Abstract
Objectives
We describe the development of a five-session psychoeducational treatment, Facilitated Integrated Mood Management (FIMM), which contains many of the core elements of longer evidence-based psychosocial treatments for bipolar disorder. FIMM incorporated a novel mood monitoring program based on mobile phone technology.
Methods
Adult patients with bipolar I and II disorders (N = 19) received six sessions (Pilot I: n = 14) or five sessions (Pilot II: n = 5) of FIMM with pharmacotherapy. Treatment facilitators were novice counselors who were trained in a three-day workshop and supervised for six months. FIMM sessions focused on identifying early signs of recurrence, maintaining regular daily and nightly routines, rehearsing mood management strategies, maintaining adherence to medications, and education about substance abuse. Patients sent daily text messages or e-mails containing ratings of their mood and sleep and weekly messages containing self-ratings on the Quick Inventory of Depressive Symptomatology (QIDS) and the Altman Self Rating Mania Scale (ASRM). Patients also completed a weekly mood management strategies questionnaire.
Results
Of the 19 patients, 17 (89.5%) completed FIMM in an average of 9.2 ± 3.4 weeks (Pilot I) and 7.6 ± 0.9 weeks (Pilot II). Patients reported stable moods on the QIDS and ASRM over a 120-day period, and on average responded to 81% of the daily message prompts and 88% of the weekly QIDS and ASRM prompts. Facilitators maintained high levels of fidelity to the FIMM manual. Patients’ knowledge of mood management strategies increased significantly between the first and last weeks of treatment.
Conclusions
Patients with bipolar disorder can be engaged in a short program of facilitated mood management. The effects of FIMM on the course of bipolar disorder await evaluation in randomized trials. The program may be a useful adjunct to pharmacotherapy in community centers that cannot routinely administer full courses of psychosocial treatment.
Keywords: electronic mail, mobile phones, mood monitoring, psychoeducation, psychosocial treatment, text messaging
“Healthcare professionals should aim to develop a therapeutic relationship with all patients with bipolar disorder, and advise them on careful and regular monitoring of symptoms (including triggers and early warning signs), lifestyle (including sleep hygiene and work patterns) an d coping strategies (1).”
Introduction
Psychosocial treatment has become a well-established adjunct to pharmacotherapy for bipolar disorder (BD) in adults and adolescents during the post-acute and maintenance phases of treatment. There are now at least 20 randomized trials of disorder-specific psychotherapies (DSPs), including cognitive behavioral therapy (CBT), family-focused therapy, interpersonal and social rhythm therapy, and group psychoeducation, all administered in combination with standard pharmacotherapy and compared to medication alone, treatment-as-usual, or other forms of therapy (2, 3). A meta-analysis of trials published up to 2005 (4) revealed that, when given in combination with pharmacotherapy, DSPs significantly reduce risk of any type of mood relapse over one- to two-year periods [odds ratio (OR) = 0.57, 95% confidence interval (CI): 0.39–0.82} and enhance social functioning (OR = 1.2, 95% CI: 0.3–2.1) relative to comparison conditions. Psychotherapy is now included in three major clinical practices guidelines for bipolar disorder (5–7).
Virtually all effective psychotherapies for BD incorporate significant elements of psychoeducation, and several studies have shown advantages of structured psychoeducational programs over nonspecific therapies of similar length (8, 9, 10). The key elements of psychoeducation are the provision of information about coping with the disorder, personalizing this information to the patient, and encouraging the practice of illness management and wellness maintenance strategies. There is, however, a significant efficacy/effectiveness gap: few evidence-based psychosocial interventions are being used in community settings (3). Certain treatment techniques – such as encouraging sleep/wake regularity, tracking medication adherence, or using a mood chart – are recommended in psychiatric treatment guidelines (e.g., 7) and in protocols designed for use in community mental health centers (e.g., 11, 12), but it is rare to find clinicians who routinely offer full courses of manual-based family-focused therapy, interpersonal social rhythm therapy, CBT, or group psychoeducation.
There are several impediments to the community implementation of DSPs for BD. First, most of these treatments are lengthy (between 12 and 21 or more sessions over periods of up to 12 months) and often too costly to provide in community care. Second, many agencies hire clinicians with little or no training in working with patients with BD and are not adequately funded to provide intensive training in evidence-based procedures. Third, several of the DSPs depend on regular (i.e., daily or at least weekly) paper-pencil tracking of moods or sleep patterns by the patient and clinician, which can prove difficult when patients are seen infrequently or travel over long distances.
This article describes the development of a five-session treatment called Facilitated Integrated Mood Management (FIMM), which incorporates many of the common elements of existing DSPs: (i) education for patients (and where relevant, family members) about the symptoms, causes, and treatment of BD; (ii) mood and sleep monitoring and management strategies; (iii) identifying prodromal signs of relapse and implementing early intervention plans; (iv) education and problem-solving related to medication usage and adherence; and (v) information about the role of alcohol or substance abuse in mood dysregulation. The treatment was designed to be administered by clinicians (hereafter referred to as facilitators) with relatively little experience with either BD or psychotherapy.
New models of intervention implementation emphasize distilling complex interventions into components that can be flexibly delivered in different settings (13). Development of FIMM was primarily guided by the UK Medical Research Council’s guidelines on Developing and Evaluating Complex Interventions (14). These guidelines provide a translational framework that includes several stages: (i) developing an intervention (identifying the evidence base, defining a coherent theoretical framework, and modeling of process and outcomes); (ii) piloting and feasibility testing (estimating recruitment and retention, determining sample sizes); (iii) evaluation (assessing effectiveness and cost-effectiveness in an experimental design, understanding change processes); and (iv) implementation (community dissemination, monitoring, and long-term follow-up). This article reports on the first two stages of this process. Specifically, we describe the development of the FIMM manual, the characteristics of the facilitators and training procedures, and the content of the sessions. We present pilot data on treatment retention, facilitators’ fidelity to the manual, patients’ symptom scores over 120 days of observation, and changes in patients’ self-reported knowledge of mood management strategies.
A novel element of FIMM is the incorporation of a system of electronic mood monitoring using the True Colours Monitoring System (15, 16). This system queries the patient in the form of daily or weekly text messages or e-mails requesting self-ratings of mood and sleep, enabling the patient and facilitator to observe fluctuations over a given treatment interval and facilitating personalized medication decisions or lifestyle adaptations. In a previous study, we found that patients were highly compliant with weekly text message or e-mail requests for ratings on depression and mania rating scales, and that longitudinal findings over an average of 36 weeks resembled longitudinal findings from observational studies (15). A secondary purpose of this article was to describe the True Colours system of mood monitoring within the context of a brief program of psychoeducation.
