Abstract
MicroRNAs (miRNAs) are regulators of gene expression in plants and animals. The biogenesis of miRNAs is precisely controlled to secure normal development of organisms. Here we report that TOUGH (TGH) is a component of the DCL1–HYL1–SERRATE complex that processes primary transcripts of miRNAs [i.e., primary miRNAs (pri-miRNAs)] into miRNAs in Arabidopsis. Lack of TGH impairs multiple DCL activities in vitro and reduces the accumulation of miRNAs and siRNAs in vivo. TGH is an RNA-binding protein, binds pri-miRNAs and precursor miRNAs in vivo, and contributes to pri-miRNA–HYL1 interaction. These results indicate that TGH might regulate abundance of miRNAs through promoting DCL1 cleavage efficiency and/or recruitment of pri-miRNAs.
Small RNAs, including microRNAs (miRNAs) and siRNAs, are sequence-specific regulators of gene expression in plants and animals (1). miRNAs are derived from imperfect stem-loop transcripts, called primary miRNAs (pri-miRNAs), which are predominately produced by DNA-dependent RNA polymerase II, whereas siRNAs are processed from perfect or near-perfect long dsRNAs (2). After generation, miRNA and siRNA are loaded into an RNA-induced silencing complex containing the Argonaute protein to guide posttranscriptional or transcriptional gene silencing (1).
In animals, pri-miRNAs are first processed to precursor miRNAs (pre-miRNAs) in the nucleus by the microprocessor containing Drosha and a dsRNA-binding protein DGCR8 (1). The resulting pre-miRNAs are then processed by Dicer in the cytoplasm to produce mature miRNAs (1). It has emerged that the activities of Drosha and Dicer are controlled to regulate miRNA expression in response to developmental and environmental signals (3). In Arabidopsis, DCL1, a dsRNA-binding protein, HYL1, and a zinc finger protein, SERRATE (SE), form a complex to process pri-miRNAs in the nucleus to pre-miRNAs and then to mature miRNAs (4–6). The accumulation of miRNAs in Arabidopsis also requires DDL, which was proposed to stabilize pri-miRNAs and to facilitate their processing (7). Recently, two cap-binding proteins, CBP80/ABH1 and CBP20, were found to be required for pre-mRNA splicing and pri-miRNA processing (8, 9). Plants also encode several classes of endogenous siRNAs, including the natural antisense transcript-derived siRNA, siRNA derived from repetitive DNA sequences (rasiRNA), and transacting siRNA (ta-siRNA) (10). In Arabidopsis, the generation of these siRNAs from long dsRNAs involves DCL1 homologues DCL2, DCL3, and DCL4, which produce 22-nt, 24-nt, and 21-nt siRNAs, respectively (11–13).
In this report, we show that TOUGH (TGH) is an important factor for miRNA and siRNA biogenesis. Loss-of-function TGH in tgh-1 reduces the activity of multiple DCLs in vitro and the accumulation of miRNA and siRNAs in vivo. In the miRNA pathway, TGH associates with the DCL1 complex and binds pri-miRNAs and pre-miRNAs. TGH is required for the efficient in vivo interaction between pri-miRNA and HYL1. These data suggest that TGH assists DCLs to efficiently process and/or recruit the precursors of miRNAs and siRNAs.
Results
TGH Is Required for Accumulation of miRNAs and siRNAs in Arabidopsis.
Three facts prompted us to test whether TGH acts in the miRNA pathway. First, TGH is an evolutionarily conserved protein across plant and animal kingdoms (14), agreeing with the fact that many components involved in miRNA biogenesis are conserved in eukaryotes (1). Second, TGH contains a G-patch and a SWAP domain (Suppressor-of-White-APricot) that often exist within RNA metabolism-related proteins (14) (Fig. S1A). Finally, like dcl1, ddl, hyl1, and abh1, which are deficient in the miRNA pathway, the tgh mutants exhibit pleiotropic developmental defects such as smaller plant size, altered leaf shape, short stature, increased branches, disordered node distribution, and reduced fertility (14–20) (Fig. S1B).
