Abstract
We studied the effect of genetic susceptibility on hexavalent chromium induced dermal adversities. The health status of population was examined from the areas of Kanpur (India) having the elevated hexavalent chromium levels in groundwater. Blood samples were collected for DNA isolation to conduct polymorphic determination of genes, namely: NQO1 (C609T), hOGG1 (C1245G), GSTT1, and GSTM1 (deletion). Symptomatic exposed subjects (n = 38) were compared with asymptomatic exposed subjects (n = 108) along with asymptomatic controls (n = 148) from a non contaminated reference community. Exposed symptomatic group consisted of 36.8% subjects who were GSTM1 null genotyped as compared to asymptomatic where only 19.4% subjects were null. The exposed subjects with GSTM1 null genotype were more susceptible to dermal adversities in comparison with wild genotyped subjects (OR = 2.42; 95% CI = 1.071–5.451). Age, smoking, gender or duration of residence were not found to have any confounding effect towards this association. Association with other genes was not statistically significant, nonetheless, possible contribution by these genes cannot be ruled out. In conclusion, variation in the polymorphic status of GSTM1 gene may influence dermal outcomes among residents from Cr(VI) contaminated areas. Further studies are therefore, needed to examine these observations among different population groups.
1. Introduction
Chromium in trivalent form is a trace metal that humans require as an important bioelement for its exceptional role in metabolic processes [1]. On the other hand, the hexavalent form of Cr [Cr(VI)] is a toxic form and is reported to have deleterious health effects on the human beings. Cr(VI) has widespread applications in industries involved in leather tanning, manufacturing chrome sulfate, paints, dyes, and so forth. Occupational exposure to Cr(VI) is reported to cause contact dermatitis among workers [2]. Due to the improper ways of waste disposal from these industries, exposure to Cr(VI) is not limited at occupational environment but expands to areas in the vicinity of human residence [3–5].
Whereas there are extensive data available on the dermatological adversities due to occupational exposure of chromium compounds, only limited information is available concerning the risk and its mechanism involved following environmental exposure. Findings of the epidemiological studies on dermal outcomes among general populations have been equivocal. In a longitudinal health effects survey conducted by the Tokyo Metropolitan Government Bureau of Sanitation [6], an increase in incidence of contact dermatitis and eczema of the hands from residents in the contaminated areas compared to the controls was reported. In the contrary, self-reported-assessment-based study conducted among residents from a contaminated site at Glasglow found no evidence of harm to the health of residents [7]. The experimental studies also dictate remarkable variations in the behavior of chromium amongst individuals. Previous studies have reported large differences in the reduction of Cr(VI) to its lower oxidation states in human plasma and blood cells of different individuals [8–10] and chromium uptake in lymphoblastic cell lines derived from three different individuals [11]. In another study on chromium-sensitive subjects, dichromate evoked a positive patch test rate in only 8% of the subjects at 0.001% and 4% at 0.01% [12]. These observations point towards interindividual variability in response towards Cr(VI) exposure among the human beings.
Twin studies show that the genetic differences account for about a quarter of the variance in adult human lifespan. Genetic differences also contribute towards selection of genetically inherited tolerance among populations exposed to environmental toxicant [13]. With expanding involvement of genetic biodiversity in deciding biological response to various agents, it seems practical to consider the role of genetic factors towards health outcomes among general population exposed to Cr(VI).
These genetic factors may correspond to those enzymes involved in the processes of Cr(VI) reduction inside the cell and the subsequent consequences [14]. So, by affecting the biological fate of Cr(VI) and its impact on various cell compartments, these genetic factors may influence the toxic impacts of Cr(VI). One out of these is NAD(P)H:quinone oxidoreductase (NQO1), also known as DT-diaphorase, reported to be involved in Cr(VI) reduction [15]. Existence of polymorphic forms of NQO1 gene among human population is well documented. A transition of base C to T in the 609 codon of NQO1 results in no detectable NQO1 activity [16] which may affect rate of Cr(VI) reduction. Following reduction of Cr(VI), the associated processes include generation of reactive oxygen species (ROS) leading to oxidative stress and DNA damage [17]. Prior studies have reported formation of 8-hydroxydeoxyguanosine (8-OHdG) base changes, an oxidation product due to occupational exposure of Cr(VI) [18, 19]. So, it is of interest to look for role of variation in 8-oxoguanine glycosylase gene (hOGG1) involved in the repair of 8-OHdG base changes [20]. A C to G nucleotide transversion at position 1,245 in exon 7 of the hOGG1 gene is associated with the substitution of cysteine (Cys) for serine (Ser) at codon 326 which affects the biological activity of hOGG1 protein [21]. Further, glutathione S-transferases (GST), xenobiotic-metabolising enzymes are involved in the metabolic detoxification of various environmental carcinogens, oxidized lipid and DNA products generated by ROS-induced damage to intracellular molecules [22]. Two of the most relevant GST isoenzymes, GSTM1 (mu) and GSTT1 (theta), are nonfunctional (due to deletion of a portion of gene) in appreciable percentage of human population. These deficiencies have been suggested to play an important role in cancer susceptibility [23–25]. Role of GSTT1 and GSTM1 polymorphism in relation to exposure towards other environmental toxicants has previously been demonstrated by our group [26, 27].