Patients and methods
Participants
Treating psychiatrists in the outpatient mood disorders program of the Warneford Hospital, University of Oxford, Oxford, UK and Oxford Health Foundation NHS Trust approached patients under their care who met the following eligibility criteria: (i) age 16 years or older; (ii) a diagnosis of bipolar I (BD-I) or bipolar II (BD-II) disorder; (iii) in regular follow-up care with the psychiatrist; (iv) not in simultaneous psychotherapy with another provider; (v) not in an acute episode of mania or mixed illness; and (vi) willing to use mobile phone text messaging or Web form e-mail. Written informed consent was obtained prior to the first FIMM session. This study was approved by the Local Research Ethics Committee.
Development of FIMM
We sought to design a brief treatment that would be practicable in most community health centers. As a first step, we undertook a survey of 31 principal investigators and therapists who took part in one of 14 published randomized trials of psychotherapy for BD published before 2008 (17). We coded the treatment elements that were present in all or most forms of manual-based therapy, and elements that were characteristic of certain treatments more than others. The results are summarized in Table 1. We were struck by the greater commonalities than differences among the treatment models, at least as reported by the clinicians who administered them.
Table 1.
Common and specific elements of psychosocial treatments for bipolar disorder (17)
Common elements (present in all or most experimental modalities)
|
Specific elements (present only in one or two experimental modalities)
|
We developed the FIMM manual to include all eight common elements. Specific elements, such as behavioral activation exercises (i.e., pleasant events scheduling), were incorporated when patients had depressive symptoms and were experiencing significant social withdrawal. Written psychoeducational materials and homework assignments were given throughout the program.
The intervention was first developed in a 14-case open trial (Pilot I), with ongoing iteration and refinement, and informed by qualitative data gathered from patients on using the True Colours system and their experiences in working with the facilitators (18). Development took place in formal, multidisciplinary meetings and local supervision meetings of a research team at Oxford University (the OXTEXT team) and during weekly or biweekly internet supervision sessions involving the facilitators and the clinical supervisor (DJM). Changes or refinements to the intervention were then tested in a second open trial (Pilot II) with five new participants.
Treatment facilitators
The four female facilitators who administered the experimental treatment in Pilots I and II were ages 24, 25, 26, and 56. Two had Psychology Bachelor’s degrees, one a Masters degree, and one a D.Phil. None had formal clinical qualifications or professional registration as a therapist. All worked at the Oxford University Department of Psychiatry and had variable durations of research experience with clinical populations (1.5, 3.0, 3.5, and 15 years). The first author (DJM) and two senior psychiatrists (JRG and GMG), who specialize in BD, trained the facilitators in administering FIMM in an initial three-day workshop. Training included lectures, videotaped examples, and role-playing of various treatment scenarios with coaching on effective responses. Over the next six months, the clinical supervisor provided biweekly supervision to the facilitators in a group setting using the electronic videoconferencing system Skype and e-mail.
True Colours system for monitoring mood
To be eligible for this pilot study, potential participants needed to be enrolled in the True Colours system and to have completed at least one month of mood monitoring before undertaking FIMM. The latter criterion over-selected for patients who were compliant with the texting procedures and perhaps, treatment in general. However, many such patients were markedly symptomatic and these symptoms became a focus for determining the short term outcome of the FIMM intervention.
The True Colours mood monitoring system, which uses text or e-mail messages (by patient preference) and validated self-rating scales of mood, was developed by clinicians and software engineers at the University of Oxford. Weekly text or e-mail messages from a central mobile phone prompt participants to complete weekly ratings on the five-item Altman Self-Rating Mania (ASRM) scale (19) and the 16-item Quick Inventory of Depressive Symptoms–Self Report (QIDS-SR) scale (20). Participants who used the text messaging option responded to text prompts with the letter ‘A’ indicating they were replying to the ASRM, or with the letter ‘Q’ indicating they were replying to the QIDS-SR, and then with a sequence of digits (5 for the ASRM, 16 for the QIDS-SR) corresponding to item responses. Rather than receiving the scale questions in each text message, the participants were supplied with wallet-sized versions of the rating scales with instructions on how to complete the ratings. For example, a participant completing the QIDS-SR would receive a text prompt, look at his or her rating card, and reply to the prompt ‘Q’ with numerical ratings for each of the 16 items (e.g., Q0330200001101111). If the participant’s text message response contained errors (e.g., too few responses, scores out of range), the system sent a reply requesting that the participant resubmit his or her responses. If the participant did not reply at all when first prompted, a reminder message was sent the following day and then again on a third day. If the third message did not prompt a response, the system did not send any more reminders and charted a ‘nonresponse’, but prompts were sent on subsequent weeks.
Participants who preferred the e-mail option received an e-mail prompt contained a Web link to open the same questionnaires. The default system sent prompts for entry every seven days. Data were stored on a central computer in the form of raw ASRM and QIDS-SR scores for each week of the study, as well as graphically, to display mood fluctuations over time. Graphical charts were sent on a weekly basis to the participant, the facilitator, and the treating psychiatrist.
The QIDS-SR is a 16-item measure of depression severity which covers the nine DSM-IV-TR (21) symptoms related to the diagnosis of major depressive disorder. Participants were asked to rate each symptom on a 0–3 scale covering the previous seven days. Depression scores on the QIDS-SR correspond to five levels of severity: none: 0–5; mild: 6–10; moderate: 11–15; severe: 16–20; very severe: 21–27. Scores < 6 typically indicate euthymia. The QIDS-SR has established psychometric properties for rating depressive symptom severity in individuals with major depressive disorder (22) and BD (23). It does not purport to distinguish between dysthymic and major depressive mood states.
Participants rated the frequency and severity of their mania-related symptoms over the course of the previous week on five individual ASRM scales that each ranged from 0–4. Total ASRM scores range from 0–20, where scores of ≥ 6 indicate significant manic or hypomanic symptoms. The ASRM has established psychometric properties for detecting mania among bipolar patients (19, 23) and is sensitive to treatment effects among patients with severe and psychotic manic or mixed episodes (23).
Because scale items do not measure the duration or functional impairment associated with symptoms, the ASRM does not distinguish between mania and hypomania. ASRM scores were used as continuous variables in order to examine the weekly severity of (hypo)manic symptoms. In a prior study using True Colours, dimensional QIDS-SR and ASRM scores distinguished between BD-I and BD-II patients and between male and female patients over an average of 36 weeks (15).
Trends in QIDS-SR and Altman scores over the 120-day study period were estimated using linear mixed models, with random effects for subject and trend over time, and an unstructured variance-covariance matrix for the random effects (24). Models were fit in Stata v11.2 using restricted maximum likelihood. Normality of residuals and random effects were checked graphically, as were the linearity of the effects.
Daily adaptation of True Colours mood monitoring
A limitation of any weekly mood rating system is that daily fluctuations in mood – often indicating emergent signs of an episode – are lost when averaging across a number of days (25). In developing FIMM, we experimented with a more fine grained system of daily mood monitoring. The system was based on an analogue study in which people at high behavioral risk for BD provided daily mood ratings within an experience sampling framework (16).
