Abstract
Background and Purpose
Ischemic tissue damage is heterogeneous, resulting in complex patterns in the widely used diffusion-weighted MRI (DWI). Our study examined the spatiotemporal characteristics of diffusion kurtosis imaging (DKI) in an animal model of transient middle cerebral artery occlusion (MCAO).
Methods
Adult male Wistar rats (N = 18) were subjected to 90min MCAO. Multi-parametric MRI were obtained during MCAO and 20 min after reperfusion, with DWI obtained using eight b-values from 250 to 3000 s/mm2 in six diffusion gradient directions. Diffusion and kurtosis lesions were outlined in shuffled images by two investigators independently. T2 MRI was obtained 24 hr after MCAO to evaluate stroke outcome.
Results
Mean diffusion (MD) lesion (23.5±8.1%, percentage of the brain slice) was significantly larger than mean kurtosis (MK) lesion (13.2±2.0%) during MCAO. MD lesion decreased significantly after reperfusion (13.8±4.3%) while MK lesion showed little change (13.0±2.5%), with their lesion size difference being insignificant.
Conclusions
We demonstrated that MD/MK mismatch recovered reasonably well upon reperfusion while regions with concurrent MD and MK deficits showed poor recovery. DKI may help stratify heterogeneous DWI lesion for enhanced characterization of ischemic tissue injury.
Keywords: acute ischemia, diffusion, kurtosis
INTRODUCTION
Diffusion-weighted imaging (DWI) detects severely damaged ischemic tissue that is likely to infarct and has been widely used in stroke imaging 1, 2. However, tissue damage within DWI deficit is heterogeneous, which may partially recover with prompt treatment. There have been no well-established techniques capable of stratifying heterogeneously damaged DWI lesion3, 4. Diffusion kurtosis imaging (DKI) is an emerging MRI technique that measures the degree of the non-Gaussian water diffusion and is sensitive to microscopic structural changes5. Indeed, DKI has been shown capable of detecting microstructural cerebral tissue changes in aging brains, acute stroke and tumor 6–8. We postulated that DKI could stratify heterogeneous DWI lesion, improving characterization of tissue injury. Our study examined the spatiotemporal characteristics of mean diffusion (MD) and kurtosis (MK) MRI, using a transient filament middle cerebral artery occlusion (MCAO) rodent model.
MATERIALS AND METHODS
Animal Model
Transient MCAO was induced in eighteen adult male Wistar rats, anesthetized under 1.5–2.0% isoflurane with heart rate and saturation of peripheral oxygen monitored online, following institution-approved guidelines. Animals were re-perfused by withdrawing filament 95 min post-MCAO. One rat deceased during MRI and was excluded from analysis.
MRI
MRI was obtained using a Bruker 4.7 Tesla small-bore scanner (5 slices, 2 mm/slice, field of view=25×25 mm2, matrix size=64×64). Multi-parametric MRI was obtained during MCAO (20–90 min post MCAO), after reperfusion (120–190 min post MCAO). DWI was acquired with b-values of 250, 500, 750, 1000, 1500, 2000, 2500 and 3000 s/mm2 (repetition time (TR)/echo time (TE)=2500/40.5 ms, number of average (NAE)=4, duration=8 min) along six diffusion directions. Cerebral blood flow (CBF) was acquired with amplitude modulated arterial spin labeling (AM-ASL) MRI (TR/TE=6500/14.8 ms, NAE=32, duration=7 min). T1-weighted images were acquired using inversion-recovery MRI, with seven delays from 250 to 3000 ms (TR/TE=6500/14.8 ms, NAE=4, duration=3.5 min); T2-weigthed images were obtained with two TEs (TR/TE(1)/TE(2)=3250/30/100 ms, NAE=16, duration=1.8 min). We also obtained follow-up T2 MRI 24 hr post-MCAO.
Data Analysis
Images were analyzed in Matlab (MathWorks, Natick, MA). DWI signal (S(b)) was fitted per-pixel using . MD and MK were obtained by averaging diffusion (Dapp) and kurtosis coefficients (Kapp) along six directions, respectively5. Apparent diffusion coefficient (ADC) was derived from S(b) = S(0) · exp(− b · ADC) using b=250 and 1,000 s/mm2, and CBF was obtained from AM-ASL MRI as described previously9. MD and MK lesions were independently outlined from shuffled images by two investigators in the central slice (2 mm behind the bregma) and averaged. Lesions were mirrored to the contralateral brain as reference region of interest (ROI). Results were reported as mean ± standard deviation (S.D.), and we used repeated measures analysis of variance (ANOVA) with Tukey’s multiple comparison test.