We understand that by affecting the individual's ability, genetic polymorphisms of NQO1, hOGG1, GSTM1, and GSTT1 may influence occurrence of dermatological outcomes among general population exposed to Cr(VI). We conducted the present study which involved residents from Cr(VI) contaminated areas of Kanpur (Uttar Pradesh, India) [5]. An earlier health impact assessment-based study conducted by us revealed significantly higher prevalence of self-reports for dermal adversities among the residents from the contaminated areas as compared to residents with similar social and demographic features living in communities without elevated Cr(VI) levels. We hypothesize that the present study may explain the reason for differences in susceptibility towards Cr(VI) exposure among the residents.
2. Materials and Methods
2.1. Selection of Area
Kanpur, a city in Uttar Pradesh province of India, lies in Indo-Gangetic plain (26.4670° North and 80.3500° East). Reportedly, there are large numbers of leather tanneries and chrome sulphate manufacturing units located in and around Kanpur. Wastes from these industries are improperly disposed which has resulted in groundwater contamination at various areas of Kanpur [5]. Levels of Cr(VI) in groundwater have reached upto 124–258 times higher than the WHO permissible limit in some areas [5, 28, 29].
We selected contaminated communities on the basis of previous reports by Central Pollution Control Board, UP. An area in the vicinity with no history of Cr(VI) contamination was also selected for a control population. To avoid any exposure misclassification, a standard diphenylcarbazide reagent method was used to estimate Cr(VI) in groundwater samples [30]. The estimated levels of Cr(VI) in groundwater from the contaminated area ranged from 8.0–38.4 ppm. The mean Cr(VI) concentration was 19.5 ± 9.4 ppm which was many folds above WHO permissible limit of 0.05 ppm. Figure 1 shows photograph of contaminated yellow water from a handpump.
Figure 1.

Photograph of yellow-colored contaminated water from a handpump.
2.2. Selection of the Study Population
Subjects were recruited through the health camps. Ethical approval for the study was obtained from Institutional Human Ethics Committee of IITR. Before inclusion, written informed consent was obtained from all participants. Inclusion criteria of subjects was age equal to or more than 18 years, duration of residence not less than 5 years, no consumption of bottled water, and no present or past occupational exposure to Cr(VI). Occupational exposure to Cr(VI) includes various types of jobs which involve use or manufacture of Cr compounds for example, leather tanneries, cement, chrome sulphate manufacturing, paint and dye synthesis, chrome plating. Nonoccupational exposure to subjects is via environment for example, air, water, food chain. In our study, the exposure to subjects was predominantly through contaminated water.
2.3. Health Examination and Record
A pretested questionnaire was used to gather demographic information. Heath information related with occurrence of dermatological symptoms, namely, itching or reddening of skin, flaky or scaly skin, and their specific histories were recorded in the questionnaire. A medical scientist also examined the subjects in accordance with recommendations outlined in the Declaration of Helsinki [31].
2.4. Blood Sample Collection
Blood samples (2-3 mL) were collected by venipuncture in EDTA-coated vacutainers (BD Biosciences), were stored at 4°C, and transported to the laboratory within 3-4 hours.
2.5. Isolation of Genomic DNA
DNA was isolated from whole blood using commercial DNA isolation kit (Qiagen). Dissolved DNA was quantitated by optical density (OD) at 260 nm using Picodrop Spectrophotometer. DNA samples were stored at −80°C in small aliquots.