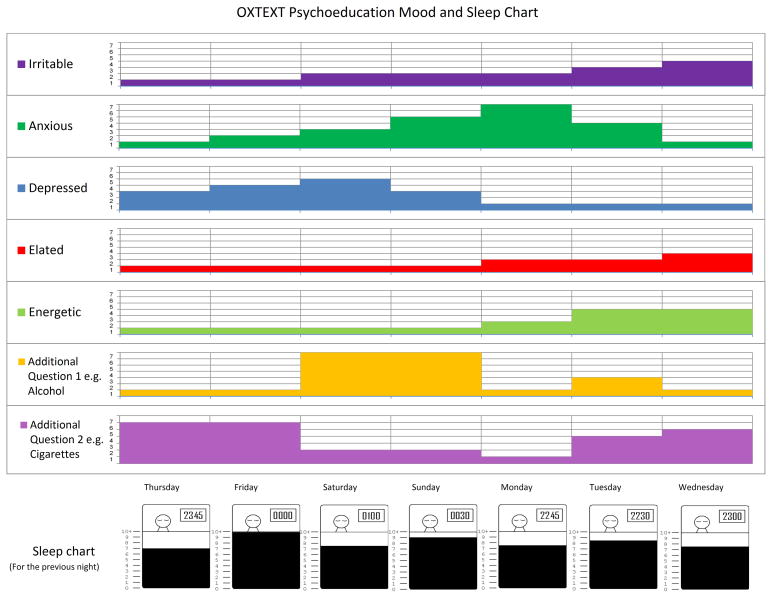
During the first psychoeducation session, participants were invited to supplement their weekly QIDS-SR and ASRM ratings with daily mood and sleep monitoring, which involved responding to two additional daily text messages or e-mails. One message asked participants to rate five moods (irritable, anxious, depressed, elated, energetic) on a scale of 1 (not at all) to 7 (very much) in terms of how they had felt in the previous 24 hours. The second message, sent at either 10 am or 12 noon asked participants to report their bedtime and the number of hours they had slept the previous night. If participants did not respond to the messages, or their response was not understood by the system they were sent another text message or e-mail prompting them to re-send their scores. Following the method piloted by Malik et al. (16), graphic charts of patients’ responses over the previous seven days were drawn and used in the sessions to make links between mood and sleep variability and as a prompt for participants to recall associated stressors in the prior week (see example in Fig. 1). Daily mood monitoring was no longer required after the psychoeducation sessions had ended.
Fig. 1.
OXTEXT Psychoeducation Mood and Sleep Chart (example).
FIMM protocol
The sections below describe the five-session FIMM protocol administered to patients in both pilot trials. A facilitator’s manual was used to guide the content of each session, alongside a range of handouts and worksheets for participants to complete during and between sessions.
The treatments offered in the two pilots differed in several ways. First, in Pilot I, patients were invited to take part in six weekly sessions, with Session 5 designated as a personalized, ‘free-form’ session to address comorbid disorders or longstanding interpersonal problems that were outside of the psychoeducational agenda. These typically included anxiety disorders, substance or alcohol abuse, chronic low self-esteem, relationship problems, or work or family conflicts. The rationale for placing this session just prior to the final session was that novel strategies for mood management might be revealed in a personalized session and would inform the mood management plan finalized in the sixth session. However, it quickly became clear that one session was not enough to address these issues adequately. Moreover, novice therapists were often not prepared to explore interpersonal problems in depth. Thus, in Pilot II, the personalized session was omitted such that the protocol was reduced to five sessions. Patients in both pilots were given referral information regarding additional specialized services for associated problems (e.g., CBT for anxiety disorders, couple therapy).
Second, in Pilot II a shared written agenda (in the form of summary bullet points) for each session was added to help the facilitator and patient develop a collaborative session plan and stay on track. A patient workbook was added to increase the involvement of patients during and between sessions. Paralleling the facilitator’s manual, it contained the handouts and worksheets for each session with a brief outline of each topic and its relevance to managing moods. This manual provided a greater structure for discussions of daily and weekly mood fluctuations, environmental triggers, and preventative strategies.
Involvement of caregivers
Although the content and format of FIMM sessions were organized primarily for the individual, participants were encouraged to invite a caregiver (spouse, parent or close friend) to sessions. Caregivers assisted the patient in identifying early warning signs of significant mood changes, risk and protective factors (e.g., one caregiver said to her husband, a teacher: ‘your mood starts changing when the school year starts, and then it changes again during Christmas’), or response strategies to use in emergency situations (e.g., agreeing to call the physician if the patient had escalating signs of mania; avoiding discussing certain ‘hot topics’ when the patient was unstable). Generally, the facilitator did not examine the history or current manifestations of couple or family relationship problems unless these problems clearly impeded the development of a mood-management plan (for example, a husband’s resentment of his wife’s reminders to take his medications). In such cases, the facilitator assisted the couple or family in developing alternative strategies that were less likely to ignite conflicts (for example, encouraging the patient to develop his own system for remembering medicines). Contact between the facilitator and caregiver was kept to a minimum between sessions. The facilitator made clear that FIMM did not replace couple or family therapy, and provided referrals as needed.
Session one: identifying the relapse signature
Facilitators began by ‘joining’ and expressing interest in the patient as a person (26). This included a brief discussion of the patient’s own goals for treatment, how the goals of psychoeducation could be adjusted to mesh with these goals, and a preview of the sessions to follow. Facilitators explained that the major objective of psychoeducation was to develop a mood management plan to curtail the severity or reduce the duration of new mood episodes. The plan, consisting of a description of early warning signs, eliciting stressors, and possible prevention or emotion regulation techniques, would be built on a week by week basis and finalized in the last session.
First, the facilitator reviewed with the patient his or her prior manic and depressive episodes, with a focus on the sequential development of symptoms: which came first, second, and third and constituted the likely prodromal signature of new recurrences. Signatures could be quite different for mania (e.g., decreased sleep duration) and depression (e.g., rumination, inactivity). When caregivers participated, they were asked to recount their perceptions of how the patient’s prior episodes had developed.
Facilitators explained that mood episodes come about due to interactions between genetic vulnerabilities, biological vulnerabilities, individual behaviors (e.g., substance abuse), and environmental stressors. They explained that mood fluctuations could include subsyndromal mood swings as well as fully syndromal episodes, and that stressors could include episodic events (e.g., losses) or chronic environmental strains (e.g., ongoing family conflict). Patients were asked for examples of environmental factors or individual risk behaviors that appeared to play a role in past episodes (examples included ‘arguments with my wife,’ ‘missing a lot of sleep because of studying,’ or ‘drinking too much’).