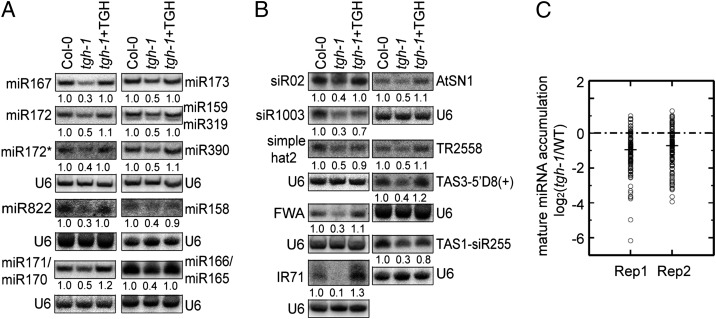
To determine whether TGH functions in miRNA biogenesis, we examined the accumulation of various DCL1-dependent miRNAs in inflorescences of tgh-1 (SALK_053445), which contains a T-DNA insertion in the 11th intron and is a potential null allele (14) (Fig. S1A). The levels of all tested miRNAs (miR158, miR159, miR166/165, miR167, miR171/170, miR172, miR173, miR319, and miR390) were reduced in tgh-1 by 50% to 70% relative to WT control [Columbia-0 (Col-0); Fig. 1A]. The expression of miR172*, the passenger strand of miR172, was also reduced in tgh-1 (Fig. 1A). Expressing a genomic copy of TGH driven by its native promoter fused with an HA tag at its C-terminal (TGH::TGH-HA) fully restored the levels of these miRNAs and miRNA172* (Fig. 1A), demonstrating that lack of TGH in tgh-1 was responsible for the defects in miRNA accumulation. We also checked the levels of miR161, miR163, miR166/165, miR167, miR172, and miR173 in mature leaves. All of them were less accumulated in tgh-1 than in WT (Fig. S1C).
Fig. 1.
tgh-1 reduces the accumulation of miRNAs and siRNAs. (A) The accumulation of miRNAs and miR172* in three genotypes. (B) The accumulation of siRNAs in three genotypes. For miR159/319: Upper band, miR159; lower band, miR319 (34). The numbers indicate the relative abundance of small RNAs among the three genotypes and represent the mean of three repeats (P < 0.05). U6 blot was used as a loading control. Col-0, WT control for tgh-1; tgh-1+TGH, tgh-1 harboring TGH genomic DNA. (C) Deep sequencing analysis of miRNAs in tgh-1 and WT. Small RNA libraries were generated from inflorescences. The miRNA abundance was calculated as reads per million, and a log2-transformed ratio of tgh-1/Wt was plotted. Each circle represents one miRNA. Thick lines indicate median values.
Next, we asked whether TGH plays a role in the accumulation of rasiRNAs and ta-siRNAs. We found that DCL4-dependent ta-siRNAs, TAS1-siR255 and TAS3-5′D8(+), DCL2-dependent IR71 and DCL3-dependent rasiRNAs, siR02, siR1003, simplehat2, AtSN1, TR2588, and FWA were reduced in abundance in tgh-1 compared with WT, and the reduction was rescued by the TGH transgene (Fig. 1B). To test the involvement of TGH in DCL4 related processing, we monitored the accumulation of DCL4-dependent miR822 (21). The levels of miR822 were lower in tgh-1 than in WT, and the defect was restored by the TGH transgene (Fig. 1A).
We further compared the transcript levels of several miRNA targets, CUC1, PHV, SAMT, PPR, and a ta-siRNA target ARF3 between WT and tgh-1, which should inform whether tgh-1 impaired miRNA and ta-siRNA function. The transcript levels of these miRNA targets were slightly increased in tgh-1 relative to WT (Fig. S1D).
TGH Does Not Affect miRNA Precision.
Although Northern blot showed that TGH affects the accumulation of miRNAs, it could not tell whether miRNA precision requires TGH. To address this question, we performed Illumina deep sequencing analysis of small RNA libraries constructed from inflorescences of WT and tgh-1. The data set was deposited into the National Center for Biotechnology Information Gene Expression Omnibus (accession no. GSE38600). We focused our analysis on miRNAs. The abundance of most miRNAs was reduced in tgh-1 relative to WT in two biological replicates (Fig. 1C). This analysis further confirmed that TGH is required for the accumulation of miRNAs. We next evaluated whether TGH affected processing precision. According to Liu et al. (22), imprecise miRNAs were defined as those that did not fall within ±2 bases of the annotated mature miRNA(s) or miRNA*(s) positions. Because evaluation on miRNA precision depends on sequencing depth (22), we analyzed only the highly expressed miRNAs. Like WT, tgh-1 contained a very low ratio of imprecise miRNAs (Dataset S1), indicating that TGH may be not required for the accurate cleavage of pri-miRNAs.
Multiple DCL Activities Are Impaired in tgh-1.
