Abstract
Background and Purpose
Effective stone comminution during shockwave lithotripsy (SWL) is dependent on precise three-dimensional targeting of the shockwave. Respiratory motion, imprecise targeting or shockwave alignment, and stone movement may compromise treatment efficacy. The purpose of this study was to evaluate the accuracy of shockwave targeting during SWL treatment and the effect of motion from respiration.
Patients and Methods
Ten patients underwent SWL for the treatment of 13 renal stones. Stones were targeted fluoroscopically using a Healthtronics Lithotron (five cases) or Dornier Compact Delta II (five cases) shockwave lithotripter. Shocks were delivered at a rate of 1 to 2 Hz with ramping shockwave energy settings of 14 to 26 kV or level 1 to 5. After the low energy pretreatment and protective pause, a commercial diagnostic ultrasound (US) imaging system was used to record images of the stone during active SWL treatment. Shockwave accuracy, defined as the proportion of shockwaves that resulted in stone motion with shockwave delivery, and respiratory stone motion were determined by two independent observers who reviewed the ultrasonographic videos.
Results
Mean age was 51±15 years with 60% men, and mean stone size was 10.5±3.7 mm (range 5–18 mm). A mean of 2675±303 shocks was delivered. Shockwave-induced stone motion was observed with every stone. Accurate targeting of the stone occurred in 60%±15% of shockwaves.
Conclusions
US imaging during SWL revealed that 40% of shockwaves miss the stone and contribute solely to tissue injury, primarily from movement with respiration. These data support the need for a device to deliver shockwaves only when the stone is in target. US imaging provides real-time assessment of stone targeting and accuracy of shockwave delivery.
Introduction
Shockwave lithotripsy (SWL) is one of the most commonly performed treatments in the United States for patients with nephrolithiasis.1–3 Over the last two decades, however, there has been a shift from SWL to ureteroscopy for the management of kidney stones.2,4 Improvements in the effectiveness of SWL could increase the use of this noninvasive therapy.
Effective stone comminution during SWL is dependent on accurate and precise three-dimensional targeting of the stone within the focal zone of the lithotripter. Inaccurate targeting, imprecise shockwave alignment, and stone movement during treatment may compromise treatment efficacy. Breathing motion can greatly influence stone targeting. The kidney and stone may move as much as 50 mm because of respiratory excursion.5–8 In vitro studies mimicking respiratory stone motion have demonstrated that stone motion of only 10 mm may significantly reduce comminution, and with greater excursion, as many as 75% of sound waves may miss the stone.6 Thus, lithotripters with a smaller focal zone may further exaggerate the effect of respiratory stone motion, while a lithotripter with a wider focal zone may offer a better chance of hitting the stone. In addition, treatment efficacy is lower for nonanesthetized patients, likely because of additional patient motion, and thus general anesthesia may be recommended for SWL.9
It has been recognized that SWL may rupture blood vessels, resulting in intraparenchymal hemorrhage or perinephric hematomas. Surrounding structures, such as the spleen, liver, and/or pancreas, may also be injured.10–12 The acute damage and renal lesions created by the SWL treatment are dose-dependent in terms of pulse amplitude and the number of shockwaves.13–18 Most patients receive the maximum number of shockwaves during their SWL, and thus efforts to ensure precise targeting of the lithotripter focal zone are warranted and restriction of the quantity of inaccurate shockwaves delivered would be advisable.1,13 The efficacy of SWL may be increased by lowering the pulse rate to 60 shocks/min, ramping the shockwave energy, and appropriate coupling of the lithotripter head.19
Despite these improvements in SWL technique, the proportion of effective shockwaves delivered during a treatment session has yet to be defined. It is likely that some portion of the shockwaves delivered miss the target stone entirely but hit the surrounding tissue. These shockwaves contribute only to kidney injury with no impact on stone comminution.1 Thus, the real-time identification of which soundwaves are targeted appropriately and when the shockwaves are likely to be effective might help the clinician to deliver the greatest number of high-quality shockwaves, perhaps with an overall decreased number of total shockwaves.
The purpose of this study is to describe the use of ultrasound (US) imaging to evaluate the accuracy of shockwave targeting during SWL treatment and as a real-time feedback mechanism to evaluate treatment efficacy.
