Abstract
Manganese (Mn) oxides are among the most reactive minerals within the environment, where they control the bioavailability of carbon, nutrients, and numerous metals. Although the ability of microorganisms to oxidize Mn(II) to Mn(III/IV) oxides is scattered throughout the bacterial and fungal domains of life, the mechanism and physiological basis for Mn(II) oxidation remains an enigma. Here, we use a combination of compound-specific chemical assays, microspectroscopy, and electron microscopy to show that a common Ascomycete filamentous fungus, Stilbella aciculosa, oxidizes Mn(II) to Mn oxides by producing extracellular superoxide during cell differentiation. The reactive Mn oxide phase birnessite and the reactive oxygen species superoxide and hydrogen peroxide are colocalized at the base of asexual reproductive structures. Mn oxide formation is not observed in the presence of superoxide scavengers (e.g., Cu) and inhibitors of NADPH oxidases (e.g., diphenylene iodonium chloride), enzymes responsible for superoxide production and cell differentiation in fungi. Considering the recent identification of Mn(II) oxidation by NADH oxidase-based superoxide production by a common marine bacterium (Roseobacter sp.), these results introduce a surprising homology between some prokaryotic and eukaryotic organisms in the mechanisms responsible for Mn(II) oxidation, where oxidation appears to be a side reaction of extracellular superoxide production. Given the versatility of superoxide as a redox reactant and the widespread ability of fungi to produce superoxide, this microbial extracellular superoxide production may play a central role in the cycling and bioavailability of metals (e.g., Hg, Fe, Mn) and carbon in natural systems.
Keywords: biomineralization, oxidative burst
Manganese (Mn) oxides are among the most reactive minerals occupying soils, sediments, and waters. In fact, the fate and transport of numerous contaminants (e.g., lead, chromium) and nutrients (e.g., phosphate) are controlled by adsorption, coprecipitation, and redox reactions mediated by Mn oxides (1). For example, Mn oxides attenuate numerous metals via substitution within the structure or adsorption to the mineral surface. The immense oxidative capacity of Mn oxides contributes significantly to the oxidation of recalcitrant organic carbon, including the degradation of lignin to bioavailable substrates that feed into the microbial food web (2). Furthermore, Mn oxides have emerged as a potential new material in a wide range of technological applications, including energy storage, electrochromism, and catalysis (3). Homogeneous oxidation of Mn(II) to Mn(III/IV) oxides by O2 is thermodynamically prohibited owing to a reactivity barrier imposed by the first electron transfer step, which controls the overall reaction rate (4). Oxidation of Mn(II) by molecular oxygen, however, may occur at mineral surfaces (5) or in the presence of high-affinity ligands (6). Within the environment, the oxidation of Mn(II) is thought to be primarily a consequence of biological activity (7). However, despite their environmental, public health, and biotechnological relevance, the biochemical pathways and physiological basis for microbial oxidation of Mn(II) to Mn oxides remains enigmatic.
The ability for microorganisms to oxidize Mn(II) to Mn(III/IV) oxides is found throughout the bacterial and fungal domains of life. Mn(II)-oxidizing fungi described to date belong to the phyla Basidiomycota and Ascomycota. The most studied group of Mn(II)-oxidizing fungi is the white rot fungi (largely Basidiomycota) because of their ability to degrade lignin and a broad range of aromatic pollutants. Mn(II) oxidation by these organisms is primarily a result of manganese peroxidase (MnP) activity (8). Oxidation of Mn(II) by MnP is hydrogen peroxide- (H2O2) dependent; the active center of MnP is oxidized by H2O2, which subsequently oxidizes two Mn(II) ions via two successive single-electron transfers (9). The Mn(III) ions form reactive chelates with fungally derived organic acid metabolites (e.g., oxalate, malonate) (10), which may then proceed to degrade phenolic units of lignin or disproportionate to Mn oxide phases.
In contrast, Mn(II) oxidation by most Ascomycete fungi, as well as by bacteria, remains poorly understood (7), and apparently serves no known physiological or ecological benefit to these organisms. Thus, the reason microbes expend energy to enzymatically oxidize Mn(II) is presently unknown (7) and brings into question any evolutionary basis for this process. The Mn(II)-oxidizing Ascomycota belong to a number of different genera, such as Pyrenochaeta, Alternaria, Phoma, and Acremonium (11, 12). Although the mechanism of Mn(II) oxidation by Ascomycete fungi remains unknown, the multicopper oxidase enzyme laccase has been implicated recently (11). Multicopper oxidases are a structurally and functionally diverse family of enzymes that have the capacity to oxidize a wide range of organic and inorganic substrates [e.g., Fe(II), diphenolics] (13). Multicopper oxidases have also been linked to Mn(II) oxidation in some bacteria, particularly the spores of Gram-positive Bacillus spp., where oxidation occurs on the outermost layer of the proteinaceous spore coat (14). More recent findings, however, indicate that laccase enzymes in some Basidiomycete fungi are responsible for the indirect production of reactive oxygen species (ROS), including superoxide (O2−) in the presence of Mn(II) and organic chelators (e.g., oxalate) (15).
