250 mg/dL) were treated with 10 mg/kg of 28G9-mIgG2a (n = 12) or control IgG (n = 7) once a week. Blood glucose was monitored. (B). Another cohort of newly onset diabetic NOD mice were treated with 10 mg/kg of 28G9-mIgG2a (n = 7), or SB/14 (n = 8), or isotype control (n = 6) once a week for 3 wk. Gray-shaded areas indicate the treatment period. 28G9-mIgG2a vs. Ctrl IgG P = 0.004; SB/14 vs. Ctrl IgG P = 0.028; 28G9 vs. SB/14 P = 0.33, Fisher’s exact test.
In another cohort of NOD mice, 86% remission rate (n = 7) was achieved in the newly onset diabetic mice with a short course of three injections of 28G9-mIgG2a antibody (Fig. 2B), compared with 0% remission rate (n = 6) in the isotype control IgG group. We included another clone of IL-7Rα antibody, SB/14 (BD Biosciences), with minimal binding to mouse Fcγ receptors (Table S1) and found a 63% remission rate (n = 8), which is statistically indistinguishable from the efficacy of 28G9-mIgG2a (Fig. 2B) (P = 0.004 for 28G9-mIgG2a vs. control IgG; P = 0.028 for SB/14 vs. control IgG; and P = 0.33 for 28G9-mIgG2a vs. SB/14, Fisher’s exact test). As expected, the CD4+ and CD8+ T-cell counts in the peripheral blood of NOD mice after short-term treatment with 28G9-mIgG2a were significantly reduced relative to those with isotype control (P < 0.01) (Fig. S3). In contrast, SB/14 did not change CD4+ and CD8+ T-cell numbers in the peripheral blood of NOD mice (not significant) (Fig. S3). Both 28G9-mIgG2a and SB/14 antibodies showed similar degrees of blockade of IL-7–mediated STAT5 phosphorylation (26). Thus, the blockade of IL-7 signaling alone appears to be sufficient to confer the long-lasting antidiabetic efficacy without affecting the circulating T-cell numbers.
Role of IL-7 in Mouse TH and TC Cell Differentiation and Type 1 Diabetes.
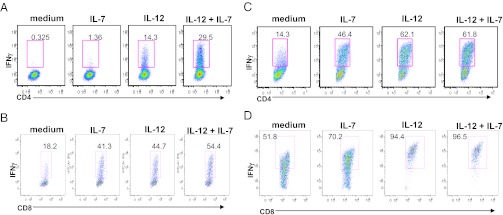
We asked whether IL-7/IL-7R signaling may regulate TH1 and TC1 cell differentiation in the NOD mice. Sorted CD4+ or CD8+ naïve T cells from NOD mice were first cultured under IL-12 alone, or IL-7 alone, or IL-12 plus IL-7 conditions. IL-12 induced IFN-γ+ producing cell differentiation in either naïve CD4+ or CD8+ cultures (Fig. 3 A and B). IL-7 alone slightly induced TH1 cell differentiation (Fig. 3A). In contrast, IL-7 alone potently induced IFN-γ–producing cells from CD8+ naïve T cells in NOD mice similar to that of IL-12 alone (Fig. 3B). Interestingly, the presence of IL-12 and IL-7 together further augment IFN-γ–producing cell differentiation from either CD4+ or CD8+ naïve T cells to a level greater than either cytokine alone.
Fig. 3.
IL-7 promotes IFN-γ+ cell development from NOD naïve CD4+ and CD8+ T cells. (A) Sorted naïve CD4+ and (B) CD8+ T cells from NOD mice were stimulated for 3 d with anti-CD3/CD28 activation beads in the presence of indicated cytokines. (C and D) After 3 d of culture in the presence of indicated cytokine shown in A, cells were rested for 48 h followed by restimulation with anti-CD3 and anti-CD28 in the presence of different cytokines (e.g., IL-12, IL-7, IL-7+IL-12), or medium as indicated for each individual FACS plot during the expansion period. CD4+ T cells (A and C) or CD8+ T cells (B and D) were analyzed for IFN-γ by flow cytometry.
