Abstract
Background
Older people are at risk for health decline and loss of independence. Lifestyle interventions offer potential for reducing such negative outcomes. The aim of this study was to determine the effectiveness and cost-effectiveness of a preventive lifestyle-based occupational therapy intervention, administered in a variety of community-based sites, in improving mental and physical well-being and cognitive functioning in ethnically diverse older people.
Methods
A randomised controlled trial was conducted comparing an occupational therapy intervention and a no-treatment control condition over a 6-month experimental phase. Participants included 460 men and women aged 60–95 years (mean age 74.9±7.7 years; 53% <$12 000 annual income) recruited from 21 sites in the greater Los Angeles metropolitan area.
Results
Intervention participants, relative to untreated controls, showed more favourable change scores on indices of bodily pain, vitality, social functioning, mental health, composite mental functioning, life satisfaction and depressive symptomatology (ps<0.05). The intervention group had a significantly greater increment in quality-adjusted life years (p<0.02), which was achieved cost-effectively (US $41 218/UK £24 868 per unit). No intervention effect was found for cognitive functioning outcome measures.
Conclusions
A lifestyle-oriented occupational therapy intervention has beneficial effects for ethnically diverse older people recruited from a wide array of community settings. Because the intervention is cost-effective and is applicable on a wide-scale basis, it has the potential to help reduce health decline and promote well-being in older people.
Trial Registration
clinicaltrials.gov identifier: NCT0078634.
Keywords: Lifestyle interventions, occupational therapy, randomised controlled trial, quality of life, ageing/geriatrics, depression, geriatrics, lifestyle, qual of life measmnt, randomised trials
Introduction
The expansion of the elderly population is likely to be accompanied by declines in physical health, mental well-being and functional ability.1–3 Fortunately, age-related declines can be delayed by engagement in a healthier lifestyle,2 4 5 a result that highlights the need to develop interventions that promote modifiable healthy behaviours in older people.
In response to this need, in 1997, our investigative team completed the University of Southern California Well Elderly study (Well Elderly 1), a randomised controlled trial of the efficacy and cost-effectiveness of a 9-month lifestyle intervention (now called Lifestyle Redesign®) designed to slow age-related declines among independently living elders.6 In this study, which included 361 elders from two large federally subsidised housing complexes, a reliable positive intervention effect was obtained cost-effectively for a wide range of outcomes, such as life satisfaction, role functioning and self-rated physical and emotional health.6–8 Although additional trials have underscored the value of lifestyle interventions for older people, such research has typically been performed in a single setting only, has involved a relatively small sample size or lacked a cost-effectiveness evaluation.9–11
This article reports on the University of Southern California Well Elderly 2 study, which assessed the Lifestyle Redesign intervention's effectiveness among ethnically diverse elders in community-based settings, the majority of whom are from populations at risk for health disparities. In contrast to efficacy, which pertains to an intervention's success under favourable conditions that maximise the experimental effect, the effectiveness of an intervention refers to its performance under less tightly controlled but more realistic circumstances that characterise complex real-world settings.12 To assess effectiveness, we (1) sampled elders from 21 locations with wide variation in agency ‘buy-in’; (2) included a 6-month intervention period to be responsive to real-world feasibility concerns and (3) sampled a high proportion of African Americans and Hispanics, the two subgroups of elders at highest risk for experiencing health disparities in the USA.13 We hypothesised that a 6-month lifestyle intervention leads to reduced decline in physical health, mental well-being and cognitive functioning among ethnically diverse older people across heterogeneous service delivery contexts and achieves these results in a cost-effective manner.
Methods
A detailed description of methodological and logistical issues surrounding the wider Well Elderly 2 study is presented in a previous publication.14 Methodology for the randomised controlled trial study component is described below.
Participants
The research participants were 460 men and women aged 60–95 years. All participants were residents of, users of or visitors to the study recruitment sites; demonstrated no overt signs of psychosis or dementia (based on a cursory screening procedure) and were able to complete the study assessment battery (with assistance, if necessary). All prospective participants completed the informed consent process prior to study entry.
Participants were recruited from 21 sites in the greater Los Angeles area, including 9 senior activity centres, 11 senior housing residences and 1 graduated care retirement community. Recruitment strategies included providing sign-up booths, giving presentations at meetings and social events and distributing flyers and posters.
Recruitment was undertaken in two successive cohorts to reduce temporal influences on study outcomes, overcome logistical difficulties, minimise interactions among participants and allow adjustments in ethnic stratification. Individuals in cohort 1 (n=205) entered the study between November 2004 and June 2005, whereas those in cohort 2 (n=255) entered the study between March and August 2006.
The study protocol was approved by the Institutional Review Board at the University of Southern California prior to participant recruitment and conformed to the principles of the Declaration of Helsinki. Furthermore, the study was monitored by a five-member data safety monitoring board.
Study design
Participants were randomly assigned to the intervention or no-treatment control condition for their first 6 months of study involvement. All participants were baseline-tested before starting and post-tested after completing their respective conditions, with outcome variables consisting of perceived health, mental (psychosocial) well-being and cognition measures. To promote experimental control, each site served as a separate research unit within which condition assignments were made. On completion of baseline testing, each participant was assigned to condition by the project managers based on a computer-generated random number sequence.
Reflecting a crossover design component, control group participants undertook the intervention during the 6-month period immediately following the trial's main experimental phase. This strategy enabled secondary analyses of pre-to-post intervention-based change involving control participants and was consistent with ethical treatment of human participants.