Superoxide is a powerful and versatile redox reactant that has been shown to play a significant role in the cycles of numerous metals, including the reduction of Fe(III) and Cu(II), as well as oxidation of Mn(II) (16–18). Recently, thermodynamic calculations have shown that although oxidation of Mn(II) [as Mn(H2O)62+] by molecular oxygen is not thermodynamically viable below a pH of 9, oxidation by superoxide is favorable over all pH conditions (4). Indeed, oxidation of Mn(II) to Mn oxides by a common marine bacterium, Roseobacter AzwK-3b, was found to be a consequence of enzymatic extracellular production of O2−, which served as the terminal oxidant of Mn(II) (19). Although extracellular superoxide production has been documented in pathogenic bacteria (20) and phytoplankton (21), very little is known about the occurrence of this process in nonpathogenic heterotrophic bacteria. In contrast, production of extracellular superoxide is widespread throughout the fungal kingdom (22), where it is involved in host defense, posttranslational modification of proteins, hyphal branching, cell signaling, and cell differentiation (22, 23). The primary enzymes responsible for ROS production in fungi are NADPH oxidases within the NOX family. These transmembrane proteins transport electrons from cytosolic NADPH via flavin adenine dinucleotide and two hemes to extracellular molecular oxygen to generate O2− and its dismutation product H2O2 (22).
Despite the universal ability of fungi to produce high concentrations of extracellular superoxide, a powerful mediator of metal redox cycling, the impact of this physiological process on metal redox transformations and metal bioavailability has been largely unexplored. Here, we use a combination of compound-specific chemical assays, microspectroscopy, and electron microscopy to explore the potential for fungal-derived ROS formed during cell differentiation to impact the cycling of metals. We focus this investigation on an Ascomycete filamentous fungus, Stilbella aciculosa, that we recently isolated from a passive treatment system used to remediate Mn-laden coal mine drainage water (12). S. aciculosa serves as a model organism because of its widespread occurrence in diverse soil and sedimentary environments, including terrestrial soils, estuarine, and marine bottom sediments (24).
Results and Discussion
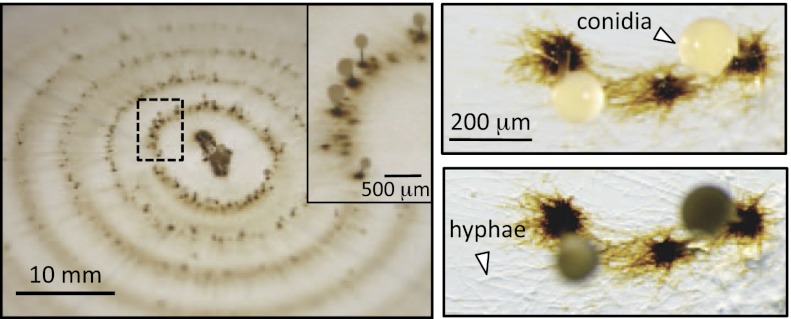
Growth of S. aciculosa on a solidified medium in the presence of soluble Mn(II) results in the formation of brown precipitates primarily at the base of reproductive structures (Fig. 1). When grown in the absence of Mn(II), these precipitates are not observed. Following inoculation at the center of the plate, S. aciculosa grows radially outward where the rate of hyphal extension is not impeded by Mn(II) at concentrations up to 1 mM (12). After ∼4 d, a ring of reproductive structures approximately 150–250 μm apart is first observed (Fig. 1). Subsequent reproductive structures form every 30–48 h, with the duration between structures increasing with age. These are asexual reproductive structures composed of synnemata (interwoven conidiophores) up to approximately 500 μm in length with cylindrical conidia at the tip. Using time-resolved microscopy, we observe the formation of visible brown precipitates (micrometer in size) followed by the initiation of the conidiophore growth. Synchrotron-based micro–X-ray fluorescence (micro-XRF) maps reveal elevated Mn at the base of synnemata and only minor levels within the conidia (Fig. 2). This distribution is in contrast to potassium (K), calcium (Ca), and zinc (Zn), where substantially higher levels are observed within the conidia relative to within or at the base of the synnemata.
Fig. 1.