Patients and Methods
We enrolled 10 patients undergoing SWL treatment for upper tract urolithiasis. After induction of anesthesia, a Healthtronics Lithotron (five cases) or Dornier Compact Delta II (five cases) shockwave lithotripter targeted the stones in three dimensions using fluoroscopy. After the appropriate low-energy pretreatment with 100 to 200 shockwaves at 1 Hz, followed by a brief protective pause, lithotripsy was performed at 1 to 2 Hz with increasing of the energy settings from 14 up to a maximum of 26 kV for the Healthtronics Lithotron and from 1 up to 5 for the Dornier Compact Delta II. Previous research has demonstrated a similar cross-sectional focal diameter for these two lithotripters (50 mm2 vs 47 mm2, respectively).20,21 All stone targeting was performed by a single, experienced SWL technician with surgeon oversight.
A commercial diagnostic US imaging system (Phillips HDI 5000 with a phased array (4–2 MHz) transducer) was used to visualize the treated kidney and stone, and video imaging was recorded for later analysis (Fig. 1). Every effort was made to orient the US probe to maximize imaging of the stone throughout respiration. Stone motion in the form of oscillation or jumping was interpreted as an accurate targeting of the shockwave focal zone onto the stone. This information was immediately available to the practitioner to determine if shockwaves were being delivered accurately or if the targeting of the SWL machine needed adjustment. Decisions about treatment, retargeting, and treatment end point were left to the discretion of the treating physician. US imaging was not regularly used in this study to determine treatment end point. A single, experienced sonographer performed all intraoperative US imaging for this study.
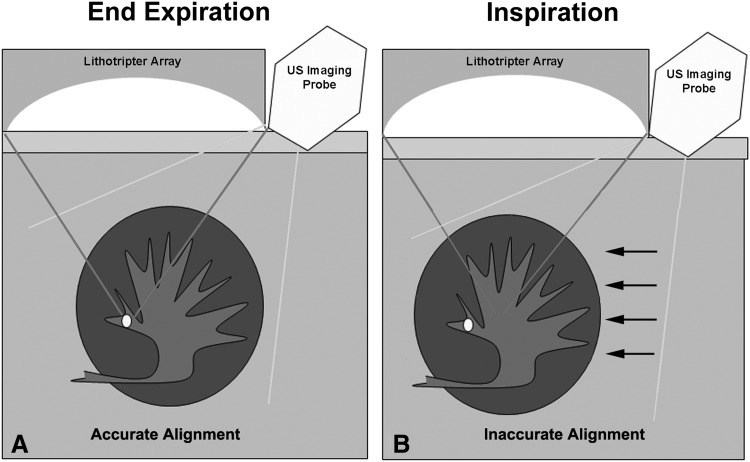
FIG. 1.
Diagram of configuration of shockwave lithotripsy machine and lithotripter array focused on the stone and the position of the associated ultrasound imager during (A) end expiration. With inspiration (B), the kidney descends with respiratory motion (arrows), moving the stone out of the lithotripter focus causing inaccurate alignment.
The primary outcome of interest was the proportion of shockwaves in the recorded session that were delivered to the stone causing motion, oscillation, or jumping denoting appropriate targeting of the lithotripter focal zone on the stone. Patient demographics, characteristics of the stone size and location, anesthesia type (general, regional, sedation), respiratory rate, and respiratory-induced distance of stone movement during the US imaging, and data from the SWL session were collected. US recordings were reviewed in a different, nonconsecutive order by two independent observers, and the accuracy of SWL targeting was determined by evaluating the proportion of the total shockwaves observed that resulted in stone motion, oscillation, or jumping. Observers only had access to information about the procedure visible in the video. They did not collaborate, and they were not trained, although they had some clinical experience with US imaging.
The overall proportion of accurately targeted shockwaves (±standard deviation) was determined. Linear regression analyses were used to evaluate trends in accuracy over time and the relationship between targeting accuracy and the patient's respiratory rate, stone size, anesthesia type, and shockwave delivery rate during the procedure.
This study received Institutional Review Board approval (#35261) from the University of Washington School of Medicine, and all patients completed appropriate informed consent.
Results
Ten patients (mean age 51±15 years) underwent treatment of 13 kidney stones with 60% male patients (Table 1). Mean stone size was 10.5±3.7 mm (range 5–18 mm), and stones were most commonly located in the renal pelvis (46%) or lower pole (38%). All stones were able to be visualized with ultrasonography, and shockwave-induced stone motion was visualized in all stones. There was no evidence of cavitation during our observations.
Table 1.