Next, we assessed the effect of IL-7 on the expansion of previously polarized TH1 or TC1 cells. Under TH1 and TC1-polarizing conditions, IL-12 expanded TH1 cells as expected (Fig. 3 C and D). When CD4+ and CD8+ naïve T cells were first stimulated in the presence of IL-7, anti-CD3 plus anti-CD28, and then rested for 48 h followed by restimulation with IL-7, we found that IL-7 increased the percentage of IFN-γ+ cells originally differentiated from either CD4+ or CD8+ naïve T-cell cultures (Fig. 3 C and D). These effects of IL-7 on TH1 cell expansion were also recapitulated in NOD.BDC2.5 mice (Fig. S4 A and B).
In contrast, neither IL-17+ nor IL-21+ T cells (Fig. S4 C–F) was increased by IL-7 treatment in NOD mice but TGF-β plus IL-6 significantly increased IL-17–producing cells in naïve CD4+ or CD8+ T cells, as expected (Fig. S4 C and D).
Furthermore, those cytokines associated with the TH1 (TNF-α, IL-2, and IFN-γ) and TH17 (IL-17A, IL-6, and IL-21) lineage were significantly reduced in both CD44+ (Fig. S4H) and CD44− (Fig. S4 G and I) cultures from 28G9-mIgG2a–treated mice compared with those from isotype control, with the only exception that IL-2 and IL-10 were elevated by 28G9-mIgG2a in the CD44− culture (Fig. S4G).
PD-1 Can Regulate the Maintenance of Peripheral Tolerance in IL-7Rα Antibody Treatment.
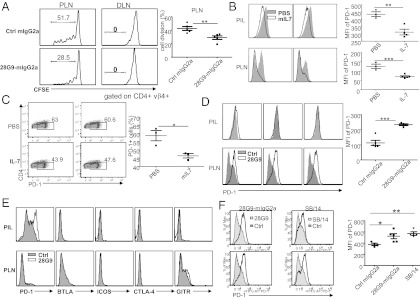
To determine the effect of anti–IL-7Rα on Teff proliferation, equal numbers of carboxyfluorescein succinimidyl ester (CFSE)-labeled Teffs (CD4+CD25−CD44+) isolated from BDC2.5.NOD mice treated with IL-7Rα antibody or isotype controls were adoptively transferred to the NOD.Rag−/− mice. The Teffs derived from IL-7Rα antibody-treated mice were significantly less proliferative than those from control treated mice (Fig. 4A).
Fig. 4.
IL-7 suppresses, whereas IL-7Rα antibody up-regulates, PD-1 in Teffs. (A) Proliferation of CFSE-labeled T cells isolated from 28G9-mIgG2a or control IgG treated BDC2.5 mice after adoptive transfer into NOD.Rag−/− mice. Flow cytometry was performed 7 d after transfer into NOD.Rag−/− in PLNs and the draining lymph node (DLN). Proliferation percentage was determined by CFSE dilution in CD4+Vβ4+ T cells. (B–E) Expression of PD-1 (x-axis of the FACS plots) by the PIL T cells or PLNs CD4+FoxP3− (Teff) cells. (B) Mice treated with PBS (gray) or 1 μg of mIL-7 (white) every other day for a total of three injections starting at 9 wk of age. (C) Lymph node T cells were isolated from NOD mice and treated with anti-CD3 and anti-CD28 in the presence of PBS or mIL-7 in vitro for 3 d. (D and E) Mice were treated once a week with control IgG-mIgG2a (gray) or 28G9-mIgG2a (white) starting at 9 wk-of-age (D) or at 12 wk-of-age (E) for 3 consecutive weeks. Graphs are representative of two independent experiments. (F) PD-1 expression in the PLN Teffs. NOD mice were treated with 28G9-mIgG2a, SB/14 or control IgG starting at 9 wk of age, each at 10 mg/kg per week for 4 wk. Mean flouorescence intensity (MFI) results are pooled from five animals in each group. Data are from one representative experiment of two independent experiments. The error bars represent SEM. *P < 0.05 and **P < 0.01 (one-way ANOVA with post tests); ***P < 0.001 (Student t test).
Then we asked whether IL-7 could affect certain key negative regulators, such as PD-1, cytotoxic T-lymphocyte antigen 4 (CTLA-4), or related molecules on the Teffs. Indeed, recombinant mouse IL-7 treatment in vivo led to reduced expression of PD-1 in the Teff of pancreatic infiltrating lymphocytes (PIL) and PLNs of the NOD mice (Fig. 4B). Similarly, in vitro IL-7 treatment suppressed PD-1 expression after T-cell activation (Fig. 4C).