Toward the end of session one, facilitators explained the procedure for tracking daily moods:
“Monitoring your mood and sleep every day may give us both insight into how much your moods tend to fluctuate and what factors in your life are associated with these fluctuations. Every day you will receive one text/e-mail message at some point between 4 pm and 5 pm asking you about your daily mood. Please text or e-mail us and tell us to what degree over the last 24 hours you have experienced each of the following five moods on a scale of 1 (not at all) to 7 (very much): Irritable, Anxious, Depressed, Elated, and Energetic.
Every morning you will also receive a separate text/e-mail message about your sleep the previous night. It will arrive at either 10 am or 12 noon. Please send a text or e-mail stating what time you went to bed last night. Finally, inform us of the number of hours you slept (to the nearest half hour).”
The facilitator then assisted the patient in registering for daily mood and sleep monitoring and practicing responding to the texts on his or her mobile phone or via e-mail on a local computer.
Session two: reviewing risk and protective factors
Facilitators began by reviewing the daily mood ratings and weekly (i.e., QIDS-SR, ASRM) symptom scale results from the previous week, emphasizing the link between mood changes and eliciting stressors. Then, they introduced the theme of the second session:
“We believe that individuals are better able to manage their mood swings when they can identify risk and protective factors. Risk factors and protective factors are those things that increase or decrease your chances of getting sick or having mood swings.”
Patients identified risk factors (e.g., substance or alcohol abuse, chaotic sleep habits) and protective factors (e.g., supportive family relationships, medication adherence, following strict sleep/wake habits) in their prior illness history. This discussion built on the themes introduced in the first session. For each risk factor, the facilitator asked the patient what she/he had learned about the relevance of the factor to his or her illness management (e.g., “So what have you concluded about your sleep/wake cycle?” “Any ideas of how your family could be more helpful when you’re depressed?”). Once again, input from caregivers on observations about risk or protective factors was solicited. The session concluded with a homework assignment – filling in a Risk and Protective Factors summary handout – and the instruction to continue with daily and weekly mood monitoring.
Session three: daily rhythm and sleep/wake regulation
This session emphasized the key role of regular daily routines and sleep/wake cycles in the course of BD (10, 27–28). At this stage, the patient had contributed two weeks of daily mood and sleep data via the True Colours monitoring system. Facilitators examined the mood and sleep charts with patients to identify the nature of any irregularities. These relationships generally fell into one of the following categories: (i) decreased sleep associated with increases in energy, elation, or irritability in the subsequent days; (ii) increased sleep associated with worsening depression or irritability; or (iii) changes in mood preceding changes in sleep.
Patients were asked to discuss any major or minor stressors that might have been associated with changes in mood or sleep. If family members or friends were present, they often added observations of their own (e.g., “she seemed agitated by the tax forms she was going to fill out the next day;” “when he starts ruminating he just sleeps and keeps to himself”). The facilitator then suggested strategies for normalizing sleep/wake and daily rhythms: (i) getting up/going to bed at the same time every weekday and on weekends; (ii) having a one-hour wind-down time before bed; (iii) avoiding caffeine or stimulants late in the day; (iv) exercising (a minimum of four hours before bedtime); (v) eating at regular times; (vi) using self-relaxation or meditation exercises; (vii) and asking assistance from family members in maintaining sleep routines (28). At the end of the session, patients were asked to make a list of sleep management strategies to practice, and to keep a log of which activities contributed to sleep problems in the forthcoming week, so that these activities could be examined in relation to daily mood ratings.
Session four: the role of medications and substance/alcohol abuse
Facilitators reviewed with the patient the medications in his or her regimen and asked the patient to discuss any concerns or misgivings he or she had about them. For example, some patients were confused as to why they were being treated with an atypical antipsychotic agent, despite having no psychotic symptoms. Patients’ or caregivers’ beliefs about medications (e.g., “I think they’re a crutch,” “I’ll eventually be able to go off of all of them”) were discussed and gently challenged if they were based on misinformation. The burden of side effects was acknowledged and, where appropriate, patients were offered advice on how to approach discussions or questions about medication with their psychiatrists. Facilitators assisted patients in developing plans to keep track of pills taken each day and to remember to refill prescriptions.
For a subset of patients, session four focused on substance or alcohol abuse. Generally, facilitators took a didactic stance, explaining that substances can worsen mood symptoms, and mood symptoms can increase the drive toward substances. The role of substance misuse in the patient’s prior manic or depressive episodes was reviewed. Caregivers were often helpful in providing examples that had been forgotten by the patient. Homework focused on keeping track of substance use during the forthcoming week so that the linkage between drinking, drug abuse, mood swings, and sleep disturbance could be clarified (see Fig. 1 example).
Session five: finalizing the mood management plan
The goal of the final session was to generate a written summary of the patient’s early warning signs, risk and protective factors, and behavioral and/or pharmacological strategies to undertake when symptoms escalated. The patient was encouraged to share the plan with the treating psychiatrist and any caregivers who had not attended, and to update it as new information (i.e., responses to medication adjustments) became available. Participants discussed obstacles to putting the plan into practice. For the three months following the final session, facilitators scheduled biweekly and then monthly half-hour phone calls to check in with the patient and determine whether s/he had been implementing the plan.
Treatment fidelity ratings
The FIMM supervisor (DJM) listened to approximately 50% of the audiotaped sessions for each case. He applied fidelity ratings using a modified version of the Therapy Competence and Adherence Scale (TCAS) (29) consisting of 21 Likert-type (range: 1–7) items covering various domains of clinician behavior: (i) effective provision of psychoeducation; (ii) explaining the mood monitoring strategies; (iii) assignment of homework; (iv) exploring prodromal symptoms; (v) developing prevention plans; and (vi) problem-solving about sleep/wake monitoring. The scales also covered nonspecific factors (rapport and alliance-building skills, pacing, session command, taking a didactic stance, addressing patients’ emotional reactions to the material) and proscribed strategies (e.g., psychoanalytic interpretations). For the present study, an overall one to seven score summarizing facilitators’ fidelity across domains was derived for each session. Inter-rater reliability for TCAS items ranged from 0.74 to 0.98 (intraclass rs, p’s < 0.001).
Bipolar Mood Management Questionnaire (BMMQ)
We developed the BMMQ (available from the corresponding author) to measure change in knowledge and behavior relevant to self-care in BD, and to guide the focus of the FIMM by establishing gaps in the patients’ knowledge base. The questionnaire comprised 20 items, such as ‘I know which early symptoms show that I am at risk of mood elevation’ or ‘At least one other person knows about my relapse prevention plan and its contents’. Patients rated their level of agreement with each statement on scales ranging from 0 = disagree through 1 = disagree a little, 2 = neither agree nor disagree, 3 = agree a little, and 4 = agree. Patients completed the questionnaire immediately prior to each of the five treatment sessions.