2.6. Genotyping for GSTT1, GSTM1 Deletion Polymorphism
Analysis for GSTT1 and GSTM1 genetic polymorphism was done by multiplex PCR [32]. DNA (50 ng) was amplified in a 20 μL reaction having 10 pmoles each of primers for GSTT1: 5′-TTCCTTACTGGTCCTCACATCTC-3′ and 5′-TCACGGGATCATGGCCAGCA-3′ and GSTM1: 5′-GAACTCCCTGAAAAGCTAAAGC-3′ and 5′-GTTGGGCTCAAATATACGGTGG-3′. As an internal control, exon 7 of the CYPlAl gene was coamplified using the primers: 5′-GAACTGCCACTTCAGCTGTCT-3′ and 5′-CAGCTGCATTTGGAAGTGCTC-3′. The PCR conditions consisted of an initial melting temperature of 94°C (5 min) followed by 35 cycles of melting (94°C, 2 min), annealing (59°C 1 min), and extension (72°C 1 min). A final extension step (72°C) of 10 min terminates the process. The PCR products from coamplification of GSTT1, GSTM1, and CYPlA1 genes were then analyzed electrophoretically on 2% agarose gel. GSTT1 and GSTM1 wild genotypes yield band of 480 basepair (bp) and 215 bp, respectively, while no band is seen in case of deletion. CYPlAl gave band of size 315 bp in all the samples.
2.7. Genotyping for NQO1 (C609T) Polymorphism
Detection of C609T transition at gene NQO1 was done using method reported by Harth et al. [33]. DNA (100 ng) was amplified using 10 pmoles of primers: 5′-GAGACGCTAGCTCTGAA CTGATT-3′ and 5′-AGCAAAATACAGATGGTGTCTCAT-3′. Thermal cycling conditions were firstly, 4 min at 94°C, 30 cycles of 1 min at 94°C, 1 min at 62°C, 1 min at 72°C and lastly 7 min at 72°C. PCR product (10 μL) of 300 bp was digested with HinfI restriction enzyme (Fermentas) and run on 2.5% agarose gel. Wild genotype (C/C) showed one 280 bp band, heterozygous (C/T) showed 3 bands of 280 bp, 164 bp, and 116 bp and mutant genotype (T/T) showed 2 bands of 164 bp and 116 bp.
2.8. Genotyping for hOGG1 (C1245G) Polymorphism
The C1245G transition at hOGG1 gene was detected using method reported by Wang et al. [34]. DNA was amplified using primers: 5′-AGGGGAAGGTGCTTGGGGAA-3′ and 5′-ACTGTCACTAGTCTCACCAG-3′. PCR consisted of an initial denaturation step for 5 min at 94°C, 35 cycles of denaturation for 20 s at 94°C, primer annealing for 20 s at 60°C, and primer extension for 40 s at 72°C, followed by a final extension step for 7 min at 72°C. PCR product (10 μL) of size 200 bp was digested using Fnu4HI restriction enzyme (Fermentas) and run on 2.5% agarose gel. Heterozygous subjects (CG) exhibited two fragments (200 and 100 bp) while homozygous wild type (C/C) and mutant (GG) genotype exhibited single fragment of 200 bp and 100 bp, respectively.
2.9. Sequencing
The gene products for GSTT1, GSTM1, NQO1 and hOGG1 were sequenced. The amount of PCR product used in sequencing for NQO1, and hOGG1 was 5 ng, while it was 7 ng for GSTM1 and 12 ng for GSTT1 [35].
2.10. Statistical Analysis
Descriptive statistics for exposed and control group were presented as mean and standard deviation. Frequencies and percentages were shown for categorical variables. Student's t-test and chi-square test were used to find out difference in distribution for socio-demographic variables among the two groups. Among exposed group, subjects afflicted with dermatological symptoms were regarded as “symptomatic” and the rest were designated as “asymptomatic”. Models were generated to analyze comparison among: (1) symptomatic exposed and asymptomatic control subjects; (2) symptomatic exposed and asymptomatic exposed subjects. Logistic regression procedures were used to estimate odds ratios (OR) with 95% confidence intervals (CI) to see influence of various genotypes on dermatological outcomes. Statistical significance was tested by chi-square test. Main predictor variables considered were genetic variants of the GSTT1, GSTM1, NQO1, and hOGG1 gene. Occurrence of the dermatological adversity (yes/no) was considered as outcome variable. The covariates analyzed were gender, age (≤35 and >35 years), smoking habit (never versus current or past), and duration of residence (≤20 and >20 years). Further, multivariate logistic regression analysis was also performed to adjust for confounding effect by the covariates. All the analyses were carried out using SPSS 13 (SPSS, Chicago, USA). A 0.05 cutoff point was set for the P value and applied in all the statistical analyses.
3. Results
3.1. Study Population and Its Characteristics
Study population comprised of a total of 146 exposed subjects (mean age ± SD: 36.29 ± 14.82 years) and 148 asymptomatic controls (mean age ± SD: 39.63 ± 14.39 years). Among exposed group, 26% (n = 38) subjects were having dermal problems, namely, itching, reddening, and crusting of skin. Duration of residence for the exposed subjects at the contaminated areas ranged from 2–79 years (mean ± SD: 24.17 ± 15.23 years). The sociodemographic characteristics of both the groups are shown in Table 1. Exposed population included 79 males and 67 females while control group consisted of 65 males and 83 females. Further, 8.8% subjects among control group and 19.2% among exposed group were smokers. There were differences in the number of smokers among two groups. So, we adjusted the risk estimates for any confounding effect by this variable.
