Microsporidia are obligate intracellular eukaryotic pathogens known to cause superficial punctate keratitis and stromal keratitis in both immunocompromised and immuno-competent individuals. Traditionally, microsporidia were classified as primitive eukaryotes; however, recent genomic evidence supports their reclassification as fungi.1
Definitive treatment for micro-sporidial keratitis has not been established. Based on the classification of microsporidia as fungi, use of topical antifungal agents may be beneficial as monotherapy. Voriconazole is available through compounding pharmacies in topical form and is effective against several fungal pathogens.2 Herein, we report 2 cases of microsporidial superficial punctate keratitis that were responsive to treatment with topical voriconazole. To our knowledge, this is the first case series of the successful treatment of microsporidial superficial punctate keratitis with topical voriconazole.
Report of Cases
Case 1
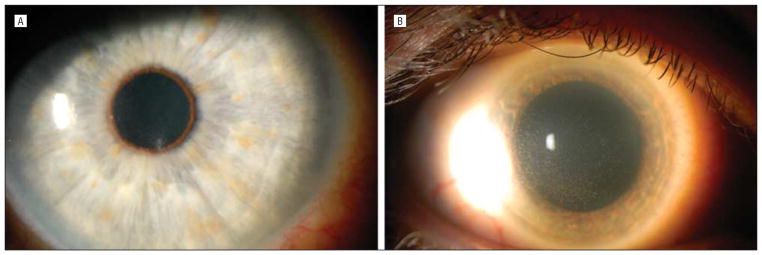
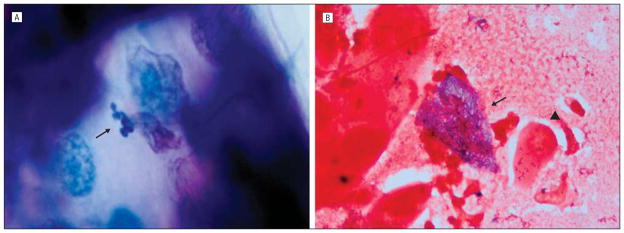
A 49-year-old white man was seen in August 2009 with foreign-body sensation, blurred vision, and redness of his right eye for 2 weeks. Medical history was significant for metastatic thyroid cancer. His chemotherapy was discontinued because of neutropenia 1 month earlier and his white blood cell count had subsequently normalized. His best-corrected visual acuity (BCVA) was 20/25 OD and 20/15 OS. Right eye slitlamp examination demonstrated 20 to 25 raised epithelial opacities diffusely throughout the cornea with subepithelial infiltrates (Figure 1A). Follicles were present on the palpebral conjunctiva. Examination was otherwise normal in the right eye. Slitlamp examination of the left eye was normal. The clinical diagnosis was viral conjunctivitis, and treatment was started with topical prednisolone acetate, 1%, 3 times daily. At 1 week, the epithelial opacities and subepithelial infiltrates were improved, so corticosteroid use was tapered and discontinued. Three weeks later, the symptoms and signs returned, and BCVA worsened to 20/30 OD. Microsporidial keratitis was suspected based on clinical appearance and time course. Microscopic examination of a diagnostic epithelial scraping revealed aggregates of intracellular organisms in the cytoplasm of epithelial cells consistent with microsporidia (Figure 2A). Steroid use was discontinued, and treatment was begun with topical voriconazole, 1%, every 2 hours. After 8 days, BCVA was 20/25 OD with improved symptoms and signs. Voriconazole use was tapered over 4 weeks. The patient had resolution of symptoms and signs at his visit after the taper. Final BCVA was 20/15 OD.
Figure 1.
Slitlamp photographs. A, Case 1 slitlamp photograph shows multiple raised epithelial opacities diffusely throughout the cornea with subepithelial infiltrates. B, Case 2 slitlamp photograph shows diffuse punctate epithelial keratopathy.
Figure 2.
Microscopic examination of a diagnostic epithelial scraping. A, Case 1. There are numerous 2.0×1.0–μm organisms in the cytoplasm of an epithelial cell (arrow) (Giemsa, original magnification ×250). B, Case 2. An epithelial cell is distended with 2.0×1.0–μm organisms in its cytoplasm (arrow) compared with a normal cell (arrowhead) (Gram, original magnification ×250).