Conversely, 28G9-mIgG2a treatment of 9-wk-old NOD mice led to an up-regulation of PD-1 in PLN Teffs (Fig. 4D) and the increase of PD-1 expression is regulated by 28G9-mIgG2a in a dose-dependent manner (Fig. S5). Treatment with 28G9-mIgG2a of 12-wk-old NOD mice was associated with a significant increase in the expression of PD-1 in PIL Teffs (Fig. 4E), without much effect on the PD-1 expression in Tregs of the PIL or PLN (Fig. S6B). This modulatory effect by IL-7Rα antibody is specific to PD-1 in that other related molecules, such as CTLA-4, inducible T-cell costimulator, glucocorticoid-induced TNF-receptor, and B- and T-lymphocyte attenuator were not affected by the IL-7Rα antibody treatment (Fig. 4E).
Although 28G9-mIgG2a and SB/14 have differential FcγR binding (Table S1) and also have distinct effect on T-cell numbers (Fig. S3), these two antibodies exhibited comparable efficacy in reversing newly onset diabetes (Fig. 2B). On the other hand, PD-1 expression was consistently increased by 28G9-mIgG2a and by SB/14 in the Teffs of PLNs at week 9 (Fig. 4F), supporting the notion that PD-1 up-regulation may contribute to the efficacy of the IL-7Rα antibodies. These results also suggest that PD-1 up-regulation by IL-7Rα antibody may not require the engagement of FcγR.
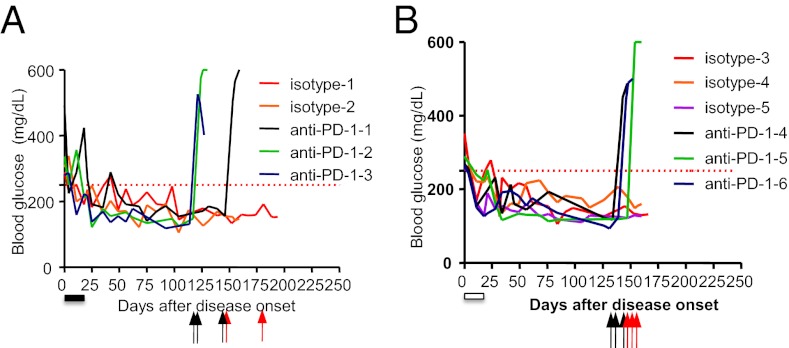
We tested whether the PD-1 pathway can regulate therapeutic efficacy in IL-7Rα antibody by taking advantage of the durable efficacy of short-term IL-7Rα antibody treatment in the newly onset diabetes. The NOD mice received a short course of treatment with either 10 mg/kg of SB/14 (treatment duration of which is indicated by the filled bar in Fig. 5A) or 28G9-mIgG2a (indicated by open bar in Fig. 5B) once weekly for 3 wk immediately after diabetic onset. As expected, such treatment of either IL-7Rα antibody reversed diabetes of these mice for over 3 mo. These IL-7Rα antibody-induced euglycemic mice, upon a single injection of anti–PD-1 (black arrows in Fig. 5) but not isotype control IgG (red arrows in Fig. 5), rapidly developed hyperglycemia within 5 d. Thus, the long-term tolerance induced by IL-7Rα antibody therapy likely requires PD-1 function.
Fig. 5.
PD-1 blockade abrogates the antidiabetic efficacy of IL-7Rα antibody. Anti–PD-1 treatment exacerbates disease in reversal paradigm. Newly diabetic NOD mice were treated with IL-7Rα antibody (A) SB/14 or with (B) 28G9-mIgG2a for a total of three doses once weekly at 10 mg/kg i.p. The treatment period for each IL-7Rα antibody was indicated by the filled and the open bars, respectively. Long-term remission of existing diabetes was observed for over 100 d. The animals reverted to normoglycemia by IL-7Rα antibody were randomly assigned to treatment of either anti–PD-1 (n = 3, or rat IgG2a isotype control (isotype, n = 2–3) once weekly at 200 μg per mouse. Black arrows indicate the timing of anti–PD-1 injections, whereas red arrows indicate that of isotype IgG injection.
IL-7Rα Antibody Treatment Increases the Frequency, but Not the Intrinsic Suppressor Activity, of Tregs.