The Well Elderly 2 study did not include a social control treatment arm. This omission, which reduced non-essential costs and logistical challenges, was justified based on our previous study's finding of no difference between a social activity control group and a no-treatment control group.6
Treatment
The intervention largely followed the one manualised in the original Well Elderly study.6 15 Key aspects of the intervention, including its crucial elements and modular content areas, are detailed in table 1. The intervention consisted of small group and individual sessions led by a licensed occupational therapist. Typically, each group had six to eight members, all recruited from the same site and treated by the same intervener. Monthly community outings were scheduled to facilitate direct experience with intervention content such as the use of public transportation. Due to the overt nature of lifestyle programmes, neither the therapists nor the treated participants were blind to the intervention. However, the interveners and participants were blind to the study design and hypotheses.
Table 1.
The Well Elderly Lifestyle Redesign® intervention
| Objective | To assist elders in developing a personally meaningful, healthy lifestyle that is sustainable within the fabric of their everyday routines |
| Responsible professionals |
|
| |
| |
| Format |
|
| |
| |
| Key elements of intervention |
|
| |
| |
| Modular content areas |
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
|
Assessment
Testing sessions typically occurred in groups of 4–29 elders and took place in recreation or meeting rooms at the study sites. Assessment of health-related quality of life, life satisfaction and depression was based on self-rated questionnaires and was overseen by trained testers who were blind to the participants' condition assignments. Spanish versions of the questionnaires were provided for individuals in a Spanish study segment (n=67). The cognitive tests were conducted individually, in a private area adjacent to the main testing room, at varying points during the testing session.
Norm-based scores on Version 2 of the 36-Item Short-Form Health Survey (SF-36v2) were used to measure perceived physical health and aspects of mental well-being.16 The SF-36 is appropriate for use with older populations17 as well as ethnically diverse samples18 and is consistent with more objective health measures.19
The Center for Epidemiologic Studies Depression (CES-D) Scale20 was used to assess depressive symptoms. The CES-D is sensitive to change in depressive status over time and has been successfully used to assess ethnically diverse older people.21 22
Life satisfaction was measured by the Life Satisfaction Index-Z (LSI-Z), a 13-item measure developed specifically for older people.23 The LSI-Z is internally consistent, possesses criterion-related validity and was sensitive to the effects of lifestyle intervention in our previous trial.6 23
Three cognitive outcome variables, immediate recall, delayed recall and recognition, were measured by the word list procedure developed by the Consortium to Establish a Registry of Alzheimer's Disease.24 Reliability and validity of the Consortium to Establish a Registry of Alzheimer's Disease Word List Memory task has been established for older people with and without dementia.25 Selective attention was measured by median reaction time on a widely used computer-based visual search task,26 with lower scores indicating higher cognitive functioning. Previous research has shown that this procedure is sensitive to individual differences in selective attention.26 A final cognitive outcome variable, psychomotor speed, was assessed by the Digit Symbol Substitution Task of the Weschler Adult Intelligence Scale–Revised,27 which is associated with general cognitive ability and physical health.28
Statistical analyses
Data quality control
All data entry, data management and statistical analyses were performed by the project's data analysis centre. Summary scores on each assessment were calculated using instrument-specific algorithms. Standard procedures were used to impute missing responses.
Intent-to-treat analysis
Power calculations were based on the assumption of an effect size of 0.32 (the average value for significant outcomes in our previous trial), a 10% attrition rate, use of a one-tailed test for adjusted change score differences and a 0.05 α level. For the initially planned sample size of 220 per group, power to detect a between-group difference was estimated to be 93%.
Student t tests or χ2 tests were used to assess (1) the equivalence of the intervention and control groups on demographic and outcome variables at baseline, (2) the differences among participants who were evaluable versus non-evaluable at the time of post-testing and (3) the association of treatment assignment with evaluable versus non-evaluable status.
To test for intervention effects, for each of the 17 outcome variables signed change scores (post-test minus baseline) were generated, and analysis of covariance was performed to determine whether change scores of the intervention group were more favourable than those of the control group. In these analyses, the study cohort and the baseline score on the outcome variable were used as covariates, along with demographic or cognitive variables significantly related to the total sample's baseline to post-test change scores.
One-tailed statistical tests, conducted at the 0.05 α level, were used to assess the hypotheses of positive treatment effects. One-sided tests were used because (1) the intervention could be justifiably linked to an expectation of positive health outcomes based on existing theory,29 (2) a positive experimental effect had previously been demonstrated in the first Well Elderly trial6–8 and (3) the directional hypotheses were prespecified prior to the start of the study.
Secondary analyses
In a secondary non-experimental analysis of the intervention's effects, pre-to-post change was examined for individuals who were initially assigned to the control group, but received the intervention during the second 6 months of their study participation. The 17 outcome variable mean change scores were each separately tested through analysis of a covariance using a mixed-effects model with repeated measurements. The covariates were those included in the primary intent-to-treat comparison. For each outcome variable, a one-sided significance test was conducted at the 0.05 α level.
Cost-effectiveness was evaluated by applying a cost per quality-adjusted life year (cost per QALY) methodology.8 SF-36v2 change scores were used to calculate differences in utility scores for the intervention and control groups. Intervention costs for the treatment group were calculated on an intent-to-treat basis and were based on an annual full-time equivalent salary of $62 400 (plus 32% fringe benefits) for the treating therapists as reported by the Bureau of Labour Statistics.30 These costs were translated into UK costs using an hourly occupational therapist wage rate of £24.31
Descriptive statistics were calculated to document the extent of treatment adherence (percentage of sessions attended) among all individuals who were in the study at the outset of their assigned intervention period within either the initial experimental or crossover phase.