RESULTS
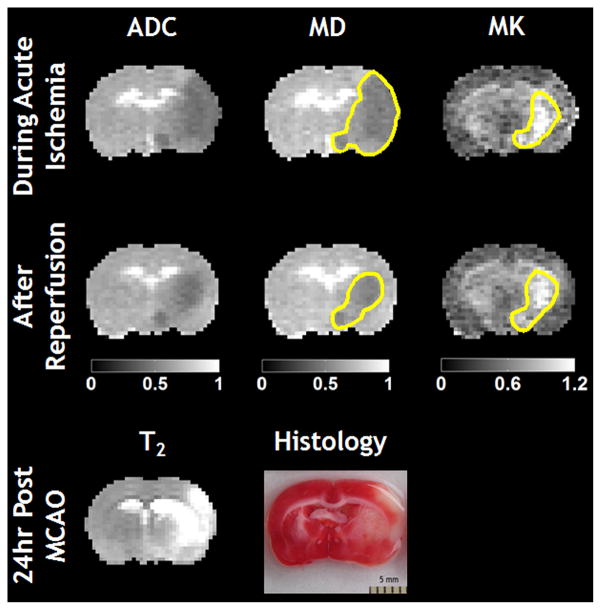
Figure 1 shows that MD lesion was significantly larger than MK deficit during MCAO. After reperfusion, MD lesion decreased to approximately the size of MK lesion, which showed negligible change. Notably, MD and ADC maps showed very similar lesion, as expected. Stroke outcome was confirmed using follow-up T2 MRI and histology.
Figure 1.
Multi-parametric ADC, MD and MK maps of a representative MCAO rat during acute ischemia and immediately after reperfusion. Tissue outcome was confirmed with follow-up T2 MRI and histology.
Figure 2 shows reperfusion–induced change in lesion size, calculated as the percentage of the brain. During MCAO, MD lesion (23.5±8.1%) was larger than MK (13.2±2.0%, p < 0.01). MD lesion decreased significantly after reperfusion, and the difference between MD (13.8±4.3%) and MK (13.0±2.5%) lesion became insignificant. However, the follow-up T2 MRI showed infarction (28.1±9.6%) significantly larger than acute MD and MK lesions (p < 0.01), likely due to severe ischemia and reperfusion injury.
Figure 2.

MD and MK lesion volumes, expressed as the percentage of the brain in the same section, during MCAO and after reperfusion. Error bars represent standard error of the mean (SEM). (** for p-value < 0.01, * for p-value < 0.05, and n.s. for non-significant)
Figure 3 compares reperfusion–induced change in multi-parametric MRI values. During MCAO, MD in ischemic lesion decreased significantly from the contralateral normal region (0.55±0.03 vs. 0.78±0.02 μm2/ms, p < 0.01), while MK was elevated (0.98±0.04 vs. 0.69±0.03, p < 0.01). Using the contralateral ROI as reference, the percentage difference between non-ischemic and ischemic tissues was −29.0±3.6% and 42.6±4.8% for MD and MK, respectively. After reperfusion, the ischemic lesion MD improved (0.60±0.04 μm2/ms, p < 0.01) but was still significantly less than the reference (p < 0.01). Moreover, ischemic tissue MK decreased significantly, yet it was still elevated from the contralateral normal tissue (0.93±0.06 vs. 0.70±0.03, p < 0.01). The percentage differences between non-ischemic and ischemic tissues were −22.7±5.3% and 33.9±10.9% for MD and MK, respectively.
Figure 3.
Comparison of MD (a) and MK (b) of the contralateral normal (con.) and ipsilateral ischemic (ipsi.) regions before and after reperfusion.
DISCUSSION
Our study found that the MD/MK mismatch recovered reasonably well upon reperfusion, while areas with concurrent MD and MK deficits showed little recovery. In comparison, T1 and T2 MRI are not sensitive to ischemic tissue injury during acute stroke. Our results suggest that MD/MK mismatch may represent mildly damaged and potentially salvageable ischemic lesion, while areas with simultaneous MD and MK deficits likely indicate aggravated cellular damage7. However, the mechanisms of diffusion and kurtosis deficits in acute stroke are complex. MD decreases in the MD/MK mismatch is likely due to cytotoxic edema10, 11. In contrast, MK is sensitive to intracellular tortuosity and viscosity changes subsequent to breakdown of cytoskeletal structures and swelling of mitochondria, likely indicating more severe tissue damage5, 7, 12–14. Nevertheless, the filament stroke model used in our study is subject to severe ischemia and reperfusion injury, and the DWI renormalization was transient15. Indeed, we found that CBF decreased significantly in the ipsilateral hemisphere from the contralateral ROI, both during MCAO (0.88±0.27 vs. 1.43±0.33 ml/g/min) and after reperfusion (1.15±0.23 vs. 1.72±0.25 ml/g/min). Importantly, reperfusion induced significant CBF increase in both hemispheres (p < 0.01). Therefore, rodent embolic stroke models that more closely resemble human stroke may be more suitable to elucidate the mechanisms of stroke DKI. Moreover, the study may be improved with remote reperfusion techniques to enable pixel-based analysis of MD and MK evolution. Furthermore, histology immediately after reperfusion may help characterize early tissue damage of MD and MK deficits, augmenting evaluation of stroke outcome currently assessed by follow-up MRI.