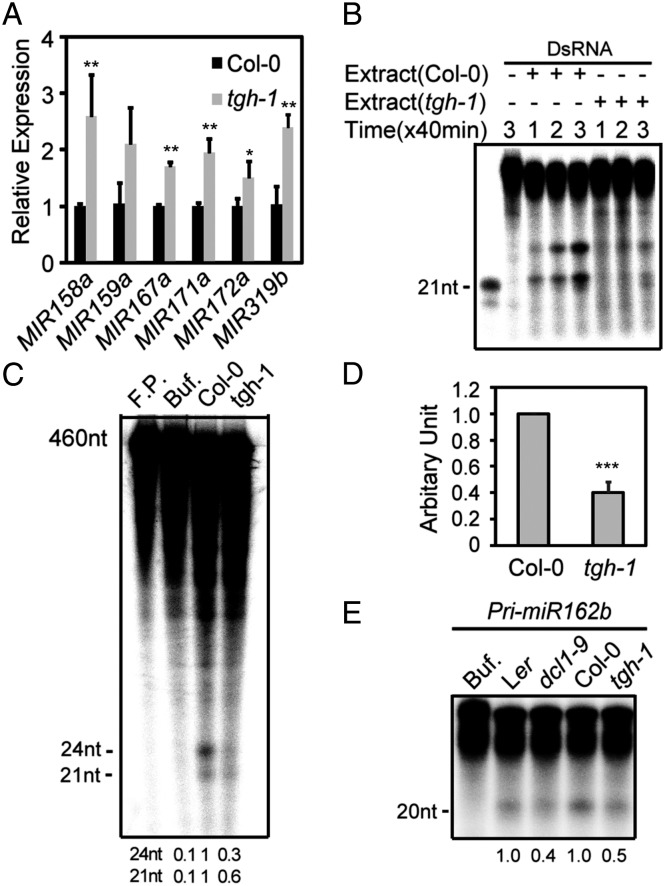
To determine at which step TGH may act in miRNA biogenesis, we examined the levels of pri-miRNAs in WT and tgh-1. Quantitative RT-PCR (qRT-PCR) analyses showed that the levels of pri-miRNAs at six loci (pri-miR158a, pri-miR159a, pri-miR167a, pri-miR171a, pri-miR172a, and pri-miR319b) were increased by 1.5 to 2.5-fold in tgh-1 relative to that in WT (Fig. 2A). This result suggested a potential defect of DCL1 activity in tgh-1. We also compared the levels of pri-miRNA from each member of miR159, miR167, and miR171 between WT and tgh-1, with the expectation to inform whether TGH equally affects the processing of each member of miRNA families. Although tgh-1 increased the levels of these pri-miRNAs, its effects on individual pri-miRNA were varied (Fig. S2B).
Fig. 2.
tgh-1 impairs multiple DCL activities. (A) Increased pri-miRNA levels in inflorescences of tgh-1. The levels of pri-miRNAs in tgh-1 were normalized to those of UBIQUITIN 5 and compared with Col. Error bars indicate SD of three technical replications (*P < 0.05 and **P < 0.01). (B) Reduced production of siRNAs from dsRNAs in the tgh-1 protein extracts. (C) Reduced production of 21- and 24-nt siRNA in the tgh-1 protein extracts. Numbers below indicate siRNA production in tgh-1 relative to control. (D) Quantification of overall siRNA production in tgh-1 extracts relative to the control extracts. Data are presented as mean and SD (n = 7; ***P < 0.001). (E) The pri-miR162b processing in tgh-1, dcl1-9, and WT. The reaction was stopped after 120 min. Numbers indicate overall miRNA production in tgh-1 and dcl1-9 extracts relative to their respective control extracts and represent the mean of three experiments (P < 0.05).
It has been established that DCL1 and DCL3 are responsible for the production of 21- and 24-nt small RNAs in an in vitro dsRNA processing assay using Arabidopsis protein extracts, respectively (23). We adapted this assay to test whether DCL1 and DCL3 activities are impaired in tgh-1. A radioactive labeled dsRNA (460 bp) was incubated with protein extracts from young flower buds of tgh-1 or WT. The reactions were stopped at 40, 80, and 120 min, and the RNAs from each reaction were extracted and resolved on a polyacrylamide gel. The production of small RNAs by tgh-1 protein extract was lower than that by WT (Fig. 2B). Quantitative analysis revealed that the overall DCL processing activity in tgh-1 was approximately 40% of that in WT (Fig. 2D). The RNAs extracted from the 120-min reaction were further resolved on a long PAGE gel to separate the 24-nt and 21-nt small RNAs. The production of 24- and 21-nt small RNAs was lower in tgh-1 extracts than in WT (Fig. 2C). These observations indicated that DCL1 and DCL3 activities are impaired in tgh-1. To test the effects of tgh-1 on DCL1-mediated miRNA maturation, we compared processing of a short form of pri-miR162b (predicted stem loop with 6-nt arms at each end; Fig. S2A) between tgh-1 and WT protein extracts. As a control of pri-miRNA processing, we included dcl1-9, which is a weak allele of dcl1 and has reduced miRNA production, as a control. Like dcl1-9, tgh-1 reduced pri-miR162b processing efficiency relative to WT (Fig. 2E).
TGH Associates with DCL1 Complex.
There are several possible ways for TGH to affect DCL1 activities. We first analyzed the expression level of several key genes in miRNA biogenesis by qRT-PCR. The abundance of DDL, CBP20, and CBP80 were comparable between WT and tgh-1 (Fig. S3A). The expression levels of DCL1, SE, and HEN1 were slightly increased in tgh-1 compared with WT, whereas the levels of HYL1 were slightly decreased (Fig. S3B). However, tgh-1 had no effect on the protein level of HYL1 and DCL1 (Fig. S3B).