Results
Description of study cohort
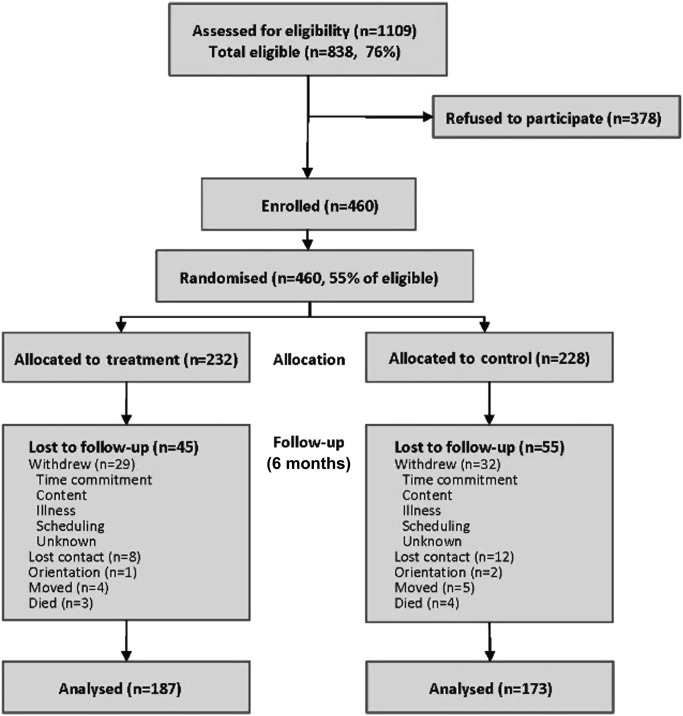
Figure 1 presents the CONSORT diagram for the study. Of 838 study eligible individuals, 460 enrolled in the study. Of the 460 participants, 232 were randomly assigned to the intervention group and 228 to the control group. A total of 360 participants completed post-testing.
Figure 1.
CONSORT diagram.
Table 2 presents baseline characteristics for all participants combined and by treatment arm. No significant differences were found between the intervention and control groups at baseline.
Table 2.
Demographic and outcome variables at baseline of sample stratified by treatment (n=460)
| Demographic variables | Treatment (n=232) | Control (n=228) | Total (n=460) | p Value* (two-sided) |
| Sex, n (%) | ||||
| Male | 70 (30.2) | 87 (38.2) | 157 (34.1) | 0.07 |
| Female | 162 (69.8) | 141 (61.8) | 303 (65.9) | |
| Age† (years) | 74.81 (7.8) | 74.90 (7.6) | 74.85 (7.7) | 0.90 |
| 60–64, n (%) | 24 (10.3) | 23 (10.1) | 47 (10.2) | 0.50 |
| 65–69, n (%) | 40 (17.2) | 39 (17.1) | 79 (17.2) | |
| 70–74, n (%) | 50 (21.6) | 44 (19.3) | 94 (20.4) | |
| 75–79, n (%) | 45 (19.4) | 59 (25.9) | 104 (22.6) | |
| 80–85, n (%) | 51 (22.0) | 38 (16.7) | 89 (19.4) | |
| 85+, n (%) | 22 (9.5) | 25 (11.0) | 47 (10.2) | |
| Race, n (%) | ||||
| White | 85 (36.6) | 87 (38.2) | 172 (37.4) | 0.44 |
| Black/African American | 78 (33.6) | 71 (31.1) | 149 (32.4) | |
| Hispanic or Latino | 49 (21.1) | 43 (18.9) | 92 (20.0) | |
| Asian | 10 (4.3) | 8 (3.5) | 18 (3.9) | |
| Other | 10 (4.3) | 19 (8.3) | 29 (6.3) | |
| Education, n (%) | ||||
| Less than high school | 72 (31.0) | 64 (28.1) | 136 (29.6) | 0.91 |
| High school graduate | 45 (19.4) | 44 (19.3) | 89 (19.4) | |
| Some college or technical school | 77 (33.2) | 81 (35.5) | 158 (34.4) | |
| Four years of college or more | 38 (16.4) | 39 (17.1) | 77 (16.7) | |
| Annual income‡, n (%) | ||||
| $0∼$11 999 | 123 (53.7) | 117 (53.2) | 240 (53.5) | 0.77 |
| $12 000–$23 999 | 51 (22.3) | 56 (25.5) | 107 (23.8) | |
| $24 000–$35 999 | 25 (10.9) | 24 (10.9) | 49 (10.9) | |
| $36 000+ | 30 (13.1) | 23 (10.4) | 53 (11.8) | |
| Income support§, n (%) | ||||
| One person | 194 (84.4) | 178 (80.2) | 372 (82.3) | 0.25 |
| More than one person | 36 (15.6) | 44 (19.8) | 80 (17.7) | |
| Retirement status, n (%) | ||||
| Retired/active (student or volunteer) | 59 (25.4) | 52 (22.8) | 111 (24.1) | 0.51 |
| Retired/inactive | 173 (74.6) | 176 (77.2) | 349 (75.9) | |
| Living situation, n (%) | ||||
| Alone | 193 (83.2) | 184 (80.7) | 377 (82.0) | 0.49 |
| Lives with others | 39 (16.8) | 44 (19.3) | 83 (18.0) | |
| Site type, n (%) | ||||
| Graduated care retirement community | 17 (7.3) | 16 (7.0) | 33 (7.2) | 0.97 |
| Senior residence-subsidised | 107 (46.1) | 101 (44.3) | 208 (45.2) | |
| Senior centre: multipurpose | 71 (30.6) | 74 (32.5) | 145 (31.5) | |
| Senior centre: parks and recreation | 37 (16.0) | 37 (16.2) | 74 (16.1) | |
| Neighbourhood score† ¶ | ||||
| Neighbourhood score (1–7) | 4.6 (1.4) | 4.6 (1.4) | 4.6 (1.4) | 0.93 |
| Outcome variables† | ||||
| SF-36 V. 2 | ||||
| Bodily pain | 43.24 (11.48) | 44.47 (11.56) | 43.85 (11.52) | 0.25 |
| Physical function | 38.65 (11.98) | 38.38 (12.33) | 38.51 (12.14) | 0.81 |
| Role physical | 40.47 (11.60) | 41.60 (10.07) | 41.03 (10.87) | 0.26 |
| General health | 44.66 (10.15) | 44.88 (10.46) | 44.77 (10.29) | 0.82 |
| Mental health | 47.35 (11.79) | 47.59 (11.32) | 47.47 (11.54) | 0.83 |
| Role emotional | 39.04 (14.04) | 40.76 (12.39) | 39.89 (13.26) | 0.17 |
| Social functioning | 44.23 (11.71) | 45.82 (10.61) | 45.02 (11.19) | 0.13 |
| Vitality | 49.86 (9.82) | 50.14 (9.75) | 50.00 (9.78) | 0.76 |
| Physical composite | 41.09 (10.06) | 41.43 (10.60) | 41.26 (10.32) | 0.72 |
| Mental composite | 46.90 (11.74) | 48.05 (10.81) | 47.47 (11.29) | 0.27 |
| Life satisfaction-Z | ||||
| 16.94 (5.56) | 16.76 (5.72) | 16.85 (5.64) | 0.73 | |
| CES-D Scale | ||||
| 14.32 (10.89) | 13.13 (10.91) | 13.73 (10.91) | 0.24 | |
| CERAD-memory | ||||
| Immediate recall | 4.07 (1.71) | 4.09 (1.48) | 4.08 (1.60) | 0.87 |
| Delayed recall | 5.02 (2.22) | 4.78 (2.23) | 4.90 (2.23) | 0.25 |
| Recognition | 18.57 (2.30) | 18.27 (2.22) | 18.42 (2.26) | 0.16 |
| Visual search | ||||
| Median reaction time | 1413.59 (647.40) | 1355.41 (596.80) | 1385 (623.40) | 0.34 |
| Psychomotor speed | ||||
| 38.85 (16.36) | 38.79 (17.08) | 38.82 (16.70) | 0.97 | |