To study the effect on Tregs, we initiated antibody treatment in 9-wk-old female NOD mice. After 3 wk of once weekly injection, 28G9-mIgG2a significantly increased the frequency of Tregs in the spleen and in the PLN (Fig. S7 A and B), and also the absolute count of Tregs in the spleen of these mice (Fig. S7C). In our experiment, the majority of Foxp3+ cells are found in the CD4+CD25hi T cells although CD4+CD25lo cells also express some level of Foxp3 (Fig. S7 E and F) (32).
We asked whether IL-7Rα antibody treatment may modulate the intrinsic suppressor activity of Tregs. Because the CD4+CD25hi T cells have consistently shown greater inhibition indices than other T-cell populations (33), we purified these cells using FACS to study their suppressor activity on Teffs. In the in vitro coculture system, the CD4+CD25hi Tregs isolated from the pancreatic lymph nodes and the spleen of IL-7Rα antibody-treated animals displayed similar suppressive capacity compared with those from isotype control-treated animals across different ratios of Tregs to Teffs (Fig. S7D). Thus, IL-7Rα antibody treatment increases the frequency and absolute counts of Tregs in some lymphoid compartments without altering the intrinsic suppressive activity of Tregs.
Discussion
The goal of this study was to examine the effects of IL-7Rα antibody on NOD mice and to understand the functional contribution of IL-7Rα to the T1D pathogenesis. We show here that IL-7Rα antibody treatment can prevent and delay the progression of T1D before onset, as well as reversing the newly onset diabetes.
Recently we demonstrated that IL-7 can enhance mouse TH1 cell expansion on the C57BL/6 background (26). In this article, we show that IL-7 promotes NOD mouse TH1 cell development, even in the absence of IL-12, consistent with our previous findings in human cord-blood cells (26). In addition, we are unique in demonstrating that IL-7 can promote CD8+ IFN-γ cell development during the differentiation and expansion stage of in vitro culture (Fig. 4 B and D). In type 1 diabetes the development of disease has usually been ascribed to a TH1 or TC response (19–25). On the other hand, the exact role of TH17 cells in the pathogenesis of T1D is not well understood. For example, highly purified TH17 from BDC2.5.NOD mice were able to transfer diabetes to NOD/SCID mice, primarily via conversion to an IFN-γ–producing TH1-like phenotype, whereas transfer of TH1 cells did not result in the generation of TH17 cells (34). IFN-γ producing TH1 and TC1 cells are important for T1D because blockade of IFN-γ or IL-12 (35–37) prevented disease, whereas IL-12 promoted disease in NOD mice (38). In our studies, the IL-7Rα–blocking antibody treatment significantly reduced TH1 and TH17 cells (Fig. 1F), as well as TH1 cytokines (IFN-γ and TNF-α) and TH17 cytokines (IL-17A, IL-6, and IL-21) (Fig. S4G). These changes likely contribute to the therapeutic efficacy.
How does IL-7Rα antibody therapy attenuate the activity of the islet targeting Teffs? The immune tolerance mediated through PD-1 may be important in restraining Teff function based on several lines of evidence: (i) the expression of PD-1 in the PIL and PLN Teffs was reduced in IL-7–treated mice (Fig. 4 B and C); (ii) PD-1 expression was up-regulated in the PLN and PIL Teffs (Fig. 4 D and E) when 28G9-mIgG2a treatment was given; (iii) SB/14, another efficacious clone of IL-7Rα antibody without T-cell–depleting ability, also increased PD-1 expression (Fig. 4F); and finally (iv) long-term tolerance induced by IL-7Rα antibodies can be abrogated by PD-1 blockade (Fig. 5 A and B).
Based on our findings that IL-7 can promote TH1 and TC1 cell development in NOD mice (Fig. 3 A–D) and down-regulates PD-1 expression (Fig. 5 B and C), we propose that IL-7Rα antibody therapy may operate through (i) the reduction of IFN-γ+ Teffs and (ii) increased PD-1 expression in the Teffs at the anatomical site of active inflammation. At the same time, the genetic association of IL-7R polymorphisms with the risk of human T1D (11, 12) may also be better appreciated from the perspective of these two immune mechanisms.