Cronbach’s alpha was calculated from week 1 data to assess internal consistency; values between 0.70 and 0.90 were regarded as optimal. Week 1 questionnaire data were compared with data from the last available questionnaire (either week 5 or week 6). When week 1 data were missing, the next observation was carried back, and when final week data were missing, the last observation was carried forward. The mean and standard deviation (SD) of the total knowledge score and the individual item scores were calculated at the first and last weeks. A pairwise t-test was conducted to determine the statistical significance of the change in total scores.
Results
Sample characteristics
Potential FIMM participants were identified from the cohort of patients already enrolled in the True Colours mood monitoring system (N = 114). Information about the FIMM pilot study was given to 29 potential participants from that cohort who had submitted mood ratings for at least one month and were identified by their clinician as being suitable for the treatment. Of the 29 who were approached, one declined for no reason, two declined due to the travel required, and seven did not reply; 19 agreed (65.5%).
Pilot I (n = 14) and Pilot II (n = 5) consisted of six male (31.6%) and 13 female (68.4%) patients who were on average 37.2 ± 11.7 years old (Table 2). Patients had been using the True Colours system for between one and 44 months, with a median of 20 months of prior use (interquartile range: 7.5–30.0). Patients were referred to the program with diagnoses of BD-I (n = 14) and BD-II (n = 5) by DSM-IV-TR criteria (21). Nine of the 17 patients (52.9%) included a family member or friend/partner in at least one of the sessions.
Table 2.
Demographic and clinical features of the patient sample
| Demographics | |
| Age, mean (SD) | 37.2 (11.7) |
| Sex, female, n (%) | 13 (68.4) |
| Diagnosis, n (%) | |
| Bipolar I disorder | 14 (72.2) |
| Bipolar II disorder | 5 (27.8) |
| Ethnicity, n (%) | |
| White | 18 (94.7) |
| Non-white | 1 (5.3) |
| Education, n (%)a | |
| Some secondary | 2 (11.1) |
| Secondary | 2 (11.1) |
| Undergraduate degree | 8 (44.4) |
| Post graduate | 6 (33.3) |
| Medication, n (%)a | |
| Antidepressants | |
| Ever | 17 (94.4) |
| Currently | 0 (0) |
| Antipsychotics | |
| Ever | 15 (83.3) |
| Currently | 2 (11.1) |
| Anxiolytics | |
| Ever | 13 (72.2) |
| Currently | 2 (11.1) |
| Mood stabilizers | |
| Ever | 17 (89.5) |
| Currently | 6 (31.6) |
| Previous therapy, n (%) | |
| Cognitive behavioral therapy | 15 (83.3) |
| Other | 13 (72.2) |
| Depression historyb | |
| Any episodes > 2 weeks, n (%) | 14 (82.4) |
| Rapid cycling, n (%) | 2 (11.8) |
| No. of hospitalizations, median (IQR) | 0 (0–1) |
| Years of impairment, median (IQR) | 15 (8–20) |
| Mania historyc | |
| Any episodes > 4 days, n (%) | 13 (81.3) |
| Rapid cycling, n (%) | 2 (11.8) |
| No. of hospitalizations, median (IQR) | 0 (0–0) |
| Years of impairment, median (IQR) | 7.5 (3.8–17.0) |
SD = standard deviation; IQR = interquartile range.
Data missing for one participant.
Data missing for two participants.
Data missing for two participants (rapid cycling, no. of hospitalizations) and for three participants (episodes > 4 days, years of impairment).
Two patients (10.5%), neither of whom had invited caregivers, withdrew from the FIMM program after three sessions. One did not give a reason and could not be contacted further. The other, who had just completed a course of CBT and felt that FIMM would not add significantly to her existing mood management plan, dropped out of treatment but continued with the follow-up.
Thus, her data were included in the longitudinal analyses. All of the remaining patients (n = 17) completed the six-session (Pilot I) or five-session (Pilot II) treatment. There was variability in the time required to complete the program (mean± SD: Pilot I: 9.2 ± 3.4, range: 5–17 weeks and Pilot II: 7.6± 0.9, range: 7–9 weeks).
Of the 19 patients, eight (42.1%) exclusively used the text message-based True Colours system, nine (47.4%) exclusively used the Web form version, and two (10.5%) used a combination of Web form and text messaging. Patients responded to an average of 81% of the daily text or e-mail prompts sent over the course of the treatment. Follow-up data were available for 18 of the 19 participants (94.7%).
Treatment fidelity
Fidelity ratings (TCAS measure) were based on 55 session tape ratings of the four facilitators, each treating four to five patients (mean 2.89 ratings/case). Although the facilitators had minimal training, they maintained high levels of fidelity across cases. The overall fidelity index, which could vary from 1 (very poor) to 7 (excellent), averaged 5.7 ± 0.7 on the first session, 5.3 ± 0.9 on the third session, and 4.9 ± 1.2 on the fifth session, indicating fidelity in the good to very good range. Of 55 sessions, 43 (78.2%) were above the threshold of 5 (good) for acceptability on the overall fidelity index.
Symptom trajectories
Assessments of symptoms were based on the QIDS-SR and ASRM scores provided weekly by patients. In the first four weeks before initiation of the FIMM sessions, the 19 participants provided an average of 3.5 scale ratings. The mean QIDS-SR score in the four weeks prior to FIMM was 7.4 ± 5.6 (scale range: 0–27) and the median ASRM score was 1 (interquartile range = 3, scale range: 0–20). Of the 19 patients, 10 (52.6%) had at least one week with a QIDS-SR score ≥ 10 over the four weeks prior to treatment, and seven (36.8%) had at least one week with an Altman score ≥ 5. Four patients (21.1%) had at least one week with significant symptoms on both scales, and six (31.6%) did not show a one-week pretreatment elevation on either scale.
Figures 2 and 3 show the trajectory of QIDS-SR and Altman scores over the 120-day period following the first FIMM. Participants averaged 88% of their expected responses to the scale queries per week over the 120-day observation period. As indicated in Figure 2, there was considerable variability in QIDS-SR total scores. The estimated trend indicated a reduction of −0.11 points on the QIDS-SR per week (95% CI: −0.33 to 0.10). The estimated trend for ASRM scores (Fig. 3) indicated an average increase of 0.08 scale points per week (95% CI: −0.05 to 0.21). These data are provided for illustrative purposes only, as they did not meet the normality assumptions of an untransformed mixed model analysis.
Fig. 2.