Age is calculated from date of birth and baseline evaluation date; in the category race, ‘Other’ includes Native Hawaiian or Other Pacific Islander, American Indian/Alaska Native, Multiracial, other and refused.
χ2 tests were performed for categorical variables, and Student t tests were performed for continuous variables.
Mean (SD).
Income: 11 refused (3 in the treatment group and 8 in the control group).
Income support: 8 refused (2 in the treatment group and 6 in the control group).
Based on project managers' ratings of the overall quality of the neighbourhood in which the site was located.
CES-D Scale, Center for Epidemiologic Studies Depression Scale; CERAD, Consortium to Establish a Registry of Alzheimer's Disease; SF-36 V. 2, Version 2 of the 36-Item Short-Form Health Survey.
Relative to individuals who were non-evaluable, evaluable participants were more often engaged in productive activities such as volunteering, caregiving or part time work (p<0.01) and had higher baseline scores on delayed recall and psychomotor speed (ps<0.05). Evaluable versus non-evaluable status was not associated with condition assignment (81% of intervention versus 76% of control participants were evaluable, p>0.05).
Intent-to-treat analysis
Table 3 summarises the results of the intent-to-treat analysis for evaluable participants. Analyses of covariance revealed a significant benefit due to the lifestyle intervention for five SF-36v2 outcomes: bodily pain (p<0.02), vitality (p<0.03), social function (p<0.04), mental health (p<0.03) and mental composite (p<0.03). Physical function and physical composite were marginally significant (p<0.10). In addition, a positive intervention effect was found for the LSI-Z (p<0.03) and CES-D Scale (p<0.03).
Table 3.
Intent-to-treat analysis of outcome change scores (post-baseline) for evaluable participants stratified by intervention group (n=360)
| Outcome variable | Intervention group | N | Baseline, mean (SD) | Post, mean (SD) | Adjusted change, mean (SEM) | p Value* (one-sided) |
| SF-36 V. 2 | ||||||
| Physical function | Treatment | 187 | 38.60 (12.06) | 39.84 (12.70) | 1.04 (0.82) | 0.09 |
| Control | 173 | 38.62 (12.35) | 38.81 (12.11) | −0.12 (0.80) | ||
| Role physical | Treatment | 187 | 40.63 (11.72) | 40.78 (11.37) | 0.66 (0.82) | 0.18 |
| Control | 173 | 41.73 (10.10) | 40.72 (9.94) | −0.15 (0.82) | ||
| Bodily pain | Treatment | 187 | 42.75 (11.60) | 44.62 (11.20) | 2.76 (0.83) | 0.02 |
| Control | 173 | 44.53 (11.30) | 44.38 (11.86) | 0.89 (0.82) | ||
| General health | Treatment | 187 | 44.74 (10.10) | 46.19 (9.85) | 1.14 (0.70) | 0.25 |
| Control | 173 | 44.75 (10.17) | 45.74 (10.48) | 0.61 (0.69) | ||
| Vitality | Treatment | 187 | 50.02 (9.90) | 51.29 (9.85) | 2.31 (0.84) | 0.03 |
| Control | 172 | 50.06 (9.47) | 49.60 (11.23) | 0.59 (0.83) | ||
| Social function | Treatment | 187 | 44.54 (11.75) | 45.36 (11.37) | 1.05 (0.89) | 0.04 |
| Control | 173 | 46.57 (9.75) | 45.00 (11.33) | −0.70 (0.88) | ||
| Role emotional | Treatment | 187 | 39.39 (14.26) | 40.72 (12.95) | 1.30 (1.03) | 0.16 |
| Control | 173 | 41.16 (12.07) | 40.69 (12.86) | 0.21 (1.02) | ||
| Mental health | Treatment | 187 | 47.75 (11.89) | 49.07 (10.70) | 2.31 (0.87) | 0.03 |
| Control | 172 | 47.48 (11.22) | 47.16 (11.81) | 0.48 (0.86) | ||
| Physical composite | Treatment | 187 | 40.83 (10.27) | 41.86 (10.68) | 1.02 (0.65) | 0.09 |
| Control | 172 | 41.51 (10.43) | 41.53 (9.99) | 0.07 (0.64) | ||
| Mental composite | Treatment | 187 | 47.41 (11.80) | 48.64 (10.63) | 1.79 (0.87) | 0.03 |
| Control | 172 | 48.28 (10.46) | 47.45 (12.01) | 0.04 (0.86) | ||
| Life satisfaction-Z | Treatment | 187 | 17.23 (5.68) | 18.00 (5.37) | 0.84 (0.40) | 0.03 |
| Control | 172 | 16.78 (5.70) | 16.85 (5.49) | −0.02 (0.39) | ||
| CES-D Scale | Treatment | 186 | 13.78 (10.80) | 12.47 (9.68) | −1.69 (0.75) | 0.03 |
| Control | 173 | 12.97 (10.54) | 13.53 (11.17) | −0.08 (0.74) | ||
| CERAD-memory | ||||||
| Immediate recall | Treatment | 180† | 4.17 (1.74) | 4.45 (1.63) | 0.26 (0.13) | 0.20 |
| Control | 167 | 4.12 (1.43) | 4.54 (1.43) | 0.38 (0.13) | ||
| Delayed recall | Treatment | 180 | 5.11 (2.25) | 5.05 (2.22) | 0.07 (0.15) | 0.38 |
| Control | 167 | 4.91 (2.18) | 4.86 (2.19) | 0.02 (0.15) | ||
| Recognition | Treatment | 180 | 18.63 (2.03) | 18.37 (2.44) | −0.12 (0.19) | 0.26 |
| Control | 166 | 18.32 (2.20) | 18.14 (2.44) | −0.16 (0.18) | ||
| Visual search | ||||||
| Median reaction time | Treatment | 166 | 1360 (565.4) | 1229 (452.1) | −137 (31.61) | 0.49 |