Demographics, Stone Characteristics, and Shockwave Lithotripsy Session Details
| N=10 patients, 13 stones | |
|---|---|
| Mean age±SD (years) | 50±15 |
| (range) | (26–78 years) |
| Male (%) | 60% |
| Female (%) | 40% |
| Mean body mass index (kg/m2)±SD | 29±6 |
| (range) | (20–39) |
| Mean stone size±SD | 10.5±3.7 mm |
| (range) | 5–18 mm |
| Stone location (%) | |
| Renal pelvis | 6 (46%) |
| Upper pole | 1 (8%) |
| Lower pole | 5 (38%) |
| Ureter | 1 (8%) |
| SWL machine | |
| Healthtronics Lithotron | 5 (50%) |
| Focal area (mm2) | 50 mm2 |
| Dornier Compact Delta II | 5 (50%) |
| Focal area (mm2) | 47 mm2 |
| SWL treatment | |
| Mean shocks±SD | 2675±303 |
| (range) | (2100–3000) |
| Frequency | 1–2 Hz |
| Mean duration of delivery of shockwaves±SD | 35±6 minutes |
| Energy settings: | |
| Healthtronics Lithitron (kVp) | 14–26 |
| Dornier Compact Delta II | 1–5 |
| Mean respiratory rate±SD | 13±4 breaths/minute |
| (range) | (8–19/minute) |
| Mean respiratory stone motion±SD | 1.5±0.3 cm |
| US imaging | |
| Average duration of US video observation | 7.1 min |
| Proportion of SWL treatment evaluated by US monitoring | 20.4% |
| Accuracy of SWL treatment (%) | |
| Subject 1 | 38% |
| Subject 2 | 46% |
| Subject 3 | 40% |
| Subject 4 | 56% |
| Subject 5 | 65% |
| Subject 6 | 71% |
| Subject 7 | 58% |
| Subject 8 | 72% |
| Subject 9 | 65% |
| Subject 10 | 85% |
| Overall±SD | 60±15% |
SD=standard deviation; SWL=shockwave lithotripsy; US=ultrasound.
An average of 2675±303 shocks (range 2100–3000) were delivered over the SWL session. US imaging was performed after the pretreatment pause. A total of 71 minutes of ultrasonographic recorded video from the 10 treatments was reviewed for an average of 7.1 minutes per SWL session representing 20.4% of the total SWL treatment session. The interobserver agreement of treatment accuracy was good; the average difference in calculated accuracy reported between observers was 5% and never differed by more than 8% for any individual ultrasonographic video.
The average respiratory-induced stone motion was 1.5±0.3 cm. One SWL was performed with sedation as the only anesthetic and had the highest respiratory rate (19 breaths/min) and the least respiratory stone movement (1.0 cm); one SWL was performed with the patient partially paralyzed in addition to general anesthesia and had the lowest respiratory rate (10 breaths/min) and the highest respiratory stone movement (1.8 cm).
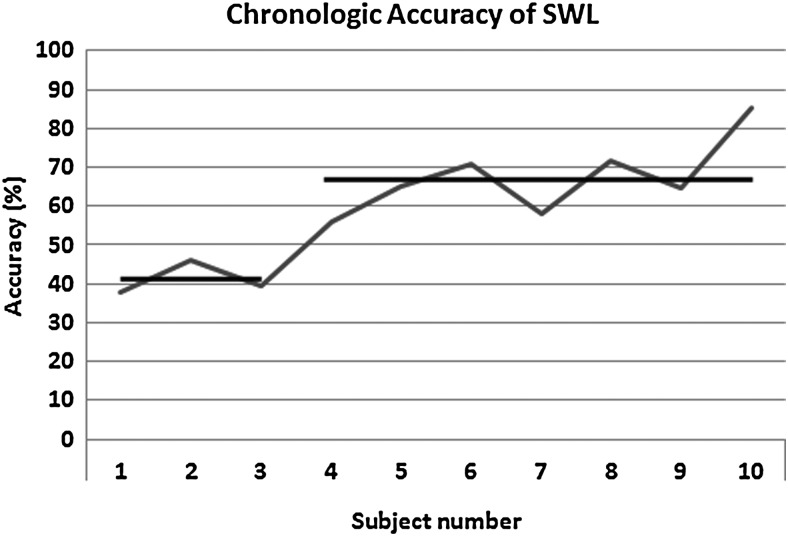
Accurate stone targeting, as interpreted by stone motion with the delivery of the shockwave, occurred for 60%±15% of delivered shockwaves. When analyzed chronologically, there was a nonlinear improvement in accuracy over the study period. After three cases performed with ultrasonograpphic monitoring, there was an improvement in average accuracy of shockwave alignment from 41%±4% up to 67%±10% accuracy (P=0.003, Fig. 2). Using linear regression analyses, there was a 4.4% (95% confidence interval 2.5–6.3%, P=0.0007) improvement in SWL accuracy with each case.