Table 1.
Demographic characteristics of the study participants.
| Characteristics | Category | Exposed group | Control group | P value |
|---|---|---|---|---|
| (N = 146) | (N = 148) | |||
| Mean age | Mean ± SD | 36.29 ± 14.82 | 39.63 ± 14.39 | 0.086a |
| Number of subjects | Males | 79 (54) | 65 (44) | 0.0805b |
| Females | 67 (46) | 83 (56) | ||
| Smoking status | Smokers | 28 (19.2) | 13 (8.8) | 0.0101b |
| Symptom category | Symptomatic | 38 (26) | — |
SD: standard deviation.
Number in parenthesis denotes percentage.
a P value for continuous variables calculated using Student's t-test.
b P value for categorical variables calculated using Chi-square test.
3.2. Genotypic Distribution
Figure 2 shows the representative gel of NQO1 C609T, hOGG1 C1245G and multiplex GSTM1 and GSTT1 genotyping. Sequences of PCR products blasted with the reference gene from the database on National Centre for Biotechnology Information (NCBI) gave homology in the range of 96–99% (Table 2).
Figure 2.
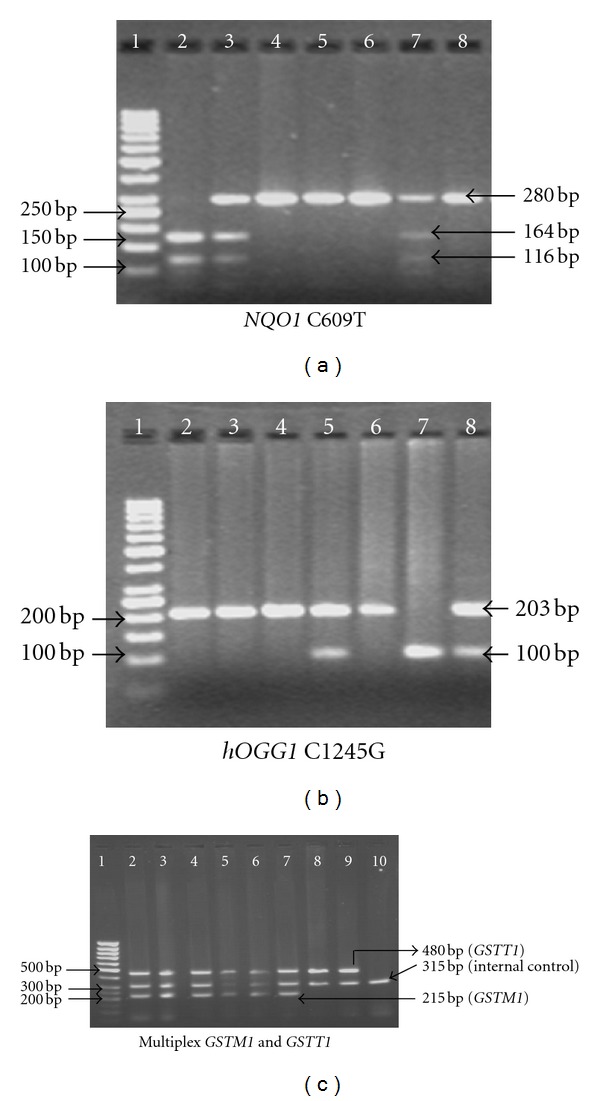
Representative gels of NQO1 C609T, hOGG1 C1245G and multiplex GSTM1 and GSTT1 genotyping.
Table 2.
Summary of accession number of individual genes and their products sequenced after purification with percentage homology.
| Gene | Accession number | Product | Product RFLP | Homology (%) |
|---|---|---|---|---|
| GSTT1 | NM_000853 | 480 bp | — | 96.6 |
| GSTM1 | NM_000561 | 215 bp | — | 97.3 |
| NQO1 C609T | NM_000903 | 300 | 280, 164, 116 | 99 |
| hOGG1 C1245G | NM_016819.3 | 200 | 100 bp | 99 |
The genotypic distribution for GSTT1 and GSTM1, NQO1 and hOGG1 genesamong exposed and control group is shown in Table 3. Frequencies for GSTT1 null and GSTM1 null were 23.3%, and 24% among exposed population and 14.2%, and 37.8% among controls. Among exposed group, 38.3%, 51.1%, and 10.6% subjects were wild, heterozygous and mutant genotyped, respectively for NQO1 C609T polymorphism and the respective frequencies among controls were 48.6%, 37.2%, and 14.2%. For hOGG1 gene, respective frequencies for wild, heterozygous and mutant genotype were 47.3%, 41.1%, and 11.6% among exposed subjects and 36.5%, 53.4%, and 10.1% among controls. Hardy Weinberg equilibrium test showed accordance for NQO1 and hOGG1 gene in both population groups. The Hardy Weinberg equilibrium could not be tested for GSTT1 and GSTM1 because of the inability of the present PCR protocol to separate heterozygous carriers of the deletion polymorphisms.