S. aciculosa growing radially outward from a central inoculation point on agar-solidified AY media (pH 7) amended with 200 μM Mn(II). Brown coloration at the base of aerial synemmata are only formed when grown in Mn(II)-supplemented media. The two images on the right illustrate the absence of coloration on hyphae with the optical focus on the conidia (Upper) and agar surface (Lower).
Fig. 2.
Micro-XRF of S. aciculosa grown on filters placed on agar-solidified AY media containing 200 μM Mn(II). Optical image (far Left) and selected elements illustrated for two fields of view. The optical image on the left is a composite of two images because of a limited field of view for the sample camera at the beamline as compared to the sample size. Elemental abundances for each field of view range from low (0 μg⋅cm−2) depicted in blue to high (Mn = 365; Zn = 10; K = 150; Ca = 50 μg⋅cm−2) in red. (Scale bars, 200 μm.)
The brown precipitates at the base of the reproductive structures are composed of oxidized Mn, indicated by positive reaction with the colorimetric dye Leucoberbelin blue, which specifically reacts with both Mn(III) and Mn(IV) but not Mn(II), and spectroscopic analysis. To determine the bulk oxidation state of the Mn associated with S. aciculosa, filter-supported cultures grown in the presence of 200 μM Mn(II) were analyzed using synchrotron-based X-ray absorption spectroscopy (XAS). Linear-combination fitting of the near edge region of the Mn K-edge XAS spectra (the X-ray absorption near edge structure or XANES region) indicates that the Mn associated with S. aciculosa (total Mn associated with all cellular material) is predominantly Mn(IV) (80%), with lesser amounts of Mn(III) (11%) and Mn(II) (9%) (Fig. S1). We spatially resolved the Mn oxidation state along reproductive structures by collecting XRF maps at several incident energies around the Mn K-edge. These energy-specific XRF maps illustrate a transition from predominantly Mn(II) within the conidia to Mn(IV) at the base of the synnemata (Fig. 3, Left). This oxidation state distribution was confirmed by collecting spot (2 × 2-μm resolution) XANES spectra at three locations along the reproductive structure (Fig. 3, Right). The micro-XANES spectra illustrate a shift to higher binding energies and hence higher oxidation states traversing from the conidia to the synnemata base.
Fig. 3.
Energy-specific XRF maps showing the location of Mn(II) and Mn(IV) species within S. aciculosa. (Left) A tricolor map for Mn(II) (green), Mn(IV) (red), and K (blue) for a representative reproductive structure also illustrated in Fig. 2. (Right) XANES spectra for points 1 through 3, with a spectral resolution of 2 μm × 2 μm. Reference spectra (dotted lines) are also included to illustrate the relative binding energies for Mn(II) (as MnCl2), Mn(III) (as Mn2O3), and Mn(IV) (as δ-MnO2).
The oxidized Mn produced by S. aciculosa exists as the common Mn oxide phase birnessite. Linear combination fitting of the Mn K-edge EXAFS (extended X-ray absorption fine structure) spectra reveals that the S. aciculosa spectra can be fully reconstructed with a single component consisting of δ-MnO2, a poorly ordered, nanocrystalline phyllomanganate with hexagonal symmetry (Fig. S1). Calcium (Ca2+) is a common interlayer cation within biogenic birnessite (25), explaining the slightly elevated Ca concentrations at the base of the synnemata relative to the surrounding hyphae or within the conidiophores (Fig. 2). Despite the large diversity in Mn oxide structures, hexagonal birnessite, similar to that observed here, is the dominant biogenic Mn oxide formed by both bacteria and fungi (25, 26). These biogenic Mn oxides are highly disordered and contain a large degree of layer site vacancies (25). These characteristics are believed responsible for the higher sorptive and oxidative capacities of biogenic versus abiotic Mn oxides. In fact, it has been suggested that organisms may intentionally oxidize Mn(II) to make birnessite on the outside of the cell to serve as an armor against uptake of toxic metals and to oxidize recalcitrant carbon to generate bioavailable carbon substrates (27). Here, the localization of Mn oxides at the base of reproductive structures implicates processes associated with cell differentiation in Mn(II) oxidation. In particular, superoxide, a known oxidant of Mn(II) (16), is believed to act as both an intracellular and extracellular cell signal for the initiation of asexual and sexual reproductive structures in fungi (28).