FIG. 2.
Graphic representation of the change in shockwave lithotripsy (SWL) accuracy over the study period. After the third study SWL treatment, the average accuracy increased from 41%±4% up to 67%±10% (P=0.003). SWL=shockwave lithotripsy.
In this small study, respiratory rate (P=0.22), stone size (P=0.06), anesthesia type (P=0.98), and shockwave treatment rate (P=0.09) during the treatment did not appear to be associated with the accuracy of delivered shockwaves.
Discussion
To our knowledge, this is the first study to quantify accurate shockwave delivery during SWL in real time. In our study, 40% of shockwaves are inaccurately targeted or otherwise miss the targeted stone. These shockwaves likely contribute only to kidney injury with no impact on the efficacy of stone comminution. Stone movement during treatment was able to be visualized in all cases using diagnostic US imaging in real time. In our experience, the loss in accuracy of shockwave delivery was primarily because of respiratory motion. Regardless of stone size, during the treatment of every stone, respiratory excursion was enough to repeatedly move the stone outside the lithotripter focal zone, at least for a brief period (Fig. 1). Compared with previous studies that report up to 5.0 cm of respiratory-induced stone motion, the stone motion in our study was rather modest, averaging only 1.5 cm, yet this was enough to cause many shockwaves to miss the stone.
Although difficult to objectively capture in this small study, because providers were allowed immediate feedback about the lithotripter targeting, we did note some instances in which the US imaging triggered the treating team to make minor adjustments of the lithotripter focal zone point to try to optimize targeting. Qualitatively, providers noted that the US image increased their confidence in SWL targeting and decreased their urge to repeatedly image the stone using fluoroscopy. This may partially explain the improvement in targeting accuracy over the study period.
These findings have important clinical ramifications. Ultrasonography could be used in the clinical setting to improve initial targeting of the lithotripter focal zone. The real-time identification of effective delivery of accurate shockwaves might help to improve overall treatment accuracy and might provide an additional mechanism to prompt the provider to make minor adjustments to the lithotripter targeting as treatment progressed. In addition, a device or trigger mechanism designed to actively adjust the focal zone to track and target the moving stone or only allow shockwave firing when the stone is on target would ensure that only accurate shockwaves are delivered. Simply gating SWL delivery to respiration does not improve SWL results, and thus triggering to the targeted stone is likely necessary.22 Prototype targeting and tracking systems have demonstrated improvements in in vitro hit rates and stone breakage of 1.5- to 2-fold.23–26 By eliminating or reducing the shockwaves that likely only contribute to injury, this could potentially decrease the total number of shockwaves delivered.
SWL causes acute tissue injury that is primarily a hemorrhagic lesion, which is dependent on the shockwave dose.13–18 Animal studies have demonstrated that hemorrhage occupies 0.3% of the functional renal volume after treatment with 1000 shockwaves, 6.1% after 2000 shockwaves, and 13.8% after 8000 shockwaves.13,18,27 Acute injury may progress to scar formation in the kidney, and in animal studies, scars from 2000 shockwaves were 10 times larger than from 1000 shockwaves.17 If every shockwave delivered was targeted perfectly, we might decrease the number of inaccurate shockwaves and potentially minimize unnecessary kidney injury without increasing the duration of the procedure.
We view this as the next step in improving efficacy of SWL. Future studies are necessary to evaluate if specific anesthesia-controlled breathing techniques improve accuracy by decreasing respiratory-induced motion and if differences in targeting accuracy translate into different stone-free rates or other clinically important outcomes. Given the degree of respiratory motion and the size of the cross-sectional focal diameter of most contemporary lithotripters, we suspect that controlled breathing techniques alone will be insufficient to improve efficacy to a clinically meaningful degree, but this remains to be seen.