| Control | 152 | 1348 (639.8) | 1235 (456.4) | −138 (31.04) | ||
| Psychomotor speed | Treatment | 171 | 39.84 (16.25) | 41.71 (17.61) | 1.34 (0.89) | 0.49 |
| Control | 160 | 39.44 (16.55) | 40.21 (18.03) | 1.31 (0.88) | ||
Covariates were (1) baseline value, (2) cohort (1, 2), (3) age (continuous), (4) sex (male, female), (5) race (white, black, Hispanic, Other), (6) education (<high school, high school, some college and college plus), (7) site (graduated care retirement community, senior residence, senior centre—multipurpose, senior centre—parks and recreation), (8) neighbourhood score (1–7), (9) CERAD-recognition, (10) psychomotor speed.
p Values were obtained using analysis of covariance.
Reasons for not completing cognitive testing: visual or other physical impairment or scheduling conflicts.
CES-D Scale, Center for Epidemiologic Studies Depression Scale; CERAD, Consortium to Establish a Registry of Alzheimer's Disease; SF-36 V. 2, Version 2 of the 36-Item Short-Form Health Survey.
A total of 405 individuals were enrolled in the study at the time they were expected to begin participating in the intervention. On average, participants attended 56% of the scheduled sessions. Sixty-nine (17%) individuals did not attend any intervention sessions. Among participants who attended more than one session, the overall attendance rate was 70%.
Among participants who received the intervention, six study-related adverse events were reported in the first 6-month experimental phase: two falls, three minor lacerations and one instance of transient dissatisfaction with the intervention. During the crossover period, in which the former control group received the intervention, four study-related adverse events were reported: two interpersonal conflicts, one partial fall and one instance of back pain following a session. No serious adverse events were reported.
Secondary analyses
Table 4 summarises the results of the change score analysis for the 137 participants who received the intervention during the second 6 months of their study involvement. For mental and physical well-being, all outcomes reflected positive change and seven outcomes were significant (ps<0.05). Among the cognitive variables, significant pre-to-post improvement was found for immediate recall (p=0.05), delayed recall (p<0.0001), recognition (p<0.01) and psychomotor speed (p<0.01).
Table 4.
Analysis of covariate-adjusted outcome change scores for control group participants who crossed over to the intervention (n=137)
| Outcome variable | N | Pre, mean (SD) | Post, mean (SD) | Adjusted change, mean (SEM) | p value* (one-sided) |
| SF-36 V. 2 | |||||
| Physical function | 137 | 38.90 (12.09) | 40.08 (12.04) | 1.18 (0.81) | 0.07 |
| Role physical | 137 | 40.46 (10.34) | 42.30 (9.81) | 1.84 (0.95) | 0.03 |
| Bodily pain | 137 | 44.40 (11.87) | 45.85 (11.06) | 1.45 (0.87) | 0.05 |
| General health | 137 | 45.77 (10.09) | 46.03 (9.24) | 0.27 (0.68) | 0.34 |
| Vitality | 136 | 50.19 (11.32) | 51.77 (9.00) | 1.53 (0.78) | 0.03 |
| Social function | 137 | 45.18 (11.44) | 46.18 (9.74) | 1.00 (0.94) | 0.15 |
| Role emotional | 137 | 40.73 (12.85) | 41.98 (11.49) | 1.25 (0.98) | 0.10 |
| Mental health | 136 | 47.33 (11.90) | 49.62 (9.90) | 2.24 (0.94) | 0.01 |
| Physical composite | 136 | 41.45 (9.95) | 42.49 (9.81) | 0.69 (0.64) | 0.07 |
| Mental composite | 136 | 47.74 (12.26) | 49.42 (10.56) | 1.63 (0.91) | 0.04 |
| Life satisfaction-Z | 136 | 16.65 (5.51) | 17.44 (5.17) | 0.79 (0.37) | 0.02 |
| CES-D Scale | 137 | 13.39 (11.05) | 11.78 (9.21) | −1.66 (0.76) | 0.01 |
| CERAD-memory | |||||
| Immediate recall | 127† | 4.60 (1.45) | 4.87 (1.71) | 0.24 (0.15) | 0.05 |
| Delayed recall | 127 | 4.99 (2.17) | 5.77 (2.30) | 0.71 (0.17) | <0.0001 |
| Recognition | 127 | 18.34 (2.35) | 18.87 (2.25) | 0.49 (0.19) | 0.01 |
| Visual search | |||||
| Median reaction time | 116 | 1222 (407.5) | 1209 (433.3) | −13.6 (27.50) | 0.31 |
| Psychomotor speed | 123 | 40.39 (17.50) | 42.99 (17.82) | 2.19 (0.96) | 0.01 |
Covariates were (1) cohort (1, 2), (2) age (continuous), (3) sex (male, female), (4) race (white, black, Hispanic, Other), (5) education (<high school, high school, some college, college plus), (6) site (graduated care retirement community, senior residence, senior centre—multipurpose, senior centre—parks and recreation), (7) neighbourhood score (1–7), (8) CERAD-recognition (pre-test), (9) psychomotor speed (pre-test).