Table 3.
Genotypic distribution for GSTT1, GSTM1, NQO1 C609T, and hOGG1 C1245G genes among exposed and control group.
| Characters | Category | Exposed group | Control group |
|---|---|---|---|
| (N = 146) n (%) | (N = 148) n (%) | ||
| GSTT1 | Wild | 112 (76.7) | 127 (85.8) |
| Null | 34 (23.3) | 21 (14.2) | |
|
| |||
| GSTM1 | Wild | 111 (76) | 92 (62.2) |
| Null | 35 (24) | 56 (37.8) | |
|
| |||
| NQO1 | Wild (C/C) | 54 (38.3) | 72 (48.6) |
| Heterozygous (C/T) | 72 (51.1) | 55 (37.2) | |
| Mutant (T/T) | 15 (10.6) | 21 (14.2) | |
|
| |||
| hOGG1 | Wild (C/C) | 69 (47.3) | 54 (36.5) |
| Heterozygous (C/G) | 60 (41.1) | 79 (53.4) | |
| Mutant (G/G) | 17 (11.6) | 15 (10.1) | |
Data for some samples are missing due to limited sample volume.
3.3. Association between Genetic Polymorphism and Occurrence of Dermal Problems
We compared the distribution for various covariates and genetic polymorphic status of GSTT1 and GSTM1, NQO1 and hOGG1 genes among symptomatic exposed (n = 38) and asymptomatic control group (n = 148). It showed no significant difference in distribution among males versus females and subjects with age ≤35 versus age >35 years (Table 4). However, smoking was found to act as a confounding factor. Exposed group consisted of greater number of smokers (21%) as compared to controls (8.8%) and exposed smokers had higher prevalence of dermal problems as compared to control nonsmokers (OR = 2.77; 95% CI = 1.055–7.272). The difference in the distribution of genetic polymorphic status for GSTT1 gene was found significant with respect to occurrence of dermal complaints. As compared to asymptomatic control group where 14.2% subjects were null genotyped, symptomatic exposed group was having 29% subjects having GSTT1 null genotype. Odds ratio showing prevalence of dermal problems among GSTT1 null genotyped as compared to wild genotyped subjects was 2.46 (95% CI = 1.064–5.705). However, after adjustment for smoking, influence of GSTT1 polymorphism did not remain significant, although the odds ratio (OR = 2.53) did not change much. Further, there were no significant differences in distribution for NQO1 (OR = 1.18), hOGG1 (OR = 0.905) and GSTM1 (OR = 0.96) gene among these groups.
Table 4.
Influence of various covariates and genotypes on dermatological adversities among exposed group compared with asymptomatic control group.
| Characters | Category | Exposed SYMP | Control ASYMP | OR (95% CI) |
|---|---|---|---|---|
| (N = 38) n (%) | (N = 148) n (%) | |||
| Sex | Males | 20 (52.6) | 65 (43.9) | 1 |
| Females | 18 (47.4) | 83 (56.1) | 0.71 (0.345–1.441) | |
|
| ||||
| Age (years) | ≤35 | 20 (52.6) | 69 (46.6) | 1 |
| >35 | 18 (47.4) | 79 (53.4) | 0.67 (0.408–1.109) | |
|
| ||||
| Smoking | Never | 30 (79) | 135 (91.2) | 1 |
| Current/past | 8 (21) | 13 (8.8) | 2.77 (1.055–7.272)∗ | |
|
| ||||
| GSTT1 | Wild | 27 (71) | 127 (85.8) | 1 |
| Null | 11 (29) | 21 (14.2) | 2.46 (1.064–5.705)∗ | |
| 2.53 (0.946–6.77)# | ||||
|
| ||||
| GSTM1 | Wild | 24 (63.2) | 92 (62.2) | 1 |
| Null | 14 (36.8) | 56 (37.8) | 0.96 (0.458–2.005) | |
|
| ||||
| NQO1 | Wild (C/C) | 16 (44.4) | 72 (48.65) | 1 |
| Mutant (C/T + T/T) | 20 (55.6) | 76 (51.35) | 1.18 (0.569–2.463) | |
|
| ||||
| hOGG1 | Wild (C/C) | 13 (34.2) | 54 (36.5) | 1 |
| Mutant (C/G + G/G) | 25 (65.8) | 94 (63.5) | 0.905 (0.428–1.915) | |
SYMP: symptomatic, ASYMP: asymptomatic, OR: odds ratio, CI: confidence interval; #odds ratio adjusted for smoking; ∗ P < 0.05.