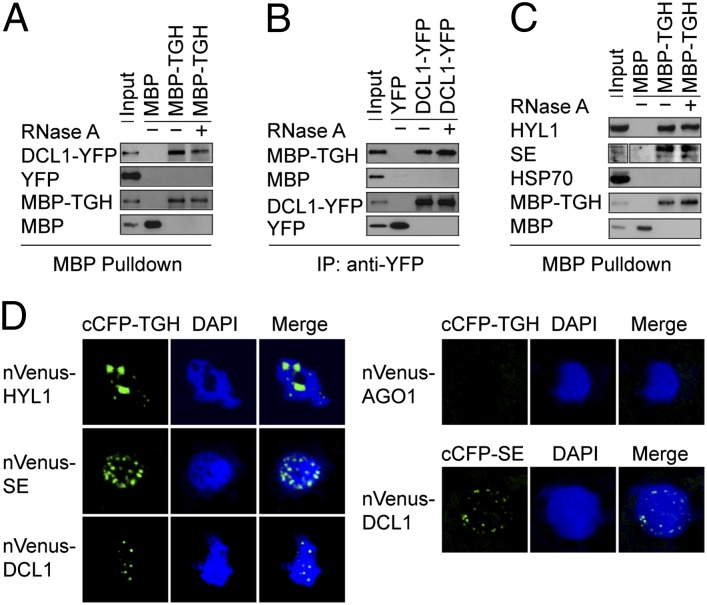
Next, we tested the association of TGH with DCL1 by using coimmunoprecipitation/pull-down assay. We expressed the recombinant TGH protein fused with a maltose-binding protein epitope at its N terminus (MBP-TGH) in Escherichia coli and the DCL1 protein fused with YFP in Nicotiana benthamiana (Fig. S3C) (7). We mixed the MBP-TGH and DCL1-YFP protein extracts and performed reciprocal pull-down assays with amylose resin and a polyclonal antibody recognizing GFP and its variants conjugated to protein A-agarose beads, respectively. Antibodies against GFP and MBP epitope detected the enrichments of DCL1-YFP in MBP-TGH precipitates and MBP-TGH in DCL1-YFP complexes, respectively (Fig. 3 A and B), indicating the TGH–DCL1 interaction. TGH is a putative RNA-binding protein, raising the possibility that the TGH–DCL1 association might be RNA-mediated. RNase A treatment abolished the RNA-mediated FDM1–AGO4 interaction (Fig. S3D) (24) but not TGH–DCL1 interaction (Fig. 3 A and B). As controls, we performed reciprocal pull-downs to test the YFP/MBP, YFP/MBP-TGH, and MBP/DCL1-YFP interactions. We did not detect any interactions among these proteins (Fig. 3 A and B). We further tested the HYL1–TGH and SE–TGH associations by using-pull down assays. MBP-TGH, but not MBP, was able to pull down HYL1 and SE from Arabidopsis protein extracts (Fig. 3C). The control protein HSP70 was not detected in the MBP-TGH precipitates. Because TGH affects 24-nt siRNA production, we tested coimmunoprecipitation between TGH and DCL3. We were able to detect the presence of MBP-TGH but not MBP in the DCL3 immunoprecipitates (Fig. S3E).
Fig. 3.
TGH associates with the DCL1 complex. (A) MBP-TGH pulls down DCL1-YFP. (B) DCL1-YFP pulls down MBP-TGH. (C) MBP-TGH pulls down HYL1 and SE. Protein precipitates were analyzed by Western blot by using anti-MBP, anti-GFP, and anti-HYL1 antibodies, respectively. One percent input proteins were used for MBP-TGH and MBP, and 2% input proteins were used for YFP, DCL1-YFP, and HYL1. (D) BiFC analysis between TGH and the components of DCL1 complex. TGH and SE were fused with cCFP whereas DCL1, HYL1, SE, and AGO1 were fused with nVenus. Respective pair of cCFP and nVenus fusion proteins was coinfiltrated into leaves, and fluorescence signals were examined ∼40 h after coinfiltration. The interaction of paired proteins will result in yellow fluorescence (green in image). More than 30 nuclei were examined for each pair, and a graph is shown. DNA was stained with DAPI to visualize the nuclei (blue).
To ascertain the association between TGH and the DCL1 complex, we performed a bimolecular fluorescence complementation (BiFC) assay. In this assay, we fused protein partners to the N-terminal fragment of Venus (nVenus) or C-terminal fragment of CFP (cCFP), respectively, and introduced paired proteins into tobacco cells by infiltration. The interaction of the two protein partners will generate a functional YFP, leading to fluorescence (25). Similar methods have been previously used to investigate the interactions among DCL1, HYL1, and SE (4, 5). BiFC signals produced from the TGH–SE, TGH–DCL1, TGH–HYL1, and SE–DCL1 (positive control) interactions were observed in distinct nuclear speckles (Fig. 3D). In contrast, only weak fluorescence signals were observed from the control AGO1–TGH pair (Fig. 3D). These results indicated that TGH is a component of the pri-miRNA processing complex.