p Values were obtained using a mixed model with repeated measurement.
Reasons for not completing cognitive testing: visual or other physical impairment or scheduling conflicts.
CES-D Scale, Center for Epidemiologic Studies Depression Scale; CERAD, Consortium to Establish a Registry of Alzheimer's Disease; SF-36 V. 2, Version 2 of the 36-Item Short-Form Health Survey.
The intervention costs averaged $783 per participant. Converting to UK wages and prices, the intervention costs averaged £472.5. The treatment group, relative to the control group, had an average QALY increment of 0.038 (p<0.02). The base case cost per QALY was estimated at $41 218. Converting to UK wages and prices the base case cost per QALY was £24 868, a number within the range that is often considered cost-effective by the UK National Institute of Health and Clinical Excellence.
Discussion
Overall, this trial demonstrated that a 6-month preventive lifestyle-oriented intervention has positive effects for a sample of ethnically diverse older people recruited from a variety of community sites. Statistically significant results were found for multiple mental well-being variables, including vitality, social function, mental health, the mental health composite index, life satisfaction and depressive symptomatology. In the case of self-rated physical health, the intervention reduced perceptions of bodily pain and led to marginally significant improvements on the physical health composite and physical function scales. In general, however, the intervention had a larger impact for mental, as opposed to physical, health and well-being.
The basic pattern of positive experimental outcomes was mirrored among individuals who received the intervention during the crossover study phase. Furthermore, the intervention's salutary effect was consistent across two different time periods, as evidenced by the lack of an interaction between the study cohort variable and experimental treatment condition in affecting outcomes (p>0.10 for all 17 dependent variables).
Because elders who are willing to undertake a research-based lifestyle intervention would potentially enrol in such an intervention in naturalistic non-research contexts, the study sample arguably approximates the pool of intervention participants in real-life applications. Based on information in figure 1 and table 2, approximately one-half of the population of ethnically diverse community-dwelling older people who live in senior housing or frequent activity centres could be expected to participate in the intervention. This participation rate, in connection with the correlation of evaluability with productive activity and selected indices of cognitive functioning, suggests that the intervention may generalise most strongly to elders who are relatively capable or motivated.
As reported in table 3, no intervention effect was found for the cognitive outcomes. Although significant gains in immediate recall, delayed recall, recognition and psychomotor speed were present for the former control participants during the time they received the intervention (table 4), similar gains were also observed 6 months after completion of the intervention in the previous experimental group, suggesting a possible practice effect. Thus, convincing evidence for an intervention effect on cognition is lacking. At least two reasons may underlie this null result. First, it is possible that the 6-month experimental duration of the study was insufficient for cognitive changes to emerge. Second, due to the intervention's relative lack of direct content pertaining to cognition, such outcomes may have been less strongly linked to the intervention than mental well-being and physical outcomes.
Strengths and limitations in relation to other studies
Previously, a limited number of randomised trials, apart from the two Well Elderly studies, have been conducted to examine the effects of lifestyle-based interventions on mental well-being or physical outcomes of older people living in community or assisted living settings.6 9–11 However, the study samples have been largely limited to white older people, the interventions have lacked an individually tailored comprehensive focus and the cost-effectiveness analyses were not performed. The Well Elderly 2 study is the first investigation that addressed these concerns while documenting the effectiveness of a lifestyle intervention under widely varying naturalistic circumstances.
The effect size estimates for significant outcomes in the primary intent-to-treat analysis ranged from 0.14 to 0.23 (mean=0.17). These values are smaller than those obtained in our previous trial, which on average exceeded 0.30. In interpreting this difference, it should be noted that the current investigation included design characteristics (eg, a more heterogeneous sample, the inclusion of relatively distal outcome measures, the enlistment of sites varying in their degree of administrative support and investment and a shortened intervention period) that are not conducive to yielding large effect sizes.12 32 By way of comparison, the effect magnitude approximates the level obtained in a large multisite trial of a lifestyle intervention for ethnically diverse care givers of relatives with dementia.33
An additional consideration concerning the magnitude of the experimental effect is that the estimated base case cost per QALY of US $41 218/ UK £24 868 is low enough for the intervention to qualify as cost-effective.34 35 Given this result, the intervention is viable as a treatment option in multi-ethnic community-based contexts.