Table 5 depicts distribution of covariates and genetic polymorphic status among symptomatic exposed (n = 38) and asymptomatic exposed group (n = 108). It was observed that the occurrence of dermal problems among exposed group was independent of gender, agecategory (≤35 versus >35 years), smoking status (never versus current or past), and duration of the residence (≤20 years versus >20 years). Analysis with genetic polymorphic status showed significant difference in distribution for GSTM1 genotypes. Exposed symptomatic group consisted of 36.8% subjects who were GSTM1 null genotyped as compared to asymptomatic where only 19.4% subjects were null. Odds ratio showed higher prevalence of dermal problems in null genotyped subjects compared to wild genotyped subjects (OR = 2.42; 95% CI = 1.071–5.451). The association with other genes, although, did not reach statistically significant level, however, higher odds ratios (OR > 20) were found in case of both hOGG1 mutant (OR = 2.07; 95% CI = 0.96–4.469) and GSTT1 null genotype (OR = 1.51; 95% CI = 0.651–3.484) compared to their respective wild genotypes. On the contrary, NQO1 genetic polymorphism showed reverse association and subjects with null genotype had lower prevalence for dermal problems as compared to those having wild genotype (OR = 0.71; 95% CI = 0.329–1.529).
Table 5.
Influence of various covariates and genotypes on dermatological adversities among symptomatic exposed compared with asymptomatic exposed group.
| Characters | Category | Exposed SYMP | Exposed ASYMP | OR (95% CI) |
|---|---|---|---|---|
| (N = 38) n (%) | (N = 108) n (%) | |||
| Sex | Males | 20 (52.6) | 59 (54.6) | 1 |
| Females | 18 (47.4) | 49 (45.4) | 1.08 (0.517–2.273) | |
|
| ||||
| Age (years) | ≤35 | 20 (52.6) | 61 (56.5) | 1 |
| >35 | 18 (47.4) | 47 (43.5) | 1.17 (0.556–2.453 | |
|
| ||||
| Smoking | Never | 30 (79) | 88 (81.5) | 1 |
| Current/past | 8 (21) | 20 (18.5) | 1.17 (0.468–2.94) | |
|
| ||||
| Duration of | ≤20 | 19 (50) | 62 (57.4) | 1 |
| residence (years) | >20 | 19 (50) | 46 (42.6) | 1.35 (0.642–2.83) |
|
| ||||
| GSTT1 | Wild | 27 (71) | 85 (78.7) | 1 |
| Null | 11 (29) | 23 (21.3) | 1.51 (0.651–3.484) | |
|
| ||||
| GSTM1 | Wild | 24 (63.2) | 87 (80.6) | 1 |
| Null | 14 (36.8) | 21 (19.4) | 2.42 (1.071–5.451)∗ | |
|
| ||||
| NQO1 | Wild (C/C) | 16 (44.4) | 38 (36.2) | 1 |
| Mutant (C/T + T/T) | 20 (55.6) | 67 (63.8) | 0.71 (0.329–1.529) | |
|
| ||||
| hOGG1 | Wild (C/C) | 13 (34.2) | 56 (51.8) | 1 |
| Mutant (C/G + G/G) | 25 (65.8) | 52 (48.2) | 2.07 (0.96–4.469) | |
SYMP: symptomatic, ASYMP: asymptomatic, OR: odds ratio, CI: confidence interval; ∗ P < 0.05.
4. Discussion
In our previous studies on the population residing in Cr(VI) contaminated areas, higher prevalence of self-reports for dermal complaints, namely, itching, reddening, and scaling of skin was observed with higher mean Cr levels (approximately 6 folds) in hair of these residents compared to controls having similar sociodemographic status (unpublished observations). However, we noticed that not all residents were at risk to the dermal outcomes. Therefore, an attempt has been made to find out genetic linkages, if any, in causing wide variability in the outcomes among Cr(VI) exposed individuals. The general population living in hexavalent chromium-contaminated areas of Kanpur provided us with the opportunity to explore the role of genetic variants in causing variable response towards dermal problems among exposed individuals and also in comparison with unexposed individuals.