TGH Binds pri-miRNAs and pre-miRNAs.
The presence of putative RNA binding domains in TGH suggested that TGH might be an RNA binding protein. We performed a pull-down assay to examine whether TGH could bind pri-miR162b, which was used for in vitro processing assay (Fig. S2A). MBP and TGH-MBP expressed in E. coli were purified with amylose resin (Fig. 4A) and incubated with radioactive labeled pri-miR162b. TGH-MBP, but not MBP, was able to retain pri-miR162b and addition of unlabeled pri-miR162b was able to wash off the radioactive signal (Fig. 4B). We also generated a radioactive-labeled premiR162b, which has a 2-nt 3′ overhang (Fig. S2A), by in vitro transcription and examined its interaction with TGH. TGH was able to bind the pre-miR162b. However, TGH-MBP could not bind a ∼460-nt dsRNA (Fig. 4B), indicating that TGH may be a single-stranded RNA binding protein. In fact, TGH bound a ∼100-nt RNA corresponding to a portion of the 5′ end of the UBIQUITIN 5 (UBQ5) ORF in vitro (Fig. 4B).
Fig. 4.
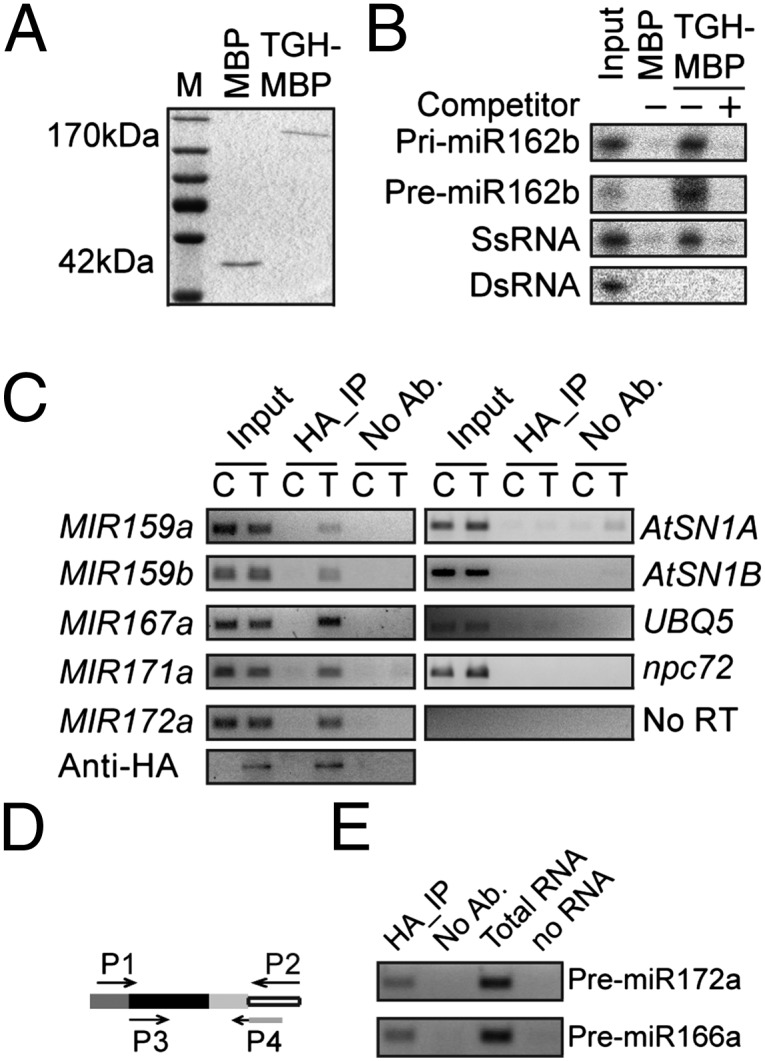
TGH is an RNA-binding protein. (A) TGH-MBP and MBP proteins used in the in vitro RNA binding assay. The proteins were resolved on an SDS-polyacrylamide gel and detected by Coomassie brilliant blue staining. (B) TGH binds pri-miR162b and premiR162b in vitro. (C) TGH binds pri-miRNA in vivo. RIP was performed with the anti-HA antibody. C, Col-0; T, tgh-1 harboring a TGH::TGH-HA transgene. One eighth immunoprecipitates was analyzed by Western blot. Input protein was 2%. No RT was performed with the pri-miR167a primers. Input RNA was 5%. (D) TGH binds pre-miRNAs in vivo. RT was performed with primer P2. The first-round PCR was done with primers P1 and P2. The Second round PCR was performed with primers P3 and P4. Primer P4 recognizes the junction between adaptor and premiRNA. Open box, adaptor; light/dark gray box, miRNA/miRNA*; black box, region between miRNA and miRNA*.