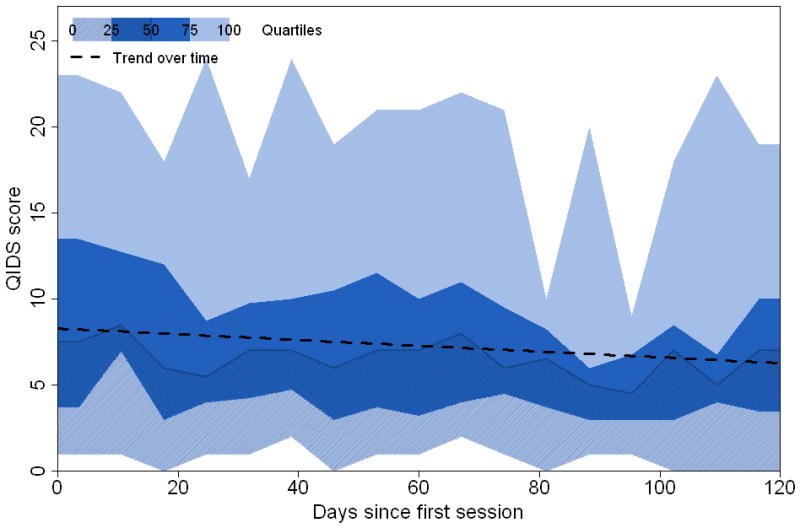
Percentiles and trend line of Quick Inventory of Depressive Symptoms–Self Report (QIDS-SR) scores. Shadings refer to weekly quantiles of the scores: minimum, 25th percentile, median, 75th percentile, and maximum.
Fig. 3.
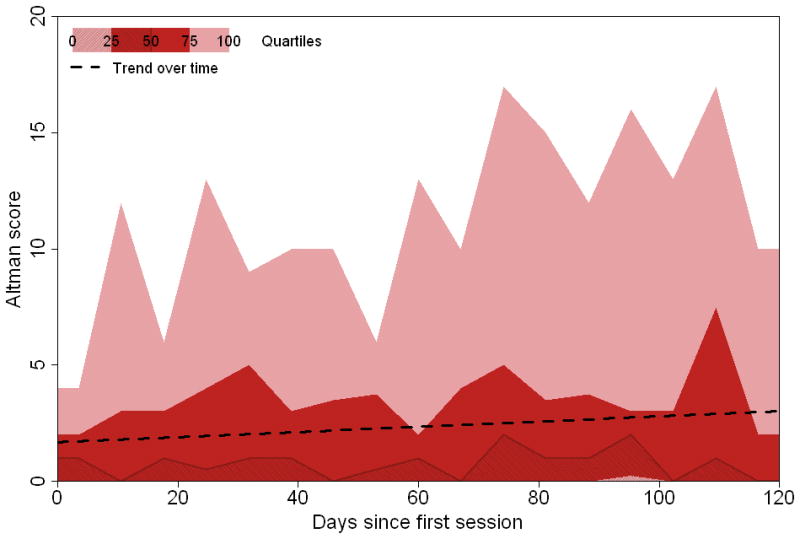
Percentiles and trend line of Altman Self-Rating of Mania scores. Shadings refer to weekly quantiles of the scores: minimum, 25th percentile, median, 75th percentile, and maximum.
Changes in patients’ knowledge of mood management strategies
Cronbach’s alpha for the 20 items of the BMMQ at week 1 was 0.76, indicating good internal consistency. The mean total score was 54.2 ± 8.8 at week 1 and 66.9 ± 9.5 at the last week of FIMM treatment. The increase in this total knowledge score was statistically significant (mean change +12.8, 95% CI: 9.5–16.1; t = −8.19, df = 18, p < 0.001; Cohen’s d = 1.39). Raw scores for individual items at the first and last week, and mean change in those scores are given in Table 3.
Table 3.
Bipolar Mood Management Questionnaire (BMMQ): individual item scores at the first and last week of treatment
| Item | First week | Last week | Change | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| 1 | I know which early symptoms show that I am at risk of mood elevation | 3.11 (1.15) | 3.58 (0.51) | + 0.47 (1.12) |
| 2 | I know which early symptoms show that I am at risk of mood depression | 3.42 (0.90) | 3.58 (0.61) | + 0.16 (0.83) |
| 3 | I know which things in my life help to keep me well | 3.37 (0.90) | 3.63 (0.60) | + 0.26 (0.65) |
| 4 | I know which things in my life may increase my risk of mood disturbance | 3.47 (0.61) | 3.79 (0.42) | + 0.32 (0.58) |
| 5 | I know how to monitor my mood | 2.74 (1.10) | 3.32 (1.06) | + 0.58 (0.90) |
| 6 | I know how to distinguish between a normal low mood and the start of depression | 1.58 (0.96) | 2.37 (1.26) | + 0.79 (1.27) |
| 7 | I know how to distinguish between a normal high mood and the start of hypomania/mania | 1.53 (1.02) | 2.89 (0.88) | + 1.37 (1.01) |
| 8 | I know how to monitor my sleep | 2.58 (1.31) | 3.58 (0.61) | + 1.00 (1.11) |
| 9 | I know why monitoring my sleep is important | 3.63 (0.76) | 3.84 (0.50) | + 0.21 (0.86) |
| 10 | I know how to improve my sleep if it is disturbed | 2.42 (1.12) | 3.32 (0.89) | + 0.89 (1.29) |
| 11 | I know what medicines to take to help control my mood | 3.53 (0.77) | 3.63 (0.83) | + 0.11 (0.94) |
| 12 | I know why I need to take medicines to control my mood | 3.79 (0.54) | 3.79 (0.54) | + 0.00 (0.33) |
| 13 | I know how to keep track of how regularly I am taking my mood medicines | 3.42 (0.90) | 3.58 (0.90) | + 0.16 (0.60) |
| 14 | I know about how genes affect my illness | 2.42 (1.31) | 3.53 (0.61) | + 1.11 (0.94) |
| 15 | I know how to monitor my use of alcohol and street drugs | 3.11 (1.15) | 3.42 (1.07) | + 0.32 (0.75) |
| 16 | I know that alcohol and street drugs can make my mood more unstable | 3.89 (0.46) | 3.84 (0.50) | −0.05 (0.23) |
| 17 | I know what to do when my mood starts to deteriorate (early stages) | 2.53 (1.22) | 3.21 (1.03) | + 0.68 (0.82) |
| 18 | I know what to do when my mood has deteriorated significantly (later stages) | 2.21 (1.55) | 2.95 (1.35) | + 0.74 (1.14) |
| 19 | I have a copy of my relapse prevention plan and keep it in a safe place | 0.63 (1.12) | 2.79 (1.51) | + 2.16 (1.68) |
| 20 | At least one other person knows about my relapse prevention plan and its contents | 0.79 (1.36) | 2.32 (1.64) | + 1.53 (1.84) |
| Total | 54.20 (8.80) | 66.90 (9.50) | + 12.80 (9.10) | |
Discussion
This paper describes a brief psychoeducational treatment (FIMM) for patients with BD. The treatment, which incorporates key components of evidence-based psychosocial treatments, was offered to patients with BD-I and BD-II in conjunction with pharmacotherapy and an electronic mood monitoring program as a means of identifying and intervening with mood exacerbations. Results suggest that patients could be engaged and retained in the brief program, were compliant with the daily and weekly versions of mood monitoring, and showed relatively stable mood scores over the course of a four-month interval. The facilitators were relatively inexperienced, with only a three-day training workshop followed by biweekly supervision sessions conducted through videoconferencing. Nonetheless, the manualized program was delivered with high levels of treatment adherence and competence.