In the present study on genetic variability, we found significant modification in the risk by GSTM1 genetic polymorphism. It was observed that absence of GSTM1 gene activity among residents from Cr(VI) contaminated areas was acting as a predisposing factor towards occurrence of dermal problems. This is suggestive of the role of GSTM1 gene in the pathogenesis of dermal problems among subjects exposed to Cr(VI).
Literature reports that a number of skin diseases are associated with oxidative stress [36] with a very specific role of GSTM1 in protection against oxidative stress [37]. GSTM1 catalyzes the conjugation of DNA hydroperoxides, namely, 4-hydroxynonenal, linoleic acid hydroperoxide which are mutagenic and cytotoxic product of lipid peroxidation [37]. Further, 5-hydroxymethyluracil, a mutagenic compound that is formed by either oxidative attack on the methyl group of the thymine base of DNA or from deamination of products formed by oxidation of 5-methylcytosine was also taken care by GSTM1 [38]. Skin allergies are more common among individuals with genetic absence of GSTM1 [39]. Arsenic-induced skin lesions [40] and non-melanoma carcinoma risk [41] are also reported in association with GSTM1 null genotype. Cr(VI) induces carcinogenesis via oxidative stress pathway [42]. The role of glutathione (GSH) in Cr(VI) reduction inside the cell has also been highlighted [43]. Increased frequency of sister chromatid exchange (SCE) among Cr workers with GSTM1 null genotype as opposed to nonnull genotype individuals is observed [44].
There are different distribution patterns of GSTM1 null genotype among different ethnic groups which ranges from 23% to 48% in African populations, 33% to 63% in Asian populations, 39% to 62% in European populations, and 23% to 62% in U.S. populations [45]. Further, interindividual as well as interethnic variations alongwith toxicants exposure to the population across the world might be showing interindividual variations in the toxicant effect relationship.
The genetic associations with other genes involved in Cr metabolism and disposition were not significant; however, this indicates possible influence of such genetic polymorphisms on the exposed population. Formation of 8-hydroxy deoxyguanosine has been demonstrated on occupationally exposed workers [18, 19]. In another study involving school children from communities near thermal power plant, greater urinary 8-OHdG concentration was found among children having elevated urinary chromium levels than those with lower urinary chromium [46]. Association of GSTT1 genetic polymorphism with dermal manifestations [47] and its influence in causing variability among chrome plating workers is also described [48]. The role of NQO1 in cellular mechanism of Cr(VI) reduction has also been introduced [15]. Advocating the role of NQO1 in Cr(VI) reduction raises possibility of increased production of lower oxidation state of chromium which is more toxic and concomitant production of ROS [14]. So, the compromised activity of NQO1 due to genetic polymorphism may give protection from damage caused by Cr(III) production inside the cell. This might be the cause behind higher prevalence of dermal outcomes among NQO1 wild genotyped subjects.
Further, elicitation of health outcomes among human population depends not only on exposure conditions on which humans have no control, but also, on factors such as smoking for which a personal choice exists. Smoking is strongly associated with numerous dermatologic conditions including squamous cell carcinoma and psoriasis, although, the evidence linking smoking and melanoma, eczema, and acne is inconclusive [49]. Synergistic effect between arsenic exposure and tobacco smoking on risk of skin lesionswas reported by Chen et al. [50]. We also observed significant influence of smoking on the dermal outcomes, in association with environmental exposure to Cr(VI). Thus, we understand that smoking status of subject should be taken into consideration while determining health risk due to a toxic environmental agent.
We accept that genetic research is not applicable for direct public health purposes, as it currently stands. Moreover, the ethical issues on revealing personal genetic information are also considerable. However, such studies can be worthwhile for the investigation of disease mechanisms, to give insights on potential therapies or to discover unidentified etiological agents involved in the case of diseases whose etiology is still unknown. These genetic studies are of high relevance for populations being chronically exposed to a toxic agent under low concentrations, a usually common scenario within residential settings.
5. Conclusion
The present study reports that GSTM1 genetic polymorphism may cause individuals bearing high-risk genotype more susceptible towards Cr(VI) exposure associated dermal outcomes. This could well explain why environmental exposures have aggravated effects, if they occur in a population of vulnerable subjects. However, more studies on role of genetic polymorphisms in association with Cr(VI) exposure among different populations are needed. With the increasing Cr(VI) toxic burden in vicinity of the human habitat, unraveling the role of such factors involved in modulation of toxic response is highly needed. Knowledge gained, thus, may not only help in screening high-risk groups but, in the long run, may also pave the path for personalized therapeutics measures.