Accordingly, using ROS-specific stains we observe ROS concentrated at the base of reproductive structures (Fig. 4), correlating with the distribution of Mn oxide precipitation (Fig. 3). Yellow, water-soluble nitroblue tetrazolium (NBT) is reduced by superoxide to form blue, water-insoluble formazan. Because the stain precipitates upon reaction with superoxide, the location of the formazan precipitates reveals the site of reaction with superoxide. Submerging (∼1 mm depth) the inoculated plate (without Mn) in an NBT solution reveals formazan precipitates only associated with reproductive structures and not the mycelial hyphae (Fig. 4). However, because of the poor photographic resolution of formazan under these conditions (appearing as a faint purple color rather than blue), we also used the soluble stain 3,3′-Diaminobenzidine (DAB), which is converted to a more easily observed insoluble brown precipitate in the presence of H2O2. In the absence of a superoxide scavenger, superoxide rapidly dismutates to hydrogen peroxide (H2O2) (29). Corresponding with the superoxide spatial distribution, H2O2 is concentrated at the base of synnemata (Fig. 4). Taken together, these results indicate that ROS and Mn oxide formation are colocalized at the base of synnemata. Although H2O2 can readily reduce Mn(III) and Mn oxides, it does not have a demonstrated ability to oxidize Mn(II) (16, 30). Taking into account that superoxide has the demonstrated ability to rapidly oxidize Mn(II) (16) forming Mn oxides (19), this codistribution of the Mn oxides and superoxide indicates that Mn(II) is being oxidized by superoxide.
Fig. 4.
S. aciculosa asexual reproductive structures grown in AY media, starting from left: without Mn(II) supplement; amended with 200 μM Mn(II); and Mn-free media stained with either NBT (for O2−) or DAB (for H2O2), illustrating concentrated stain at the base of synnemata and not the supporting mycelium. The contrast and brightness on the NBT-stained image was increased to illustrate the light purple stain at the base of the reproductive structure. (Scale bars, 200 μm.)
In further support of superoxide-mediated Mn(II) oxidation, addition of superoxide scavengers, such as superoxide dismutase (SOD) and divalent copper, inhibits the formation of Mn oxides. In particular, Cu(II), an effective and rapid scavenger of superoxide (18, 19), shows a concentration dependent inhibition of Mn oxide formation (Fig. 5). At high Cu(II) concentrations (≥100 μM), Mn oxides are not observed and the number of reproductive structures is significantly (>90%) decreased. The decline in asexual reproduction is a consequence of a loss of superoxide because superoxide is required for initiation of cell differentiation (see ref. 23 and references therein). In contrast, a chemically similar divalent cation, Zn(II), which cannot catalyze superoxide dismutation, does not have a significant impact on either observed Mn oxide formation or reproductive structure formation (Fig. 5). Considering that the dismutation of superoxide by SOD produces H2O2, inhibition of Mn oxide formation in the presence of SOD further implicates superoxide in Mn(II) oxidation and not H2O2. NADPH oxidases have been found to play a key role in superoxide and hydrogen peroxide formation in multicellular organisms, including fungi (see reviews in refs. 22, 23, and 31). In particular, deletion of nox genes in a variety of fungi blocks cell differentiation, including asexual and sexual development, which correlates to the inability of the nox mutants to generate superoxide (28, 32–34).
Fig. 5.

Typical S. aciculosa asexual reproductive structures grown on Mn(II)-amended AY media in the presence of 200 μM Mn(II), and either 50 μM Cu, 100 μM Cu, 100 μM Zn, or 50 μM DPI. (Scale bars, 200 μm.)
Indeed, here superoxide-mediated Mn oxide formation by S. aciculosa is associated with the activity of NADPH oxidase enzymes. Addition of 50 μM diphenylene iodonium chloride (DPI), a known inhibitor of transmembrane oxidoreductases and other NAD(P)H binding enzymes (35), results in complete inhibition of Mn oxide formation (Fig. 5). DPI has been used frequently in fungal research to eliminate the activity of the NOX family of NADPH oxidase enzymes to explore various processes attributed to their activity (23). Of relevance here, DPI has been previously shown to inhibit cell differentiation in several Ascomycete fungi, including for example fruiting body formation in Aspergillus nidulans (33). Similarly, here addition of DPI completely inhibits asexual reproductive structure formation by S. aciculosa, as well as Mn oxide formation (Fig. 5). NADPH oxidases are enzymes inserted within the plasma membrane that use cytosolic NADPH to reduce O2 to superoxide on the outer aspect of the membrane. Superoxide is converted to hydrogen peroxide by either spontaneous dismutation or by the catalytic activity of a cell wall-associated superoxide dismutase (23). Here, we detect both extracellular superoxide and hydrogen peroxide at the base of asexual reproductive structures (Fig. 4), which are absent in the presence of NADPH oxidase inhibitors (Fig. 5). These findings implicate a causal relationship between NADPH oxidase, superoxide production, asexual reproduction, and Mn oxide precipitation.