Although the degree of symptomatic change was nonsignificant over the brief study period, it is worth noting that the trajectory of patients’ depressive symptoms was toward improvement. A decrease in QIDS-SR scores among BDI-I and BD-II patients was also observed in our first True Colours study (12). Equally noteworthy in the present study was a numerical increase in (hypo)mania scores over this same interval. It is possible that these decreases or increases reflect regression to the mean among patients who were selected to have minimal symptoms at the beginning of the protocol, although a control arm would have been necessary to test this hypothesis. It is also possible that the ASRM captures increases in positive emotion as well as clinically significant (hypo)mania symptoms in a proportion of patients. Examination of the degree to which week-to-week increases in elevated mood (as reported through text messaging or e-mail) covary with changes in the behavioral symptoms of mania may shed light on the clinical significance of minor fluctuations in ASRM scores.
Few studies have used computer technology to collect mood data from bipolar patients. Schärer et al. (30) adapted the National Institute of Mental Health Prospective Life-Chart Form for use on a handheld computer and found that patients preferred the device to paper and pencil charting, and reported less social stigma when using the device in public. Bauer et al. (31) recruited 80 individuals to use a software system installed on their home computers to record mood, medication, and sleep data. Participants only missed 6.1% of the 114 days for which data were requested. Chinman et al. (32) compared in-clinic, computer-assisted, self-report mood ratings provided by 45 individuals with bipolar disorder to mood ratings made by trained interviewers, and found high correlations between the two sources.
Taken together with the observed compliance rate of 88% in the present study, it can be concluded that patients with BD adopt technology-assisted mood monitoring methods at least as readily as paper and pencil methods. However, we do not know whether adoption and compliance will be satisfactory in all age groups, socioeconomic classes, or countries, or in patients with different comorbid disorders or levels of illness severity or chronicity. Future research should examine which subgroups of patients are most amenable to electronic mood monitoring, and which subgroups require more extensive coaching.
Patients reported increases in their awareness of mood management strategies from the beginning to the end of FIMM treatment. Items on the BMMQ showing the largest change included the ability to distinguish between a normal pleasant mood and the beginning of a hypo(manic) episode, knowing how to monitor sleep and improve sleep consistency, awareness that BD runs in families, rehearsing a mood management plan, and informing significant others of the content of this plan. It is noteworthy that these content items were a focus of FIMM treatment. It is possible, of course, that patients could have gained similar knowledge from routine outpatient treatment or from reading a self-help workbook. Further, increases in knowledge about BD may not translate into increases in implementation of the relevant self-care behaviors. Randomized trials comparing FIMM to brief supportive interventions or bibliotherapy will be necessary to clarify whether changes in knowledge – leading to increased use of mood management strategies – mediate the effects of psychosocial interventions on the symptomatic course of BD.
The sample size was small (N = 19) and the follow-up period (120 days) limited. Thus, we cannot determine whether FIMM is associated with greater mood stability and longer delays prior to recurrence than usual care. It is also possible that regular mood monitoring alone may achieve the same effects as a facilitated program administered by mental health professionals. A randomized trial of the longer-term incremental costs and cost-effectiveness of combining FIMM with regular mood monitoring, as compared to mood monitoring alone, is currently underway.
The piloting in phase I convinced us that problems related to marital or family relationships, vocational disability, stigma, or low self-esteem could not be adequately addressed in this brief treatment model. Comorbid disorders, including substance abuse or dependence, personality pathology, or attention deficit hyperactivity disorder would also require more specialized (and more intensive) programs. We believe there are limits to all manualized approaches to patient care and a generic psychoeducation package is never going to supplant personalized therapist care in more complex co-morbid cases. Nevertheless, our hypothesis remains that the approach described here is a necessary and sometimes sufficient foundation for effective psychosocial intervention. There are advantages to having a well-specified generic treatment that is deliverable by the larger number of mental health professionals with basic rather than advanced level therapy experience. In the future it might be possible to develop additional extended modules for specific comorbidities.
FIMM is not free of clinician training costs, nor are the costs of technical assistance for supporting text-based data entry by patients negligible. Nonetheless, the training and time to administer the treatment is an order of magnitude less than other forms of manualized therapy. Also, having a simple clinicians’ manual may facilitate training of a wider base of mental health professionals without the level of psychosocial expertise needed to implement specialized interventions. Hence, FIMM may be more feasible to administer in mental health systems that cannot regularly offer patients full courses (e.g., 6–9 months) of evidence-based therapy. Further evaluation of the FIMM approach is necessary to provide estimates of its overall resource requirements and the effects of its budget on implementation in community care centers.
We did not examine whether this brief treatment could have been made even more economical through group administration. Group psychoeducation has been found to be an effective adjunct to pharmacological treatment in relapse prevention and long-term symptom management, although existing group models usually require maintenance sessions for up to three years (8, 33, 34). Nonetheless, relapse prevention planning within a group setting, especially if the early warning symptoms and behaviors can be individualized, may provide a cost-effective alternative to FIMM.
In this study, approximately half of the patients attended treatment with a family member (typically a spouse or partner). Family members may be able to spot the onset of prodromal symptoms of mania or depression more rapidly and more accurately than patients. One study found that patients randomized to a 21-session family psychoeducational intervention were less likely to relapse, and were less likely to require hospitalization if they did relapse than patients randomly assigned to a 21-session individual psychoeducational treatment over a two-year follow-up (6). Future studies should examine whether facilitated mood monitoring is more effective when family members are engaged in the relapse prevention process.
A central premise of most forms of psychoeducation is that understanding the genetic and neural bases of mood disorder helps to engage patients as partners in treatment and reduce their experiences of illness stigma (17). The specific details in psychoeducational programs must be continuously updated as the science evolves, while acknowledging to patients the areas in which we have less certainty. In developing FIMM, we were aware that the science of bipolar disorder was changing rapidly. The main emphasis in this treatment, however, is less on providing information than skill development using the individual’s unique mood data.
In summary, this study suggests that a program of daily mood monitoring is a feasible adjunct to weekly monitoring of depression and mania symptoms. Although it is plausible that patients would have provided daily mood ratings without a facilitator, we suspect that the weekly facilitated discussions of day-to-day mood fluctuations, with education regarding the likely causes of those fluctuations (e.g., sleep changes, stress), increased the patients’ sense of self-efficacy regarding their ability to manage the disorder. Moreover, the skills for managing mood may become self-sustaining once patients learn what works for them.
Acknowledgments
This article presents independent research commissioned by the National Institute for Health Research (NIHR) under Research Programme Grant for Applied Research: RP-PG-0108-10087. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. EAH was supported by a Wellcome Trust Clinical Fellowship (Ref: 088217). DJM was supported by grants MH073871 and MH077856 from the National Institute of Mental Health, National Institute of Health.