This study has limitations. Only 20% of the total SWL procedure was captured in our US imaging. It is possible that the accuracy at different portions of the procedure would vary, although we did not identify this in any of our videos. An experienced SWL technician performed all lithotripter targeting during the procedures. It is possible that targeting from other sonographers might vary and that her ability to capture and display the movement of the stone (or lack of movement) may have improved over the study period. US targeting during the procedure required the skill of an experienced US technologist, and the reproducibility of this step has not been evaluated.
Given the study size, we were likely underpowered to rigorously evaluate some of our secondary end points, such as correlation of shockwave accuracy with the patient's respiratory rate, stone size, anesthesia type, and shockwave delivery rate. It is possible that shockwaves delivered during respiratory excursion that failed to move the stone might still exert some effect. Although not formally tested in this study, we believe that the failure to move the stone is a marker of the stone not being within the target focal zone, and thus these soundwaves do not appreciably contribute to stone comminution.
Overall, this study has demonstrated that a substantial number of delivered shockwaves miss the stone because of inaccurate targeting of the shockwave focal zone or stone movement during treatment. If these shockwaves could be eliminated, reserved, or redirected onto the stone, it is possible that the efficacy of SWL could be improved with potentially less renal injury. This may provide the basis for devices to control targeting so that shockwaves are only delivered when the stone is within the focal zone.
Conclusions
Respiratory motion, misalignment, and stone movement during SWL may lead to as many as 40% of shockwaves missing the stone entirely. This energy contributes only to kidney injury, and if these shockwaves could be redirected or eliminated with a targeting device, SWL efficacy could potentially be improved. US imaging represents a noninvasive way to provide real-time assessment of shockwave delivery accuracy.
Abbreviations Used
- SWL
shockwave lithotripsy
- US
ultrasound
Disclosure Statement
No competing financial interests exist.
Acknowledgments
The authors would like to thank Dr. Jonathan Wright for his assistance with patient recruitment and Wei Lu for his help with data management and storage. This work was supported by grants NIH DK48331, NIH DK92197, and NSBRI through NASA NCC 9–58.
References
- 1.Lingeman JE. McAteer JA. Gnessin E. Evan AP. Shock wave lithotripsy: Advances in technology and technique. Nat Rev Urol. 2009;6:660–670. doi: 10.1038/nrurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerbl K. Rehman J. Landman J, et al. Current management of urolithiasis: Progress or regress? J Endourol. 2002;16:281–288. doi: 10.1089/089277902760102758. [DOI] [PubMed] [Google Scholar]
- 3.Pearle MS. Lingeman JE. Leveillee R, et al. Prospective, randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2005;173:2005–2009. doi: 10.1097/01.ju.0000158458.51706.56. [DOI] [PubMed] [Google Scholar]
- 4.Matlaga BR American Board of Urology. Contemporary surgical management of upper urinary tract calculi. J Urol. 2009;181:2152–2156. doi: 10.1016/j.juro.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Boucher L. Rodrigue S. Lecomte R. Bénard F. Respiratory gating for 3-dimensional PET of the thorax: Feasibility and initial results. J Nucl Med. 2004;45:214–219. [PubMed] [Google Scholar]
- 6.Cleveland RO. Anglade R. Babayan RK. Effect of stone motion on in vitro comminution efficiency of Storz Modulith SLX. J Endourol. 2004;18:629–633. doi: 10.1089/end.2004.18.629. [DOI] [PubMed] [Google Scholar]
- 7.Moerland MA. van den Bergh AC. Bhagwandien R, et al. The influence of respiration induced motion of the kidneys on the accuracy of radiotherapy treatment planning, a magnetic resonance imaging study. Radiother Oncol. 1994;30:150–154. doi: 10.1016/0167-8140(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz LH. Richaud J. Buffat L, et al. Kidney mobility during respiration. Radiother Oncol. 1994;32:84–86. doi: 10.1016/0167-8140(94)90452-9. [DOI] [PubMed] [Google Scholar]