Mn oxides formed during cell differentiation are located extracellularly, consistent with the distribution of ROS stains as well as the localization of NADPH oxidase activity observed in other Ascomycete fungi in previous studies (see, for example, ref. 28). Microscopy images show that the DAB (H2O2) and formazan (O2−) precipitates are present as a diffuse halo of precipitates around the hyphae at the base of the synemmata, indicating that the ROS are reacting with the stain extracellularly (Fig. S2). Superoxide, being a charged species, has limited ability to cross cell membranes, and thus the site of precipitation of formazan most likely reflects the site of superoxide production. These results are consistent with oxidative bursts of extracellular superoxide observed around reproductive structures by other fungi, including the fruiting bodies of the filamentous fungus Podospora anserine (28). Using transmission electron microscopy (TEM), Mn oxides are only observed adjacent to the exterior of hyphal surfaces located at the base of synnemata (Fig. 6). Mn oxide precipitates are observed within the voids between the aerial hyphae (conidiophore) cells comprising the synnemata, where the Mn oxides have a rumpled sheet appearance indicative of the Mn oxide phase birnessite, consistent with the EXAFS results (Fig. S1). Some precipitates are nucleated on the hyphal surface, growing radially from the site of nucleation and resulting in a nearly spherical morphology (Fig. 6). Energy-dispersive X-ray spectroscopy confirmed that the black precipitates were composed of Mn and O, but Mn was not detected within the hyphal cells (Fig. S3). Taken together, these observations indicate that cell differentiation and subsequent extracellular superoxide production in S. aciculosa is responsible for Mn(II) oxidation by this organism. In the presence of Mn(II), cell differentiation results in superoxide-mediated Mn oxide formation at the site of superoxide production, which is adjacent to the hyphae at the site of conidiophore development.
Fig. 6.
TEM images of cross-sections through hyphal cells located at the base of reproductive structures. The images illustrate the morphology of Mn oxides and relationship between hyphal cells (unstained = white) and Mn oxide (black) precipitation. Right image illustrates the boxed region in the left image.
Conclusions
Here we show that extracellular superoxide produced during cell differentiation is responsible for Mn(II) oxidation by the common Ascomycete fungi S. aciculosa. Interestingly, these observations are equivalent to those obtained for a common marine bacterium, Roseobacter sp. AzwK-3b, where the oxidation of Mn(II) was found to be a function of extracellular superoxide production by a soluble enzyme that is either exuded by the organisms or loosely associated with the outer aspect of the outer membrane. For both Roseobacter and Stilbella, quenching of superoxide and addition of an oxidoreductase inhibitor (DPI) results in inhibition of Mn oxide formation implicating NAD(P)H oxidoreductases in Mn(II) oxidation. For both organisms, superoxide production is extracellular and thus available to react with numerous organic and inorganic compounds in the surrounding aqueous milieu. Here we show that biologically generated superoxide will impact the cycling of Mn. Given the versatility of superoxide as a redox reactant, biologically generated superoxide will impact other metals and contaminants (e.g., Hg, Cu, Fe) as well as numerous organic compounds. Considering the widespread ability of fungi to produce superoxide, this extracellular production of superoxide by microbes should have important consequences on the bioavailability of metals and carbon in natural systems.
Similar to Roseobacter bacteria, these findings suggest that Mn(II) oxidation may be an unintentional trait of this organism, where it is an accidental side reaction of extracellular superoxide production. To date, the physiological reason for Mn(II) oxidation by bacteria and Ascomycete fungi is unknown, and this process has not been linked to energy conservation by these organisms (12, 36). The rate and extent of growth by these organisms is not enhanced in the presence of Mn(II) (12, 36) and superoxide formation by Roseobacter and Stilbella does not appear to be a response to Mn(II)—for example, cell differentiation is not enhanced in the presence of Mn(II)—and thus we hypothesize that for these organisms Mn(II) oxidation is likely an unintentional side reaction. Interestingly, this finding also introduces a surprising homology between some prokaryotic and eukaryotic organisms in the mechanisms responsible for Mn(II) oxidation. Furthermore, although the reasons for superoxide formation by Roseobacter sp. are still unknown, a similar class of enzymes appear to be involved (NADH oxidases) (19). Thus, these findings suggest that NAD(P)H oxidases may be contributors to the cycling of metals in natural systems and thus identifying the mechanisms and environmental conditions regulating NAD(P)H activity may allow for better predictions of the fate and bioavailability of carbon and metals within the environment.
Materials and Methods
Fungal Strains and Growth Conditions.