Footnotes
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.National Collaborating Centre for Mental Health. Bipolar disorder: The management of bipolar disorder in adults, children and young people, in primary and secondary care. London: National Institute for Health and Clinical Excellence; 2006. [PubMed] [Google Scholar]
- 2.Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165:1408–1419. doi: 10.1176/appi.ajp.2008.08040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miklowitz DJ, Scott J. Psychosocial treatments for bipolar disorder: cost-effectiveness, mediating mechanisms, and future directions. Bipolar Disord. 2009;11 (Suppl 2):110–122. doi: 10.1111/j.1399-5618.2009.00715.x. [DOI] [PubMed] [Google Scholar]
- 4.Scott J. Psychotherapy for bipolar disorders-efficacy and effectiveness. J Psychopharmacol. 2006;20:46–50. doi: 10.1177/1359786806063078. [DOI] [PubMed] [Google Scholar]
- 5.Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord. 2005;7 (Suppl 3):5–69. doi: 10.1111/j.1399-5618.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 6.United States Department of Veterans Affairs and Department of Defense. [accessed Dec 15, 2011];Clinical Practice Guideline for Management of Bipolar Disorder in Adults. http://www.healthquality.va.gov/bipolar/bd_305_full.pdf.
- 7.Goodwin GM Consensus Group of the British Association for Psychopharmacology. Evidence-based guidelines for treating bipolar disorder: revised second edition --recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2009;23:346–388. doi: 10.1177/0269881109102919. [DOI] [PubMed] [Google Scholar]
- 8.Colom F, Vieta E, Sanchez-Moreno J, et al. Group psychoeducation for stabilised bipolar disorders: 5-year outcome of a randomised clinical trial. Br J Psychiatry. 2009;194:260–265. doi: 10.1192/bjp.bp.107.040485. [DOI] [PubMed] [Google Scholar]
- 9.Rea MM, Tompson M, Miklowitz DJ, Goldstein MJ, Hwang S, Mintz J. Family focused treatment vs. individual treatment for bipolar disorder: results of a randomized clinical trial. J Cons Clin Psychol. 2003;71:482–492. doi: 10.1037/0022-006x.71.3.482. [DOI] [PubMed] [Google Scholar]
- 10.Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- 11.Reiser RP, Thompson LW. Bipolar Disorder. Cambridge, MA: Hogrefe and Huber; 2005. [Google Scholar]
- 12.Bauer MS, McBride L. Structured Group Psychotherapy for Bipolar Disorder: The Life Goals Program. 2. New York: Springer; 2003. [Google Scholar]
- 13.Kilbourne AM, Neumann MS, Pincus HA, Bauer MS, Stall R. Implementing evidence-based interventions in health care: application of the replicating effective programs framework. Implement Sci. 2007;2:42. doi: 10.1186/1748-5908-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bopp JM, Miklowitz DJ, Goodwin GM, Stevens W, Rendell JM, Geddes JR. The longitudinal course of bipolar disorder as revealed through weekly text messaging: a feasibility study. Bipolar Disord. 2010;12:327–334. doi: 10.1111/j.1399-5618.2010.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik A, Goodwin GM, Holmes EA. Contemporary approaches to frequent mood monitoring in bipolar disorder. J Exp Psychopathol. doi: 10.5127/jep.014311. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miklowitz DJ, Goodwin GM, Bauer M, Geddes JR. Common and specific elements of psychosocial treatments for bipolar disorder: a survey of clinicians participating in randomized trials. J Psychiatr Pract. 2008;14:77–85. doi: 10.1097/01.pra.0000314314.94791.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell NC, Murray E, Darbyshire J, et al. Designing and evaluating complex interventions to improve health care. BMJ. 2007;3:455–459. doi: 10.1136/bmj.39108.379965.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman EG, Hedeker D, Peterson JL, Davis JM. The Altman Self-Rating Mania Scale. Biol Psychiatry. 1997;42:948–955. doi: 10.1016/S0006-3223(96)00548-3. [DOI] [PubMed] [Google Scholar]
- 20.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2000. (Text Revision) (DSM-IV-TR) [Google Scholar]
- 22.Trivedi MH, Rush AJ, Ibrahim HM, et al. The inventory of depressive symptomatology, clinician rating (IDS-C) and self-report (IDS-SR), and the quick inventory of depressive symptomatology, clinician rating (QIDS-C) and self-report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 23.Altman E, Hedeker D, Peterson JL, Davis JM. A comparative evaluation of three self-rating scales for acute mania. Biol Psychiatry. 2001;50:468–471. doi: 10.1016/s0006-3223(01)01065-4. [DOI] [PubMed] [Google Scholar]
- 24.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 25.Holmes EA, Geddes JR, Colom F, Goodwin GM. Mental imagery as an emotional amplifier: Application to bipolar disorder. Behav Res Therapy. 2008;46:1251–1258. doi: 10.1016/j.brat.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Miklowitz DJ. Bipolar Disorder: A Family-focused Treatment Approach. 2. New York: Guilford Press; 2008. [Google Scholar]
- 27.Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- 28.Frank E. Treating Bipolar Disorder: A Clinician’s Guide to Interpersonal and Social Rhythm Therapy. New York: Guilford Publications; 2005. [Google Scholar]
- 29.Weisman AG, Okazaki S, Gregory J, et al. Evaluating therapist competency and adherence to behavioral family management with bipolar patients. Fam Proc. 1998;37:107–121. doi: 10.1111/j.1545-5300.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- 30.Schärer LO, Hartweg V, Valerius G, et al. Life charts on a palmtop computer: first results of a feasibility study with an electronic diary for bipolar patients. Bipolar Disord. 2002;4 (Suppl 1):107–108. doi: 10.1034/j.1399-5618.4.s1.51.x. [DOI] [PubMed] [Google Scholar]
- 31.Bauer M, Grof P, Rasgon N, Gyulai L, Glenn T, Whybrow P. New computer based tool for the longitudinal study of bipolar disorder. Aspects of Affect. 2005;2:101–108. [Google Scholar]
- 32.Chinman M, Young AS, Schell T, Hassell J, Mintz J. Computer-assisted self-assessment in persons with severe mental illness. J Clin Psychiatry. 2004;65:1343–1351. doi: 10.4088/jcp.v65n1008. [DOI] [PubMed] [Google Scholar]
- 33.Simon GE, Ludman EJ, Bauer MS, Unutzer J, Operskalski B. Long-term effectiveness and cost of a systematic care program for bipolar disorder. Arch Gen Psychiatry. 2006;63:500–508. doi: 10.1001/archpsyc.63.5.500. [DOI] [PubMed] [Google Scholar]
- 34.Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder: Part II. Impact on clinical outcome, function, and costs. Psychiatr Serv. 2006;57:937–945. doi: 10.1176/ps.2006.57.7.937. [DOI] [PubMed] [Google Scholar]