Case 2
A 63-year-old white man with no medical history was referred for left eye redness and irritation. Treatment elsewhere had included topical prednisolone acetate, 1%. A diffuse punctate epitheliopathy with a “dendritiform pattern” subsequently developed. Treatment with topical trifluorothymidine was started; however, the signs did not improve, and the patient was referred for further evaluation. At presentation in our clinic, BCVA measured 20/20 OD and 20/30 OS. Slitlamp examination of the right eye was normal. The left eye showed a diffuse punctate epithelial keratopathy (Figure 1B). We suspected microsporidial infection and performed an initial epithelial scraping, results of which were inconclusive. At 2 weeks, his visual acuity remained at 20/30 OD with worsening punctate epithelial keratopathy. Another scraping was performed. Microscopic examination of this specimen revealed aggregates of intraepithelial organisms consistent with microsporidia (Figure 2B). Trifluorothymidine use was discontinued, and treatment was begun with topical voriconazole, 1%, every 2 hours. Visual acuity improved to 20/20 OD, and clinical signs improved over 1 to 2 weeks. Use of voriconazole drops was tapered over the course of 4 weeks, and there was no recurrence of signs or symptoms.
Comment
An optimal mono-therapy for microsporidia keratitis does not currently exist despite trials of oral and topical agents. Treatment with numerous topical medications has been attempted without success. Oral medications, such as albendazole and itraconazole, have shown efficacy, but the risk of systemic adverse effects and drug-drug interactions exists.3 Combinations of medications, both topical and oral, have been attempted with varying success.
Given the efficacy of azoles systemically, we treated our patients with a topical azole (voriconazole, 1%) to target the superficial cornea locally. Voriconazole has proven efficacy in treating ocular fungal infections secondary to its broad spectrum of activity and excellent ocular penetration.4 In human eyes, Hariprasad et al5 demonstrated aqueous concentrations exceeding the minimum inhibitory concentration required to inhibit the growth of 90% of organisms of most fungal pathogens, often by 5-fold, after use of topical voriconazole, 1%, every 2 hours for 24 hours.
It is possible microsporidia is a self-limited disease in immunocompetent patients.6 We know that our first patient was immunosuppressed. Our second patient was healthy, but the human immunodeficiency virus status was unknown. This patient did not respond to topical steroids. Once treated with topical voriconazole, the patient improved quickly.
Both of our patients had epithelial debridement to establish the diagnosis. Debridement alone may be therapeutic; however, our second patient only improved after the second debridement was accompanied by treatment with voriconazole.
We conclude that topical voriconazole, 1%, is an effective treatment for keratitis due to microsporidia. Both patients were treated for approximately 6 weeks with complete resolution of signs and symptoms. Neither patient had complaints of adverse effects during treatment. As such, we believe topical voriconazole, 1%, should be further investigated as a potential treatment of choice for patients with microsporidial superficial punctate keratitis.
Acknowledgments
Funding/Support: Supported in part by Research to Prevent Blindness.
Footnotes
Financial Disclosure: None reported.
References
- 1.Lee SC, Corradi N, Byrnes EJ, III, et al. Microsporidia evolved from ancestral sexual fungi. Curr Biol. 2008;18(21):1675–1679. doi: 10.1016/j.cub.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Badriyeh D, Leung L, Davies GE, Stewart K, Kong D. Successful use of topical voriconazole 1% alone as first-line antifungal therapy against Candida albicans keratitis. Ann Pharmacother. 2009;43(12):2103–2107. doi: 10.1345/aph.1m318. [DOI] [PubMed] [Google Scholar]
- 3.Loh RS, Chan CM, Ti SE, Lim L, Chan KS, Tan DT. Emerging prevalence of microsporidial keratitis in Singapore: epidemiology, clinical features, and management. Ophthalmology. 2009;116(12):2348–2353. doi: 10.1016/j.ophtha.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Vemulakonda GA, Hariprasad SM, Mieler WF, Prince RA, Shah GK, Van Gelder RN. Aqueous and vitreous concentrations following topical administration of 1% voriconazole in humans. Arch Ophthalmol. 2008;126(1):18–22. doi: 10.1001/archophthalmol.2007.8. [DOI] [PubMed] [Google Scholar]
- 5.Hariprasad SM, Mieler WF, Holz ER, et al. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol. 2004;122 (1):42–47. doi: 10.1001/archopht.122.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Sahu SK, Sharma S, Nayak SS, Kar S. Clinical trial of 0.02% polyhexamethylene biguanide versus placebo in the treatment of microsporidial keratoconjunctivitis. Am J Ophthalmol. 2010;150(1):110–115. e2. doi: 10.1016/j.ajo.2010.01.038. [DOI] [PubMed] [Google Scholar]