S. aciculosa isolate DS2rAY2a (12) cultures were inoculated in a Hepes-buffered (20 mM; pH 7) AY medium, consisting of 0.25 g⋅L−1 sodium acetate, 0.15 g⋅L−1 yeast extract, and 1 mL⋅L−1 trace element stock (10 mg⋅L−1 CuSO4•5H2O, 44 mg⋅L−1 ZnSO4•7H2O, 20 mg⋅L−1 CoCl2•6H2O, 13 mg⋅L−1 Na2MoO4•2H2O) and supplemented with MnCl2 (0–200 μM), CuCl2 (0–200 μM), ZnCl2 (0–200 μM), or DPI (50 μM). Stab cultures were first initiated in Petri dishes containing agar-solidified [2% (wt/vol) agar] AY media without supplements and fungal mycelia were sampled with a sterile coring device (8-mm diameter). The mycelia-containing agar plugs were positioned either on new AY agar medium surfaces or on cellulose acetate membrane filters (0.2-μm pore size, 47-cm diameter) that had been placed directly on agar-solidified AY media. The filters provided a solid support for the cultures to grow and also allowed diffusion of water-soluble nutrients and metals through the filter, as Mn(II) oxidation was observed on the top surface of the filter. Cultures were incubated in both light and dark conditions at room temperature for ∼14–20 d.
ROS and Mn Oxide Detection.
Extracellular ROS were detected using stains that precipitate upon reaction with ROS, thus maintaining spatial distributions of ROS. Plates were both point inoculated and flooded with NBT chloride or DAB for superoxide and hydrogen peroxide detection, respectively. The NBT assay involved addition of 2.5 mM NBT in 5 mM 3-(N-morpholino)propanesulfonate-NaOH, pH 7.6, which upon reaction with O2− forms a blue precipitate. The DAB assay involved addition of 2.5 mM DAB and 5 purpurogallin unit/mL of horseradish peroxidase (type VI from Sigma) in potassium phosphate buffer, pH 6.9, which forms a red precipitate upon reaction with H2O2. For both assays, plates were incubated with the stain for 30 min in the dark. Then, excess reagents were decanted, the plates were incubated for an additional 2–3 h, and imaged using a stereomicroscope (as described below). Mn(III/IV) oxides were confirmed by applying the colorimetric dye Leucoberbelin blue (37), which oxidizes and turns blue in the presence of Mn(III) and Mn(IV).
XRF and XAS.
Bulk and microfocused XAS was conducted at the Stanford Synchrotron Radiation Lightsource on beam line 2–3 (see SI Materials and Methods for bulk XAS methods and results). XAS analysis was conducted on filter (cellulose acetate, 0.2-μm pore size, 47-cm diameter) supported S. aciculosa cultures (38), incubated in the presence of 200 μM Mn(II) for 20 d. The whole filter, with the attached fungi (including mycelia, spores, reproductive structures, and so forth), was easily removed from the media surface and used directly for analysis (no preservation or sample alteration). Using filter-supported fungal cultures alleviates issues associated with beam-induced photoreduction of Mn oxide phases grown directly in or on carbon-based agar. Spatially resolved (microscale) XRF and XAS were conducted by collecting spectra at select points of interest or defining and rastering a defined region. The beam size on the sample was 2 × 2 μm. Monochromatic X-rays were selected using a Si(111) Φ = 90 double crystal monochromator. Multiple elements were mapped simultaneously by collecting fluorescence on a multichannel Si Vortex detector (SII Nano Technology) using an incident monochromator energy of 13,200 eV, which is above the absorption edge of all of the elements of interest. Maps were also collected at several discrete incident energies (6,553, 6,558, 6,562 eV) in continuous raster scanning mode to collect the fluorescence at several distinguishing points within the Mn absorption edge. XANES spectra were collected at spots of interest to confirm the oxidation state at discrete locations. The line-shapes (peak position and peak shape) of the XANES spectra were used to compare the relative proportions of Mn(II), Mn(III), and Mn(IV) in the Mn oxides (25, 26). Fluorescence maps and XANES spectra were analyzed using the MicroAnalysis Toolkit (39) and SIXPACK (40), respectively.
Microscopy.
For light microscopy, stab cultures were initiated in agar-solidified AY media supplemented with 200 μM MnCl2 and grown for ∼14 d. Radially growing cultures were imaged using a SZX16 Zoom Stereo Microscope (Olympus America) fitted with an Olympus DP72 Microscope Digital Camera. For TEM, cultures were grown on filter-supported media supplemented with 200 μM MnCl2. Cultures were grown for ∼14 d, fixed in 2.5% glutaraldehyde, washed three times in PBS buffer, subjected to an ethanol dehydration series, and embedded in LR White resin and cured at 60 °C overnight. Hardened resin blocks were sectioned to 70 nm with a Diatome 45° diamond knife using a Leica UCT ultramicrotome and mounted on 100-mesh copper grids with formvar support film coated with carbon. Unstained sections were imaged with an FEI Tecnai T-12 cryo-TEM. Thin sections were also examined using a JEOL 2010 high resolution TEM (HR-TEM) equipped with an Oxford ISIS Energy-dispersive X-ray spectroscopy microanalysis system.
