To the Editor
Rituximab is a chimeric mouse-human IgG1 monoclonal antibody that targets CD20, a transmembrane protein expressed on B cells. It has demonstrated success in two prospective case series in pemphigus (1;2), a potentially fatal blistering disease caused by autoantibodies to desmoglein skin adhesion proteins. The production of human anti-chimeric antibodies (HACA) to the murine fragments of rituximab has previously been described in pemphigus and other autoimmune disease patients (3–7), although a direct functional effect of these HACA has not been reported. Here we describe a pemphigus patient treated with rituximab who developed inhibitory IgG HACA associated with infusion reactions and lack of therapeutic response (i.e. complete or partial remission of disease) (8).
The index patient is an African-American woman who developed severe mucosal pemphigus vulgaris in 1987 at the age of 21. She failed prior therapy with prednisone, gold, cyclosporine, cyclophosphamide, azathioprine, and mycophenolate mofetil. On evaluation she had severe painful erosions on the gingiva, palate, buccal mucosa, tongue, and posterior pharynx. Due to refractory severe disease, she received her first course of rituximab (375 mg/m2 weekly x 4) in January 2007. Her disease improved but did not remit, with persistent mild gingival erosions. She began a second course of rituximab using the rheumatoid arthritis regimen, receiving a 1000 mg dose in July 2009. She failed to receive the second dose because of hospitalization for syncope. Workup revealed iron-deficiency anemia, and the patient gradually improved with oral iron supplementation. She returned in March 2010 with widespread erosions on the buccal mucosa and gingiva, at which time rituximab was re-initiated. The patient experienced an infusion reaction 2.5 hours into the second infusion (cumulative dose 450 mg), with itching of the hands and feet, followed by tongue and throat swelling. Vital signs and pulse oximetry were normal. Symptoms abated after treatment with intravenous hydrocortisone and diphenhydramine. 75 minutes after restarting the infusion (cumulative dose 900 mg), the patient experienced chest tightness, flushing, and diaphoresis. Pulse was 107; other vital signs and pulse oximetry were normal. Electrocardiogram demonstrated sinus tachycardia with T-wave inversion in anterior leads. The infusion was stopped, and her symptoms resolved without treatment within 30 minutes.
The patient’s disease improved slightly after the course, with resolution of buccal mucosal erosions but persistence of severe gingival erosions. She was retreated with rituximab in December 2010 but did not demonstrate a therapeutic response. 30 minutes into the second infusion (cumulative dose 250 mg), she reported flushing and throat swelling; vital signs and pulse oximetry were normal. She was treated with intravenous hydrocortisone, and the infusion was restarted at a slower rate. 90 minutes later (cumulative dose 550 mg), she experienced itching of the hands and feet that responded to treatment with hydrocortisone, diphenhydramine, and ranitidine, allowing for completion of the infusion.
Given the patient’s course (summarized in Table 1), we hypothesized that the patient may have HACA. Using institutional review board approved protocols, the patient’s serum samples were obtained retrospectively from the clinical laboratory that had performed ELISA to measure desmoglein 3 autoantibody levels (Table 1). One sample was not available. We first used crisscross serial dilution analysis to optimize a competitive sandwich ELISA to quantitate serum HACA. Unlabeled rituximab (5 μg/mL) was coated on ELISA plates. HRP-labeled rituximab (62.5 ng/mL) and serial dilutions of rat anti-rituximab monoclonal antibody (as a surrogate standard, AbD Serotec) were incubated on rituximab-coated plates simultaneously, followed by development with tetramethylbenzidine substrate. Concurrently, dilutions of patient sera were tested to quantitate serum HACA based on the standard curve. The patient’s serum demonstrated 109 ng/mL HACA in July 2010 and 6748 ng/mL HACA in February 2011 (Table 1). No reaction was observed when human myeloma IgG1 was used as the coating antigen.
Table 1. Patient clinical and serologic characteristics.
| Date | Desmoglein 3 ELISA (U/mL) | Serum HACA (ng/mL) | Date | Rituximab dose | Infusion reaction | Disease endpoint |
|---|---|---|---|---|---|---|
| 4/29/05 | 205 | negative | ||||
| 1/17/07 | 375 mg/m2 × 4 | none | No | |||
| 4/2/09 | 148 | n.d. | ||||
| 7/31/09 | 1000 mg × 1 | none | No | |||
| 3/8/10 | 1000 mg, 900 mg | angioedema (2nd dose) | No | |||
| 7/15/10 | 155 | 109 | ||||
| 12/10/10 | 1000 mg x 2 | angioedema (2nd dose) | No | |||
| 2/10/11 | 138 | 6748 | ||||
HACA, human anti-chimeric antibodies, n.d., not determined.
To determine the isotype of rituximab HACA and rule out rheumatoid factor (immunoglobulins against IgG Fc), we tested an indirect ELISA using Fab’ fragments of rituximab (5 μg/mL) as the coating antigen on ELISA plates. 49 normal human sera, diluted 1:25 and detected with HRP-labeled anti-human IgG Fc, were used to establish a cut-off value at 3 standard deviations above the mean optical density value. The patient’s serum was positive when detected with anti-human IgG; no reaction was observed when detected with anti-human IgE or secondary antibody alone.