Next, we tested whether TGH binds pri-miRNA and premiRNAs in vivo. Seedlings of tgh-1 complementation plants harboring the TGH::TGH-HA transgene were subjected to RNA immunoprecipitation (RIP) (26). The presence of pri-miRNA in the TGH complex was then examined with RT-PCR. All the tested pri-miRNAs were present in the TGH–HA complex but not in the immunoprecipitates from nontransgenic plants and “no-antibody” controls (Fig. 4C). We did not find the interaction between TGH and RNA controls AtSN1B RNA, which is transcribed from the flanking region of AtSN1 siRNA target locus (26), npc72 (27), and UBQ5 mRNA (Fig. 4C). This result indicated that TGH might specifically interact with some RNAs in vivo. However, we did not detect AtSN1A RNA (Fig. 4C), which likely is a ra-siRNA–generating RNA, in the TGH–HA complex. One possible explanation is that TGH might transiently interact with the DCL3 complex. Alternatively, it may be because that the substrates of DCL3 are dsRNAs. To examine the association of TGH with pre-miRNA in vivo, TGH-bound RNAs were ligated to a 3′ adaptor, and then reverse transcription and nested PCR were performed to detect the pre-miRNAs (Fig. 4D). This assay allowed us to detect pre-miR172a and pre-miR166a in the TGH complex (Fig. 4D). Because TGH did not bind perfect dsRNA, the interaction between TGH and pre-miRNAs indicated that TGH might bind the loop or the bulge of pre-miRNAs.
tgh-1 Impairs HYL1–pri-miRNA Interaction.
Based on the association of TGH with pri-miRNA and its processing complex, we tested whether TGH contributes to the association between pri-miRNAs and HYL1. HYL1 is a component of DCL1 complex, and its association with pri-miRNA is essential for pri-miRNA processing (28). We examined HYL1–pri-miRNA interaction in WT and tgh-1 by RIP by using antibody against HYL1. A similar amount of HYL1 was immunoprecipitated from the protein extracts of tgh-1 and WT (Fig. 5A). RT-PCR and qRT-PCR analysis revealed that the amount of HYL1-bound pri-miR167a and pri-miR171a was reduced in tgh-1 relative to WT (Fig. 5 B and C). We included hyl1-2 as a negative control in this experiment. No HYL1 and associated RNAs were immunoprecipitated by HYL1 antibody from hyl1-2 (Fig. S4).
Fig. 5.

TGH contributes to in vivo HYL1–pri-miRNA interaction. (A) Detection of HYL1 protein after immunoprecipitation. Immunoprecipitation was performed with the anti-HYL1 antibody. (B) and (C) association between HYL1 and pri-miR171a and pri-miR167a was impaired in tgh-1. C, Col-0; t, tgh-1. One eighth immunoprecipitates were analyzed by Western blot. Input protein was 2% of total input proteins. The amount of pri-miR167a and pri-miR171a was determined by qRT-PCR and normalized to the input. AtSN1B was used as a negative control (*P < 0.05 and **P < 0.01).
Discussion
In conclusion, TGH is an important component of miRNA and siRNA biogenesis. Several lines of evidence demonstrate that TGH has a role in promoting miRNA maturation. The facts that lack of TGH in tgh-1 reduces the accumulation of miRNAs and increases the levels of pri-miRNAs and the association of TGH with the DCL1 complex, pri-miRNAs, and pre-miRNAs demonstrate that TGH has a role in promoting miRNA maturation. However, TGH shall have additional important functions in plants because tgh-1 has severe morphological phenotypes, whereas its effects on the levels of miRNAs appear to be less than those of dcl1-9.
In the miRNA pathway, TGH may have two non-mutually exclusive activities. First, TGH may contribute to the interaction between pri-miRNA and the DCL1 complex, which is supported by the reduced amount of pri-miRNA in the HYL1 complex from tgh-1. Second, TGH may have a role in modulating DCL1 activity, as DCL1-dependent in vitro pri-miRNA and dsRNA processing is impaired in the TGH-depleted extracts. However, TGH may not affect miRNA precision because tgh-1 contains a very low ratio of imprecise miRNAs. TGH affects the accumulation of DCL4-dependent miR822. The reduction of ta-siRNA and ra-siRNA levels indicates that TGH may have a role in siRNA biogenesis. However, the direct role of TGH in ta-siRNA processing needs further investigation, because DCL1-dependent miRNAs is also required for ta-siRNA biogenesis (29, 30). The reduction of DCL3-dependent 24-nt small RNA production in tgh-1 protein extracts indicates that TGH may act as a cofactor of DCL3 to facilitate dsRNA processing (Fig. 2). However, TGH may not contribute to the DCL3–dsRNA association, as it does not bind dsRNAs in vitro. Clearly, this hypothesis needs to be further examined.