Unanswered questions and future research
One unanswered question pertains to the absence of a readiness-to-change screen. It has been recommended that controlled trials should exclude individuals who are unlikely to adhere to the study protocol or benefit from the intervention.32 Underscoring this notion, behavioural and health promotion studies reveal a strong relationship between subject readiness-to-change and protocol adherence and study outcomes.36 We believe that the study dropout rate might have been mitigated, or the experimental effect increased, if we had used readiness-to-change as a criterion for admissibility and used such scores to modify the intervention protocol.
Several potential future research extensions should be noted. First, although in the current study the intervention demonstrated excellent reach in extending to urban, community-dwelling older people from different age, gender, racial/ethnic and socioeconomic strata (see figure 1 and table 2), there is a need to test the benefits, as well as the cost-effectiveness, of lifestyle-oriented interventions when administered to groups with varying daily living circumstances such as those who reside in nursing homes or rural environments. Second, there is a need to document which aspects of complex multifaceted lifestyle interventions are most important in producing positive outcomes. Finally, in designing future lifestyle interventions for older people, inclusion of modular content that directly addresses cognitive functioning should be considered. In testing for such cognitive benefits, a follow-up period longer than that used in the current study should be included insofar as such effects may develop slowly over time.
Conclusions
In conclusion, the Well Elderly 2 study documented the effectiveness of the intervention when (1) applied to a sample of older people at high risk for experiencing health disparities, (2) delivered within a 6-month time interval and (3) implemented in various community settings. The findings of this study are particularly relevant today when escalating healthcare costs are being attributed largely to the ageing population and related increases in depressive symptomatology, poor mental well-being and chronic illness.1 37 38 As a consequence, healthcare policy specialists and governmental agencies are recognising that preventive and wellness care for older people must be a key element in healthcare provision.1 39 The results of the Well Elderly 2 study demonstrate that because the intervention is cost-effective and applicable on a wide-scale basis, it has the potential to promote physical and mental well-being in this population.
What is already known on this subject.
Older people are at increased risk for declines in mental well-being, physical functioning and loss of independence.
Lifestyle interventions have been shown to reduce age-related declines under carefully controlled conditions.
Full-scale trials of the effectiveness and cost-effectiveness of comprehensive lifestyle interventions on health outcomes administered to ethnically diverse elders in a variety of community settings are lacking.
What this study adds.
A lifestyle-oriented occupational therapy intervention promotes elder's mental well-being across community-based settings in a cost-effective manner.
This intervention is widely applicable to those at high risk for poor health due to low socioeconomic status.
Acknowledgments
We thank program officer Sidney Stahl of the National Institute on Aging for his continuing support and helpful comments throughout the conduct of the project. The investigative team also expresses gratitude to the members of the data and safety monitoring board for their careful oversight and helpful suggestions: Sidney Stahl, NIA Behavioural and Social Research Program; Kenneth Ottenbacher, University of Texas-Medical Branch; Nancy Gibbs, Southern California Kaiser Permanente Medical Group; Thomas Belin, University of California at Los Angeles and Eleanor Durr of Altadena, CA. We also thank Carolyn Ervin, Peng Zhao and James Gardner for assisting in the early stages of data monitoring and analysis. Finally, we thank Stephanie Mielke and Janis Wise for their ongoing administrative support of the project, without whose diligent efforts this study could not have been initiated or completed.
Footnotes
Funding: This research was supported by National Institutes of Health grant R01 AG021108 from the National Institute on Aging.
Competing interests: None.
Ethics approval: This study was conducted with the approval of the institutional review board at the University of Southern California, and all participants gave informed consent.
Contributors: FC, as principal investigator, obtained funding for the study and is guarantor. SPA, FC, JJ, MC, DM, JB, BW, C-PC, MJ-M, JH, BC, BGK and DAG provided substantial contributions to conception and design of the study. DM, JB, C-PC, MJ-M and BC acquired the data. SPA, MC, DM, C-PC, RW, MYL, CL, JH and DAG analysed the data. SPA, FC, JJ, MC, DM, JB, AM, C-PC, RW, MYL, CL, MJ-M, JH, BC, BGK and DAG interpreted the data. SPA, FC, JJ, MC, DM, AM, MYL, CL, JH and BC drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final version.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.National Institute on Aging Why Population Aging Matters: A Global Perspective. Washington, DC: U.S. National Institutes of Health, 2007. http://www.nia.nih.gov/ResearchInformation/ExtramuralPrograms/BehavioralAndSocialResearch/GlobalAging.htm (accessed 7 Jul 2009). [Google Scholar]
- 2.Low G, Molzahn AE. Predictors of quality of life in old age: a cross-validation study. Res Nurs Health 2007;30:141–50 [DOI] [PubMed] [Google Scholar]
- 3.Smith J, Borchelt M, Maier H, et al. Health and well-being in the young old and oldest old. J Soc Issues 2002;58:715–32 [Google Scholar]
- 4.Rowe JW, Kahn RL. Successful aging. Aging (Milano) 1998;10:142–4 [PubMed] [Google Scholar]