Supplementary Material
Acknowledgments
The authors thank Alice Dohnalkova (Environmental Molecular Sciences Laboratory) for assistance in transmission electron microscopy collection and analysis, and three anonymous reviewers for their valuable contributions to this manuscript. Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a national user facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. The Stanford Synchrotron Radiation Lightsource Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program. A portion of this research was also performed using the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory. This project was funded by the National Science Foundation, Grant EAR-0846715 (to C.M.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203885109/-/DCSupplemental.
References
- 1.Huang PM. Kinetics of redox reactions on manganese oxides and its impact on environmental quality. In: Sparks DL, Suarez DL, editors. Rates of Soil Chemical Processes. no. 27. Madison, WI: Soil Science Society of America; 1991. pp. 191–230. [Google Scholar]
- 2.Sunda WG, Kieber DJ. Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substrates. Nature. 1994;367:62–64. [Google Scholar]
- 3.Izawa K, et al. Photoelectrochemical oxidation of methanol on oxide nanosheets. J Phys Chem B. 2006;110:4645–4650. doi: 10.1021/jp056210l. [DOI] [PubMed] [Google Scholar]
- 4.Luther GW., III The role of one- and two-electron transfer reactions in forming thermodynamically unstable intermeidates as barriers in multi-electron redox reactions. Aquat Geochem. 2010;16:395–420. [Google Scholar]
- 5.Junta JL, Hochella MF., Jr Manganese(II) oxidation at mineral surfaces: A microscopic and spectroscopic study. Geochim Cosmochim Acta. 1994;58:4985–4999. [Google Scholar]
- 6.Duckworth OW, Sposito G. Siderophore-manganese(III) interactions. I. Air-oxidation of manganese(ll) promoted by desferrioxamine B. Environ Sci Technol. 2005;39:6037–6044. doi: 10.1021/es050275k. [DOI] [PubMed] [Google Scholar]
- 7.Tebo BM, Johnson HA, McCarthy JK, Templeton AS. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 2005;13:421–428. doi: 10.1016/j.tim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Glenn JK, Akileswaran L, Gold MH. Mn(II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1986;251:688–696. doi: 10.1016/0003-9861(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 9.Wariishi H, Valli K, Gold MH. Manganese(II) oxidaiton by manganese peroxidase from the Basidiomycete Phanerochaete chrysosporium. J Biol Chem. 1992;267:23688–23695. [PubMed] [Google Scholar]
- 10.Perez J, Jeffries TW. Roles of manganese and organic acid chelators in regulating lignin degradation and biosynthesis of peroxidases by Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:2402–2409. doi: 10.1128/aem.58.8.2402-2409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyata N, Tani Y, Iwahori K, Soma M. Enzymatic formation of manganese oxides by an Acremonium-like hyphomycete fungus, strain KR21-2. FEMS Microbiol Ecol. 2004;47:101–109. doi: 10.1016/S0168-6496(03)00251-4. [DOI] [PubMed] [Google Scholar]
- 12.Santelli CM, et al. Promotion of Mn(II) oxidation and remediation of coal mine drainage in passive treatment systems by diverse fungal and bacterial communities. Appl Environ Microbiol. 2010;76:4871–4875. doi: 10.1128/AEM.03029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases. Chem Rev. 1996;96:2563–2606. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 14.Dick GJ, Torpey JW, Beveridge TJ, Tebo BM. Direct identification of a bacterial manganese(II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Appl Environ Microbiol. 2008;74:1527–1534. doi: 10.1128/AEM.01240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlosser D, Höfer C. Laccase-catalyzed oxidation of Mn(2+) in the presence of natural Mn(3+) chelators as a novel source of extracellular H(2)O(2) production and its impact on manganese peroxidase. Appl Environ Microbiol. 2002;68:3514–3521. doi: 10.1128/AEM.68.7.3514-3521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansard SP, Easter HD, Voelker BM. Rapid reaction of nanomolar Mn(II) with superoxide radical in seawater and simulated freshwater. Environ Sci Technol. 2011;45:2811–2817. doi: 10.1021/es104014s. [DOI] [PubMed] [Google Scholar]
- 17.Voelker BM, Sedlak DL. Iron reduction by photoproduced superoxide in seawater. Mar Chem. 1995;50:93–102. [Google Scholar]