TGH is an evolutionarily conserved protein in plant and animals. Given the similarity of small RNA pathways among different organisms, it will not be a surprise that the TGH homologues from other organisms have a role in RNA silencing. The reduced expression of TGH homologue from Caenorhabditis elegans has been shown to cause embryonic lethality or developmental defects in genome-wide RNAi screens (31–33), consistent with the role of miRNA in regulating developmental processes of plants and animals.
Materials and Methods
Plant Materials, Complementation Assay, Deep Sequencing, and RNA Analysis.
Plant materials, complementation assay, deep sequencing, and RNA analysis are described in SI Materials and Methods. Data generated from deep sequencing of small RNA libraries were deposited in the National Center for Biotechnology Information (accession no. GSE38600).
Dicer Activity Assay.
Dicer activity assay was performed according to Qi et al. (23). DNA template for dsRNA and pri-miR162b was amplified by using T7 promoter anchored primers (Table S1). The DNA templates for dsRNAs contain the T7 promoter at both ends. Resulting DNAs were used for in vitro transcription under the presence of [α-32P]UTP. RNAs were resolved on 6% (wt/vol) native PAGE gel and eluted with buffer containing 300 mM NaCl and 15 mM EDTA. After passing through a Spin-X filter, purified RNAs were precipitated with ethanol. For Dicer activity assay, RNAs were incubated with 30 μg protein in 20 μL reaction buffer containing 100 mM NaCl, 1 mM ATP, 0.2 mM GTP, 1.2 mM MgCl2, 25 mM creatine phosphate, 30 μg/mL creatine kinase, and 4 U RNase Inhibitor at room temperature. RNAs were extracted, precipitated, and resolved on PAGE gel. Radioactive signals were detected with a PhosphorImager and quantified by ImageQuant version 5.2.
Coimmunoprecipitation Assay.
MBP-TGH or MBP alone were expressed in E. coli BL21 and extracted according to the manufacturer’s protocol (NEB). Protein extracts containing DCL1-YFP or YFP from N. benthamiana were obtained according to Yu et al. (7). Half the MBP-TGH or MBP lysate was mixed with the DCL1-YFP or YFP lysate, respectively. The mixed lysate was incubated with anti-GFP (and GFP variants) antibodies coupled to protein A-agarose beads (Clontech) or amylose resin (NEB) for 4 h to overnight. The precipitates were washed five times with protein extraction buffer and resolved by SDS/PAGE. Anti-YFP (Covance) and anti-MBP (NEB) antibodies were used to detect DCL1-YFP/YFP and MBP-TGH/MBP, respectively, by Western blot analysis. Anti-HYL1 (catalog no. AS06136) and anti-SE (catalog no. AS09 532) antibodies were obtained from Agrisera.
BiFC Assay.
Paired constructs were coexpressed in N. benthamiana leaves for 40 h and subjected to confocal microscopy (Fluoview 500 workstation; Olympus) for imaging. BiFC signals were excited at 488 nm and detected with a narrow barrier filter (505–525 nm, BA505–525; Olympus). Nuclei were visualized by DAPI staining.
Supplementary Material
Acknowledgments
We thank Drs. David Holding and Heriberto Cerutti from the University of Nebraska–Lincoln for critically reading the manuscript. This work was supported by National Science Foundation Grants MCB-1121193 (to B.Y.) and an Enhancing Interdisciplinary Teams Grant from the University of Nebraska–Lincoln (to B.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE38600).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204915109/-/DCSupplemental.