- 5.Hendricks J, Hatch LR. Theorizing lifestyle: exploring agency and structure in the life course. In: Bengston VL, Gans D, Putney N, et al., eds. Handbook of theories of aging. New York, NY: Springer Publishing Company, 2009:435–54 [Google Scholar]
- 6.Clark F, Azen SP, Zemke R, et al. Occupational therapy for independent-living older adults. A randomized controlled trial. JAMA 1997;278:1321–6 [PubMed] [Google Scholar]
- 7.Clark F, Azen SP, Carlson M, et al. Embedding health-promoting changes into the daily lives of independent-living older adults: long-term follow-up of occupational therapy intervention. J Gerontol B Psychol Sci Soc Sci 2001;56:P60–3 [DOI] [PubMed] [Google Scholar]
- 8.Hay J, LaBree L, Luo R, et al. Cost-effectiveness of preventive occupational therapy for independent-living older adults. J Am Geriatr Soc 2002;50:1381–8 [DOI] [PubMed] [Google Scholar]
- 9.Peri K, Kerse N, Robinson E, et al. Does functionally based activity make a difference to health status and mobility? A randomised controlled trial in residential care facilities (The Promoting Independent Living Study; PILS). Age Ageing 2008;37:57–63 [DOI] [PubMed] [Google Scholar]
- 10.Scott JC, Conner DA, Venohr I, et al. Effectiveness of a group outpatient visit model for chronically ill older health maintenance organization members: a 2-year randomized trial of the cooperative health care clinic. J Am Geriatr Soc 2004;52:1463–70 [DOI] [PubMed] [Google Scholar]
- 11.Wallace JI, Buchner DM, Grothaus L, et al. Implementation and effectiveness of a community-based health promotion program for older adults. J Gerontol A Biol Sci Med Sci 1998;53:M301–6 [DOI] [PubMed] [Google Scholar]
- 12.Glasgow RE, Lichtenstein E, Marcus AC. Why don't we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health 2003;93:1261–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smedley B, Stith A, Nelson A, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: Institute of Medicine, 2003:29–79 [PubMed] [Google Scholar]
- 14.Jackson J, Mandel D, Blanchard J, et al. Confronting challenges in intervention research with ethnically diverse older adults: the USC Well Elderly II Trial. Clin Trials 2009;6:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandel D, Jackson J, Zemke R, et al. Lifestyle Redesign: Implementing the Well Elderly Program. Bethesda, MD: AOTA Press, 1999:1–61 [Google Scholar]
- 16.Ware J, Kosinski M, Dewey J. How to Score Version 2 of the SF-36(r) Health Survey. Lincoln, RI: QualityMetric Incorporated, 2000:23–102 [Google Scholar]
- 17.Hayes V, Morris J, Wolfe C, et al. The SF-36 health survey questionnaire: is it suitable for use with older adults? Age Ageing 1995;24:120–5 [DOI] [PubMed] [Google Scholar]
- 18.Madsen F. Quality of life questionnaires for all respiratory diseases, every language and ethnic minorities. Are alternatives available? Respir Med 2000;94:187–9 [DOI] [PubMed] [Google Scholar]
- 19.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radloff L. The CES-D scale: a self report depression scale for research in the general population. Appl Psych Meas 1977;1:385–401 [Google Scholar]
- 21.Foley K, Reed P, Mutran E, et al. Measurement adequacy of the CES-D among a sample of older African Americans. Psychiatr Res 2002;109:61–9 [DOI] [PubMed] [Google Scholar]
- 22.Lewinsohn PM, Hoberman HM, Rosenbaum M. A prospective study of risk factors for unipolar depression. J Abnorm Psychol 1988;97:251–64 [DOI] [PubMed] [Google Scholar]
- 23.Wood V, Wylie ML, Sheafor B. An analysis of a short self-report measure of life satisfaction: correlation with rater judgments. J Gerontol 1969;24:465–9 [DOI] [PubMed] [Google Scholar]
- 24.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39:1159–65 [DOI] [PubMed] [Google Scholar]
- 25.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 1994;44:609–14 [DOI] [PubMed] [Google Scholar]
- 26.Lupien S, Lecours AR, Lussier I, et al. Basal cortisol levels and cognitive deficits in human aging. J Neurosci 1994;14:2893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weschler D. WAIS-R: Weschler Adult Intelligence Scale Revised. New York, NY: Psychological Corporation, 1981:1–20 [Google Scholar]
- 28.Lezak MD. Neuropsychological assessment. 3rd edn New York, NY: Oxford University Press, 1995:376–9 [Google Scholar]
- 29.Jackson J, Carlson M, Mandel D, et al. Occupation in lifestyle redesign: the well elderly study occupational therapy program. Am J Occup Ther 1998;52:326–36 [DOI] [PubMed] [Google Scholar]
- 30.U.S. Bureau of Labor Statistics Occupational Employment and Wages, May 2006. http://www.bls.gov/oes/2006/may/oes291122.htm (accessed 14 Jan 2009).
- 31.National Institute for Health and Clinical Excellence Mental Well Being and Older People: Costing Report. London: 2008. http://guidance.nice.org.uk/PH16/CostReport/pdf/English (accessed 14 Jan 2009). [Google Scholar]
- 32.Vamvakas EC. Rationale, objectives, and interpretation of randomized controlled trials. J Clin Apher 1997;12:130–9 [DOI] [PubMed] [Google Scholar]
- 33.Gitlin LN, Belle SH, Burgio LD, et al. Effect of multicomponent interventions on caregiver burden and depression: the REACH multisite initiative at 6-month follow-up. Psychol Aging 2003;18:361–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hay JW. Where's the value in health care? Value Health 2006;9:141–3 [DOI] [PubMed] [Google Scholar]
- 35.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733–44 [DOI] [PubMed] [Google Scholar]
- 36.Prochaska J, Norcross J. Stages of change. Psychotherapy 2001;38:443–8 [Google Scholar]
- 37.Centers for Disease Control and Prevention The State of Aging and Health in America 2007. U.S. Department of Health and Human Services, 2007. [updated 2008 June 30]. http://www.cdc.gov/aging/saha.htm (accessed 14 Jan 2009). [Google Scholar]
- 38.Allen J. Older People and Wellbeing. London Institute for Public Policy Research, 2008. http://www.ippr.org.uk (accessed 7 Jul 2009). [Google Scholar]
- 39.Baucus M. Call to Action: Health Reform 2009. U.S. Senate Finance Committee, 2008. http://finance.senate.gov/healthreform2009/home.html (accessed 14 Jan 2009). [Google Scholar]