Like many discoveries, our findings raise new questions, such as the precise intracellular signaling pathways that mediate PD-1 up-regulation, and whether these signals differ from those that mediate the cell proliferation and IFN-γ expression in the IL-7 target cells. Several reports showed that blocking PD-1/PD-L1 signaling by neutralizing antibody or by genetic deletion of PD-1 or PD-L1 exhibited significantly elevated IFN-γ–producing cells in several autoimmune diseases (28, 36, 39–42). Of note, PD-1/PD-L1 signaling was shown to inhibit IFN-γ production during naïve T-cell activation. PD-1 or PD-L1–deficient NOD mice displayed significantly higher IFN-γ production, which resulted in the rapid onset of diabetes and the early onset of insulitis (28, 36). A recent report showed that PD-1/PD-L1 signaling converts human TH1 cells into Tregs in vitro and in vivo, thereby preventing human-into-mouse xenogeneic graft-vs.-host disease. Recipient of TH1 cells plus T cells expressing PD-L1 had a reduced number of T-bet+ T cells and an increased number of Foxp3+ T cells (43). In this connection, we also noted an increased frequency and also absolute number of Tregs in certain—but not all—lymphoid compartments in NOD mice treated with IL-7Rα antibody. The sparing of Tregs by IL-7Rα antibody therapy is consistent with the relatively low level of expression of IL-7Rα in Tregs (Fig. S2A) (26, 44, 45).
What variables of the newly diabetic mice may have contributed to the durable remission of NOD mice by anti–IL-7Rα? To begin to address this question, we combined several reversal experiments and analyzed the age of onset and blood glucose value at onset of all of the newly onset NOD mice treated with either isotype control or one of the two anti–IL-7Rs (28G9-mIgG2a and SB/14) across several experiments. Our preliminary post hoc observations raise the possibility that animals with a less-aggressive course of newly onset diabetes (i.e., with a later age of onset and with lower blood-glucose levels at onset) may be more responsive to the IL-7Rα antibody therapy (Fig. S8 B and E). This possibility will have to be confirmed by future prospective studies. If substantiated, this theory may have important implications for the clinical development of IL-7Rα antibody therapy in human T1D.
Previously it was reported that, in the preventative paradigm, TNF-α and anti–TNF-α treatments in young vs. adult NOD animals exhibited opposite outcome (46, 47). The anti-CD3 therapy also did not show protection when administered to 4-wk-old, prediabetic NOD mice. Thus, it is reasonable to ask whether, in the preventative paradigm, anti–IL-7Rα might exert any age-dependent effect on NOD pathogenesis or not. We treated some NOD mice starting at 4 wk of age with 28G9-mIgG2a or with isotype control IgG for three injections (at 4-, 7-, and 10-wk-old) and found that 50% mice in the 28G9-mIgG2a treated group did not develop diabetes, and merely 20% of the control IgG treated mice exhibited normaglycemia (Fig. S9). These results further suggest the unique potential of preventing the clinical onset of T1D in certain high-risk groups by targeting the IL-7Rα pathway, as oppose to the TNF-α or anti-CD3–targeting drugs.
In conclusion, we show that IL-7 suppressed PD-1 expression after T-cell activation, and IL-7 can promote IFN-γ+ cell development in CD4+ and CD8+ T cells, both T-cell subsets of which contribute to the pathogenesis of T1D. Anti–IL-7Rα treatment can prevent diabetes and can also reverse newly established disease in NOD mice through the combination of targeting IFN-γ–producing T cells and keeping pathogenic T cells in check via PD-1 up-regulation. Our findings provide the mechanistic insight to the genetic association of IL-7R with human T1D, as well as the scientific rationale for the IL-7Rα–blocking antibody as a unique therapy for the patients with T1D.
Materials and Methods
Mice.
NOD.Lt mice were obtained from The Jackson Laboratory and were housed in groups of five under specific pathogen-free conditions in Rinat-Pfizer animal facilities. All animals used were 7- to 8-wk-old females, unless specifically noted. All animal experiments were conducted according to the protocols approved by the Institutional Animal Care and Use Committee of Rinat, Pfizer.
Antibody Treatment.