CONCLUSION
We showed that MD/MK lesion mismatch recovered reasonably well upon reperfusion while regions with concurrent MK and MD deficits responded poorly. DKI may augment DWI for improved characterization of ischemic tissue injury.
Acknowledgments
Funding Sources: This study was partially supported by NSFC-30900365, AHA/SDG-0835384N, NIH/NIBIB-1K01EB009771 and NIH/NCRR-P41RR14075.
We thank Ms. Eusemann for editorial assistance.
Footnotes
DISCLOSURE The authors have no conflicts of interest.
References
- 1.Moseley ME, Cohen Y, Mintorovitch J, Chileuitt L, Shimizu H, Kucharczyk J, et al. Early detection of regional cerebral ischemia in cats: Comparison of diffusion- and t2-weighted mri and spectroscopy. Magn Reson Med. 1990;14:330–346. doi: 10.1002/mrm.1910140218. [DOI] [PubMed] [Google Scholar]
- 2.Neumann-Haefelin T, Wittsack HJ, Wenserski F, Siebler M, Seitz RJ, Modder U, et al. Diffusion- and perfusion-weighted mri. The dwi/pwi mismatch region in acute stroke. Stroke. 1999;30:1591–1597. doi: 10.1161/01.str.30.8.1591. [DOI] [PubMed] [Google Scholar]
- 3.Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weiller C, et al. Severe adc decreases do not predict irreversible tissue damage in humans. Stroke. 2002;33:79–86. doi: 10.1161/hs0102.100884. [DOI] [PubMed] [Google Scholar]
- 4.Ringer TM, Neumann-Haefelin T, Sobel RA, Moseley ME, Yenari MA. Reversal of early diffusion-weighted magnetic resonance imaging abnormalities does not necessarily reflect tissue salvage in experimental cerebral ischemia. Stroke. 2001;32:2362–2369. doi: 10.1161/hs1001.096058. [DOI] [PubMed] [Google Scholar]
- 5.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 6.Raab P, Hattingen E, Franz K, Zanella FE, Lanfermann H. Cerebral gliomas: Diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254:876–881. doi: 10.1148/radiol.09090819. [DOI] [PubMed] [Google Scholar]
- 7.Jensen JH, Falangola MF, Hu C, Tabesh A, Rapalino O, Lo C, et al. Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR Biomed. 2011;24:452–457. doi: 10.1002/nbm.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JJ, Lin WY, Lu CS, Weng YH, Ng SH, Wang CH, et al. Parkinson disease: Diagnostic utility of diffusion kurtosis imaging. Radiology. 2011;261:210–217. doi: 10.1148/radiol.11102277. [DOI] [PubMed] [Google Scholar]
- 9.Sun PZ, Wang EF, Cheung JS. Imaging acute ischemic tissue acidosis with ph-sensitive endogenous amide proton transfer (apt) mri. Neuroimage. 2012;60:1–6. doi: 10.1016/j.neuroimage.2011.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 11.Cvoro V, Marshall I, Armitage PA, Bastin ME, Carpenter T, Rivers CS, et al. Mr diffusion and perfusion parameters: Relationship to metabolites in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2010;81:185–191. doi: 10.1136/jnnp.2008.168393. [DOI] [PubMed] [Google Scholar]
- 12.Wu EX, Cheung MM. Mr diffusion kurtosis imaging for neural tissue characterization. NMR Biomed. 2010;23:836–848. doi: 10.1002/nbm.1506. [DOI] [PubMed] [Google Scholar]
- 13.Dijkhuizen RM, de Graaf RA, Tulleken KA, Nicolay K. Changes in the diffusion of water and intracellular metabolites after excitotoxic injury and global ischemia in neonatal rat brain. J Cereb Blood Flow Metab. 1999;19:341–349. doi: 10.1097/00004647-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 14.van Pul C, Jennekens W, Nicolay K, Kopinga K, Wijn PF. Ischemia-induced adc changes are larger than osmotically-induced adc changes in a neonatal rat hippocampus model. Magn Reson Med. 2005;53:348–355. doi: 10.1002/mrm.20353. [DOI] [PubMed] [Google Scholar]
- 15.Aronowski J, Strong R, Grotta JC. Reperfusion injury: Demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]