- 9.Ozgur A. Yalm Iker N. Extracorporeal shock wave lithotripsy of renal pelvis stones with PCK stonelith lithotripter. Int Urol Nephrol. 2005;37:9–11. doi: 10.1007/s11255-004-6085-2. [DOI] [PubMed] [Google Scholar]
- 10.Coe FL. Favis MJ. Pak CC, et al. Kidney Stones: Medical and Surgical Management. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- 11.Kaude JV. Williams CM. Millner MR, et al. Renal morphology and function immediately after extracorporeal shock-wave lithotripsy. AJR Am J Roentgenol. 1985;145:305–313. doi: 10.2214/ajr.145.2.305. [DOI] [PubMed] [Google Scholar]
- 12.McAteer JA. Evan AP. The acute and long-term adverse effects of shock wave lithotripsy. Semin Nephrol. 2008;28:200–213. doi: 10.1016/j.semnephrol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connors BA. Evan AP. Blomgren PM, et al. Reducing shock number dramatically decreases lesion size in a juvenile kidney model. J Endourol. 2006;20:607–611. doi: 10.1089/end.2006.20.607. [DOI] [PubMed] [Google Scholar]
- 14.Connors BA. Evan AP. Willis LR, et al. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11:310–318. doi: 10.1681/ASN.V112310. [DOI] [PubMed] [Google Scholar]
- 15.Delius M. Enders G. Xuan ZR, et al. Biological effects of shock waves: Kidney damage by shock waves in dogs—dose dependence. Ultrasound Med Biol. 1988;14:117–122. doi: 10.1016/0301-5629(88)90178-0. [DOI] [PubMed] [Google Scholar]
- 16.Delius M. Jordan M. Eizenhoefer H, et al. Biological effects of shock waves: Kidney haemorrhage by shock waves in dogs—administration rate dependence. Ultrasound Med Biol. 1988;14:689–694. doi: 10.1016/0301-5629(88)90025-7. [DOI] [PubMed] [Google Scholar]
- 17.Morris JS. Husmann DA. Wilson WT. Preminger GM. Temporal effects of shock wave lithotripsy. J Urol. 1991;145:881–883. doi: 10.1016/s0022-5347(17)38482-3. [DOI] [PubMed] [Google Scholar]
- 18.Willis LR. Evan AP. Connors BA, et al. Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. J Am Soc Nephrol. 1999;10:1753–1762. doi: 10.1681/ASN.V1081753. [DOI] [PubMed] [Google Scholar]
- 19.Rassweiler JJ. Knoll T. Köhrmann KU, et al. Shock wave technology and application: An update. Eur Urol. 2011;59:784–796. doi: 10.1016/j.eururo.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evan AP. McAteer JA. Williams JC, et al. Shock wave physics of lithotripsy: Mechanisms of shock wave action and progress toward improved SWL. In: Moore RG, editor; Bishoff JT, editor; Loenig S, editor; Docimo SG, editor. Textbook of Minimally Invasive Urological Surgery. London, UK: Informa Healthcare; 2005. pp. 425–438. [Google Scholar]
- 21.Lingeman JE. Lifshitz DA. Evan AP. Surgical management of urinary lithiasis. In: Campbell MF, editor; Walsh PC, editor; Retik AB, editor. Campbell's Urology. 8th. Philadelphia, PA: Saunders; 2002. p. 3399. [Google Scholar]
- 22.Sade M. Guler C. Esen AA. Kirkali Z. Does respiratory gating improve extracorporeal shockwave lithotripsy results? J Endourol. 1994;8:329–330. doi: 10.1089/end.1994.8.329. [DOI] [PubMed] [Google Scholar]
- 23.Bohris C. Bayer T. Lechner C. Hit/Miss monitoring of ESWL by spectral Doppler ultrasound. Ultrasound Med Biol. 2003;29:705–712. doi: 10.1016/s0301-5629(02)00773-1. [DOI] [PubMed] [Google Scholar]
- 24.Chang CC. Liang SM. Pu YR, et al. In vitro study of ultrasound based real-time tracking of renal stones for shock wave lithotripsy: Part 1. J Urol. 2001;166:28–32. [PubMed] [Google Scholar]
- 25.Orkisz M. Farchtchian T. Saighi D, et al. Image based renal stone tracking to improve efficacy in extracorporeal lithotripsy. J Urol. 1998;160:1237–1240. [PubMed] [Google Scholar]
- 26.Thomas JL. Wu F. Fink M. Time reversal focusing applied to lithotripsy. Ultrason Imaging. 1996;18:106–121. doi: 10.1177/016173469601800202. [DOI] [PubMed] [Google Scholar]
- 27.Willis LR. Evan AP. Connors BA, et al. Shockwave lithotripsy: Dose-related effects on renal structure, hemodynamics, and tubular function. J Endourol. 2005;19:90–101. doi: 10.1089/end.2005.19.90. [DOI] [PubMed] [Google Scholar]