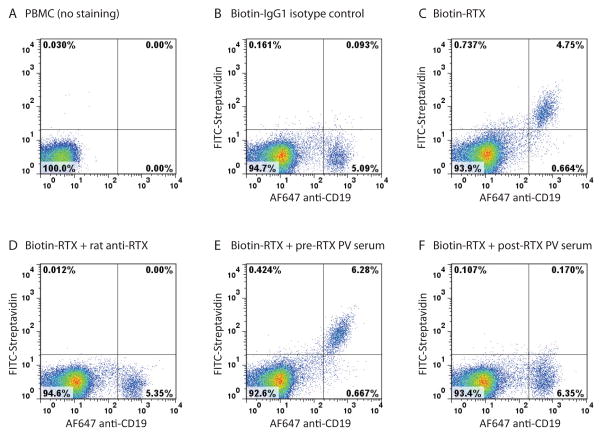
We next determined whether HACA could prevent the binding of rituximab to B cells. Peripheral blood mononuclear cells (PBMCs), obtained by Ficoll gradient centrifugation of whole blood from healthy donors, were incubated for one hour at 4°C with biotinylated rituximab (1 μg/mL) that had been pre-incubated with rat anti-rituximab (2 μg/mL), HACA-negative patient serum prior to rituximab therapy (diluted 1:20), or HACA-positive patient serum after rituximab therapy (diluted 1:20), followed by staining with FITC-streptavidin plus Alexa Fluor 647 mouse anti-human CD19 to identify B cells. A FACSCalibur flow cytometer with CellQuest software was used for data acquisition, and FlowJo software was used for data analysis. Incubation of PBMCs with biotinylated rituximab but not biotinylated human IgG1 isotype control showed congruent labeling of the rituximab (CD20+) and CD19+ B cell population (Figure 1B,C). Pre-incubation of rituximab with rat anti-rituximab blocked rituximab binding to B cells (Figure 1D). Pre-incubation of rituximab with the HACA-high positive patient serum, but not HACA-negative patient serum, similarly abolished rituximab labeling of B cells (Figure 1E,F). No significant inhibition of rituximab labeling was observed with the HACA-low positive patient serum.
Figure 1. HACA-positive PV serum blocks the binding of RTX to B cells.
Flow cytometry analysis of PBMCs incubated with biotinylated human IgG1 isotype control (B), biotinylated rituximab (biotin-RTX) (C), biotin-RTX + rat anti-RTX (D), biotin-RTX + HACA-negative PV serum prior to RTX therapy (E), and biotin-RTX + HACA-positive PV serum after RTX therapy (F). Samples were unstained (A), or stained with FITC-streptavidin and Alexa Fluor (AF) 647 anti-human CD19.
Previous studies of rituximab in autoimmune disease patients have described a correlation between HACA and incomplete B cell depletion in systemic lupus erythematosus (3;4), serum sickness-like reactions in Sjogren’s syndrome and immune thrombocytopenic purpura (5;6), and partial clinical response in pemphigus (7). To our knowledge this is the first demonstration of a direct pathologic role for rituximab HACA in blocking the binding of rituximab to B cells. The appearance of HACA coincided with the development of infusion reactions and a lack of therapeutic response. B cell counts were not available for this patient, as monitoring of B cell depletion is not routinely performed in pemphigus patients after rituximab therapy. It is possible that incomplete B cell depletion due to an incomplete rituximab course in 2009 could have contributed to the development of HACA. Prior studies have also associated clinical response to rituximab with Fc-gamma receptor polymorphisms (9). The Fc-gamma receptor genotypes of this patient are unknown; therefore we cannot rule out additional factors that may have contributed to HACA development. Interestingly, the patient only experienced infusion reactions during the second administration of drug in each of her third and fourth courses, suggesting that perhaps the first infusion may have boosted HACA production.
In conclusion, we report the development of inhibitory IgG HACA in a pemphigus patient who did not achieve disease remission after rituximab therapy. Currently there are no commercially available assays to measure rituximab HACA. Future studies should prospectively monitor B cell depletion and HACA levels in autoimmune disease patients treated with rituximab to better define who may develop HACA and the functional consequences of HACA development.
Acknowledgments
This study was supported by the Department of Dermatology at the University of Milan (LL), the National Institute of Arthritis and Musculoskeletal and Skin Diseases AR053505 and AR057001 (ASP), and the Penn Skin Disease Research Center (P30-AR057217).
Abbreviations
- HACA
human anti-chimeric antibodies
- PBMC
peripheral blood mononuclear cells
- RTX
rituximab
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Joly P, Mouquet H, Roujeau JC, D'Incan M, Gilbert D, Jacquot S, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007 Aug 9;357(6):545–52. doi: 10.1056/NEJMoa067752. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006 Oct 26;355(17):1772–9. doi: 10.1056/NEJMoa062930. [DOI] [PubMed] [Google Scholar]
- 3.Albert D, Dunham J, Khan S, Stansberry J, Kolasinski S, Tsai D, et al. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann Rheum Dis. 2008 Dec;67(12):1724–31. doi: 10.1136/ard.2007.083162. [DOI] [PubMed] [Google Scholar]
- 4.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004 Aug;50(8):2580–9. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 5.Goto S, Goto H, Tanoshima R, Kato H, Takahashi H, Sekiguchi O, et al. Serum sickness with an elevated level of human anti-chimeric antibody following treatment with rituximab in a child with chronic immune thrombocytopenic purpura. Int J Hematol. 2009 Apr;89(3):305–9. doi: 10.1007/s12185-009-0269-6. [DOI] [PubMed] [Google Scholar]
- 6.Pijpe J, van Imhoff GW, Spijkervet FK, Roodenburg JL, Wolbink GJ, Mansour K, et al. Rituximab treatment in patients with primary Sjogren's syndrome: an open-label phase II study. Arthritis Rheum. 2005 Sep;52(9):2740–50. doi: 10.1002/art.21260. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt E, Hennig K, Mengede C, Zillikens D, Kromminga A. Immunogenicity of rituximab in patients with severe pemphigus. Clin Immunol. 2009 Sep;132(3):334–41. doi: 10.1016/j.clim.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol. 2008 Jun;58(6):1043–6. doi: 10.1016/j.jaad.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003 Nov 1;21(21):3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]