- 18.Voelker BM, Sedlak DL, Zafiriou OC. Chemistry of superoxide radical in seawater: Reactions with organic Cu complexes. Environ Sci Technol. 2000;34:1036–1042. [Google Scholar]
- 19.Learman DR, Voelker BM, Vazquez-Rodriguez AI, Hansel CM. Formation of manganese oxides by bacterially generated superoxide. Nat Geosci. 2011;4:95–98. [Google Scholar]
- 20.Huycke MM, et al. Extracellular superoxide production by Enterococcus faecalis requires demethylmenaquinone and is attenuated by functional terminal quinol oxidases. Mol Microbiol. 2001;42:729–740. doi: 10.1046/j.1365-2958.2001.02638.x. [DOI] [PubMed] [Google Scholar]
- 21.Rose AL, Salmon TP, Lukondeh T, Neilan BA, Waite TD. Use of superoxide as an electron shuttle for iron acquisition by the marine cyanobacterium Lyngbya majuscula. Environ Sci Technol. 2005;39:3708–3715. doi: 10.1021/es048766c. [DOI] [PubMed] [Google Scholar]
- 22.Aguirre J, Ríos-Momberg M, Hewitt D, Hansberg W. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 2005;13:111–118. doi: 10.1016/j.tim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Scott B, Eaton CJ. Role of reactive oxygen species in fungal cellular differentiations. Curr Opin Microbiol. 2008;11:488–493. doi: 10.1016/j.mib.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Seifert KA. A monography of Stilbella and some allied hyphomycetes. Stud Mycol. 1985;27:1–235. [Google Scholar]
- 25.Webb SM, Tebo BM, Bargar JR. Structural characterization of biogenic Mn oxides produced in seawater by the marine Bacillus sp. strain SG-1. Am Mineral. 2005;90:1342–1357. [Google Scholar]
- 26.Bargar JR, et al. Biotic and abiotic products of Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1. Am. Min. 2005;90:143–154. [Google Scholar]
- 27.Tebo BM, Ghiorse WC, van Waasbergen LG, Siering PL, Caspi R. Bacterially mediated mineral formation: Insights into manganese(II) oxidation from molecular genetic and biochemical studies. In: Banfield JF, Nealson KH, editors. Geomicrobiology: Interactions Between Microbes and Minerals, Reviews in Mineralogy. Vol 35. Washington, DC: Mineralogical Society of America; 1997. pp. 225–266. [Google Scholar]
- 28.Malagnac F, Lalucque H, Lepère G, Silar P. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet Biol. 2004;41:982–997. doi: 10.1016/j.fgb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Zafiriou OC. Chemistry of superoxide ion-radical (O2-) in seawater. I. pKa (HOO) and uncatalyzed dismutation kinetics studied by pulse radiolysis. Mar Chem. 1990;31:31–43. [Google Scholar]
- 30.Sunda WG, Huntsman SA. Photoreduction of manganese oxides in seawater. Mar Chem. 1994;46:133–152. [Google Scholar]
- 31.Takemoto D, Tanaka A, Scott B. NADPH oxidases in fungi: Diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet Biol. 2007;44:1065–1076. doi: 10.1016/j.fgb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Semighini CP, Harris SD. Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics. 2008;179:1919–1932. doi: 10.1534/genetics.108.089318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lara-Ortíz T, Riveros-Rosas H, Aguirre J. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol. 2003;50:1241–1255. doi: 10.1046/j.1365-2958.2003.03800.x. [DOI] [PubMed] [Google Scholar]
- 34.Cano-Domínguez N, Alvarez-Delfín K, Hansberg W, Aguirre J. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot Cell. 2008;7:1352–1361. doi: 10.1128/EC.00137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansel CM, Francis CA. Coupled photochemical and enzymatic Mn(II) oxidation pathways of a planktonic Roseobacter-Like bacterium. Appl Environ Microbiol. 2006;72:3543–3549. doi: 10.1128/AEM.72.5.3543-3549.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krumbein WE, Altmann HJ. New method for detection and enumeration of manganese oxidizing and reducing microorganisms. Helgol Wiss Meeresunters. 1973;25:347–356. [Google Scholar]
- 38.Santelli CM, Webb SM, Dohnalkova AC, Hansel CM. Diversity of Mn oxides produced by Mn(II)-oxidizing fungi. Geochim Cosmochim Acta. 2011;75:2762–2776. [Google Scholar]
- 39.Webb SM. 2006. SMAK: Sam’s Microprobe Analysis Kit, v.0.25 (Stanford Synchrotron Radiation Laboratory, Melo Park, CA) [Google Scholar]
- 40.Webb SM. SIXPack a graphical user interface for XAS analysis using IFEFFIT. Phys Scr. 2005;T115:1011–1014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.