References
- 1.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen X. MicroRNA biogenesis and function in plants. FEBS Lett. 2005;579:5923–5931. doi: 10.1016/j.febslet.2005.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 4.Fang Y, Spector DL. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr Biol. 2007;17:818–823. doi: 10.1016/j.cub.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujioka Y, Utsumi M, Ohba Y, Watanabe Y. Location of a possible miRNA processing site in SmD3/SmB nuclear bodies in Arabidopsis. Plant Cell Physiol. 2007;48:1243–1253. doi: 10.1093/pcp/pcm099. [DOI] [PubMed] [Google Scholar]
- 6.Song L, Han MH, Lesicka J, Fedoroff N. Arabidopsis primary microRNA processing proteins HYL1 and DCL1 define a nuclear body distinct from the Cajal body. Proc Natl Acad Sci USA. 2007;104:5437–5442. doi: 10.1073/pnas.0701061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu B, et al. The FHA domain proteins DAWDLE in Arabidopsis and SNIP1 in humans act in small RNA biogenesis. Proc Natl Acad Sci USA. 2008;105:10073–10078. doi: 10.1073/pnas.0804218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laubinger S, et al. Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008;105:8795–8800. doi: 10.1073/pnas.0802493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory BD, et al. A link between RNA metabolism and silencing affecting Arabidopsis development. Dev Cell. 2008;14:854–866. doi: 10.1016/j.devcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Vazquez F. Arabidopsis endogenous small RNAs: highways and byways. Trends Plant Sci. 2006;11:460–468. doi: 10.1016/j.tplants.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Bouché N, Lauressergues D, Gasciolli V, Vaucheret H. An antagonistic function for Arabidopsis DCL2 in development and a new function for DCL4 in generating viral siRNAs. EMBO J. 2006;25:3347–3356. doi: 10.1038/sj.emboj.7601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Z, Allen E, Wilken A, Carrington JC. DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2005;102:12984–12989. doi: 10.1073/pnas.0506426102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson IR, et al. Dissecting Arabidopsis thaliana DICER function in small RNA processing, gene silencing and DNA methylation patterning. Nat Genet. 2006;38:721–725. doi: 10.1038/ng1804. [DOI] [PubMed] [Google Scholar]
- 14.Calderon-Villalobos LI, et al. The evolutionarily conserved TOUGH protein is required for proper development of Arabidopsis thaliana. Plant Cell. 2005;17:2473–2485. doi: 10.1105/tpc.105.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hugouvieux V, Kwak JM, Schroeder JI. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell. 2001;106:477–487. doi: 10.1016/s0092-8674(01)00460-3. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen SE, Running MP, Meyerowitz EM. Disruption of an RNA helicase/RNAse III gene in Arabidopsis causes unregulated cell division in floral meristems. Development. 1999;126:5231–5243. doi: 10.1242/dev.126.23.5231. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Liu J, Cheng Y, Jia D. HEN1 functions pleiotropically in Arabidopsis development and acts in C function in the flower. Development. 2002;129:1085–1094. doi: 10.1242/dev.129.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke JH, Tack D, Findlay K, Van Montagu M, Van Lijsebettens M. The SERRATE locus controls the formation of the early juvenile leaves and phase length in Arabidopsis. Plant J. 1999;20:493–501. doi: 10.1046/j.1365-313x.1999.00623.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu C, Fedoroff N. A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell. 2000;12:2351–2366. doi: 10.1105/tpc.12.12.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris ER, Chevalier D, Walker JC. DAWDLE, a forkhead-associated domain gene, regulates multiple aspects of plant development. Plant Physiol. 2006;141:932–941. doi: 10.1104/pp.106.076893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajagopalan R, Vaucheret H, Trejo J, Bartel DP. A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev. 2006;20:3407–3425. doi: 10.1101/gad.1476406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Axtell MJ, Fedoroff NV. The helicase and RNaseIIIa domains of Arabidopsis Dicer-Like1 modulate catalytic parameters during microRNA biogenesis. Plant Physiol. 2012;159:748–758. doi: 10.1104/pp.112.193508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi Y, Denli AM, Hannon GJ. Biochemical specialization within Arabidopsis RNA silencing pathways. Mol Cell. 2005;19:421–428. doi: 10.1016/j.molcel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Xie M, Ren G, Costa-Nunes P, Pontes O, Yu B. A subgroup of SGS3-like proteins act redundantly in RNA-directed DNA methylation. Nucleic Acids Res. 2012;40:4422–4431. doi: 10.1093/nar/gks034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh I, Hamilton AD, Regan L. Antiparallel leucine zipper-directed protein reassembly: Application to the green fluorescent protein. J Am Chem Soc. 2000;122:5658–5659. [Google Scholar]
- 26.Wierzbicki AT, Haag JR, Pikaard CS. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell. 2008;135:635–648. doi: 10.1016/j.cell.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben Amor B, et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009;19:57–69. doi: 10.1101/gr.080275.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang SW, et al. Structure of Arabidopsis HYPONASTIC LEAVES1 and its molecular implications for miRNA processing. Structure. 2010;18:594–605. doi: 10.1016/j.str.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen E, Xie Z, Gustafson AM, Carrington JC. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121:207–221. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Yoshikawa M, Peragine A, Park MY, Poethig RS. A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev. 2005;19:2164–2175. doi: 10.1101/gad.1352605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamath RS, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 32.Waters K, Yang AZ, Reinke V. Genome-wide analysis of germ cell proliferation in C. elegans identifies VRK-1 as a key regulator of CEP-1/p53. Dev Biol. 2010;344:1011–1025. doi: 10.1016/j.ydbio.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebeisen M, Drysdale J, Roy R. Suppressors of the cdc-25.1(gf)-associated intestinal hyperplasia reveal important maternal roles for prp-8 and a subset of splicing factors in C. elegans. RNA. 2008;14:2618–2633. doi: 10.1261/rna.1168408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bologna NG, Mateos JL, Bresso EG, Palatnik JF. A loop-to-base processing mechanism underlies the biogenesis of plant microRNAs miR319 and miR159. EMBO J. 2009;28:3646–3656. doi: 10.1038/emboj.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.