For IL-7Rα antibody treatment, mice were injected intraperitoneally with 10 mg/kg or 3 mg/kg body weight of anti–IL-7Rα mAb once weekly starting at week 9, or after disease onset, as indicated in each figure. For PD-1 blockade experiments, PD-1 (RMP1-14) (29, 48) were purchased from eBioscience. In the reversal paradigm, three to four injections of 28G9-mIgG2a or SB/14 were given in diabetic mice and reverted diabetes-free for 3 mo. One dose of anti–PD-1 (200 μg per mouse) was given in the previously 28G9-mIgG2a or SB/14-treated mice. Animals were monitored for blood-glucose measurement and mice were considered diabetic if blood-glucose levels were >250 mg/dL on two consecutive draws. A detailed description of Ab generation, mouse T-helper cell differentiation, flow cytometry, immunohistochemistry, ELISA, cytometry bead assay, CFSE labeling, adoptive transfer, and in vitro suppression assay are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Gilbert for flow cytometry, M. Karnoub for statistical analysis, and D. Malashock and Y. Abdiche for the biosensor assay.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 12270.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203795109/-/DCSupplemental.
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Castaño L, Eisenbarth GS. Type-I diabetes: A chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 3.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 4.Lee LF, et al. The role of TNF-alpha in the pathogenesis of type 1 diabetes in the nonobese diabetic mouse: Analysis of dendritic cell maturation. Proc Natl Acad Sci USA. 2005;102:15995–16000. doi: 10.1073/pnas.0508122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatenoud L, Bluestone JA. CD3-specific antibodies: A portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 6.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 7.Vyse TJ, Todd JA. Genetic analysis of autoimmune disease. Cell. 1996;85:311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- 8.Gregory SG, et al. Multiple Sclerosis Genetics Group Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 9.Hafler DA, et al. International Multiple Sclerosis Genetics Consortium Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 10.Lundmark F, et al. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 11.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 12.Todd JA, et al. Genetics of Type 1 Diabetes in Finland Wellcome Trust Case Control Consortium Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Tan JT, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan JT, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calzascia T, et al. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci USA. 2008;105:2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 18.Peschon JJ, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santamaria P. The long and winding road to understanding and conquering type 1 diabetes. Immunity. 2010;32:437–445. doi: 10.1016/j.immuni.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Healey D, et al. In vivo activity and in vitro specificity of CD4+ Th1 and Th2 cells derived from the spleens of diabetic NOD mice. J Clin Invest. 1995;95:2979–2985. doi: 10.1172/JCI118006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh N, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest. 1993;92:2313–2322. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 23.Nagata M, Santamaria P, Kawamura T, Utsugi T, Yoon JW. Evidence for the role of CD8+ cytotoxic T cells in the destruction of pancreatic beta-cells in nonobese diabetic mice. J Immunol. 1994;152:2042–2050. [PubMed] [Google Scholar]
- 24.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 25.Sumida T, et al. Prevention of insulitis and diabetes in beta 2-microglobulin-deficient non-obese diabetic mice. Int Immunol. 1994;6:1445–1449. doi: 10.1093/intimm/6.9.1445. [DOI] [PubMed] [Google Scholar]
- 26.Lee LF, et al. IL-7 promotes T(H)1 development and serum IL-7 predicts clinical response to interferon-β in multiple sclerosis. Sci Transl Med. 2011;3:93ra68. doi: 10.1126/scitranslmed.3002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fife BT, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Pellegrini M, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 32.You S, et al. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci USA. 2007;104:6335–6340. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belghith M, et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 34.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicoletti F, et al. The effects of a nonimmunogenic form of murine soluble interferon-gamma receptor on the development of autoimmune diabetes in the NOD mouse. Endocrinology. 1996;137:5567–5575. doi: 10.1210/endo.137.12.8940385. [DOI] [PubMed] [Google Scholar]
- 36.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trembleau S, Penna G, Gregori S, Gately MK, Adorini L. Deviation of pancreas-infiltrating cells to Th2 by interleukin-12 antagonist administration inhibits autoimmune diabetes. Eur J Immunol. 1997;27:2330–2339. doi: 10.1002/eji.1830270930. [DOI] [PubMed] [Google Scholar]
- 38.Trembleau S, et al. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J Exp Med. 1995;181:817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salama AD, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandner SE, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 41.Sandner SE, et al. Mechanisms of tolerance induced by donor-specific transfusion and ICOS-B7h blockade in a model of CD4+ T-cell-mediated allograft rejection. Am J Transplant. 2005;5:31–39. doi: 10.1111/j.1600-6143.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 42.Latchman YE, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amarnath S, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3(111) doi: 10.1126/scitranslmed.3003130. 111ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cope AP, et al. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang XD, et al. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fife BT, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.