Acknowledgments
The authors are grateful to Dr. K. C. Gupta, Director, IITR for his support and encouragement throughout the study. The authors also acknowledge Mr. Rakesh Jaiswal (NGO “Eco-friends”) for local support at Kanpur. The authors thank Mr. Ramsurat and Ms. Mumtaz Jahan for their technical support during the study. The authors are also thankful to all the study participants for their cooperation in this study. P. sharma acknowledges the fellowship support from University Grant Commission, New Delhi.
References
- 1.Morris GS, Guidry KA, Hegsted M, Hasten DL. Effects of dietary chromium supplementation on cardiac mass, metabolic enzymes, and contractile proteins. Nutrition Research. 1995;15(7):1045–1052. [Google Scholar]
- 2.NIOSH. Comments on the Occupational Safety and Health Administration request for information on occupational exposure to hexavalent chromium (CrVI) OSHA Docket No. H-0054a. NIOSH policy statements. Cincinnati, Ohio, USA: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, 2002.
- 3.Zhang J, Li X. Chromium pollution of soil and water in Jinzhou. Chinese Journal of Preventive Medicine. 1987;21(5):262–264. [PubMed] [Google Scholar]
- 4.Burke T, Fagliano J, Goldoft M, Hazen RE, Iglewicz R, McKee T. Chromite ore processing residue in hudson county, New Jersey. Environmental Health Perspectives. 1991;92:131–137. doi: 10.1289/ehp.9192131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CPCB. Report on Groundwater quality in Kanpur, status, sources and control measures. Central Pollution Control Board. no. GWQS/8/1996-97, 1997.
- 6.TMBGS. Survey of Health Effects from Chromium Contamination: Fourth Report. Tokyo Metropolitan Government Bureau of Sanitation Tokyo, Japan, March, 1987.
- 7.McCarron P, Peters TJ. Self reported health of people in an area contaminated by chromium waste: interview study. British Medical Journal. 2000;320(7226):11–15. doi: 10.1136/bmj.320.7226.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett GE, Dodge DG, O’Flaherty E, et al. In vitro reduction kinetics of hexavalent chromium in human blood. Environmental Research. 1998;78(1):7–11. doi: 10.1006/enrs.1998.3840. [DOI] [PubMed] [Google Scholar]
- 9.Kerger BD, Paustenbach DJ, Corbett GE, Finley BL. Absorption and elimination of trivalent and hexavalent chromium in humans following ingestion of a bolus dose in drinking water. Toxicology and Applied Pharmacology. 1996;141(1):145–158. doi: 10.1006/taap.1996.0271. [DOI] [PubMed] [Google Scholar]
- 10.Finley BL, Kerger BD, Corbett GE, et al. Human ingestion of chromium (VI) in drinking water: pharmacokinetics following repeated exposure. Toxicology and Applied Pharmacology. 1997;142(1):151–159. doi: 10.1006/taap.1996.7993. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Kluz T, Salnikow K, Costa M. Comparison of the cytotoxicity, cellular uptake, and DNA-protein crosslinks induced by potassium chromate in lymphoblast cell lines derived from three different individuals. Biological Trace Element Research. 2002;86(1):11–22. doi: 10.1385/BTER:86:1:11. [DOI] [PubMed] [Google Scholar]
- 12.Zelger J, Wachter H. On the relationships between chromate and dichromate allergy. A contribution to the analysis of chromium (VI) allergy. Dermatologica. 1966;132(1):45–50. [PubMed] [Google Scholar]
- 13.Medina MH, Correa JA, Barata C. Micro-evolution due to pollution: possible consequences for ecosystem responses to toxic stress. Chemosphere. 2007;67(11):2105–2114. doi: 10.1016/j.chemosphere.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V. Reduction of chromium (VI) and its relationship to carcinogenesis. Journal of Toxicology and Environmental Health B. 1999;2(1):87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- 15.De Flora S, Morelli A, Basso C, Romano M, Serra D, De Flora A. Prominent role of DT-diaphorase as a cellular mechanism reducing chromium (VI) and reverting its mutagenicity. Cancer Research. 1985;45(7):3188–3196. [PubMed] [Google Scholar]
- 16.Kelsey KT, Ross D, Traver RD, et al. Ethnic variation in the prevalence of a common NAD(P)H quinone oxidoreductase polymorphism and its implications for anti-cancer chemotherapy. British Journal of Cancer. 1997;76(7):852–854. doi: 10.1038/bjc.1997.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutation Research. 2003;533(1-2):3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Hodges NJ, Ádám B, Lee AJ, Cross HJ, Chipman JK. Induction of DNA-strand breaks in human peripheral blood lymphocytes and A549 lung cells by sodium dichromate: association with 8-oxo-2-deoxyguanosine formation and inter-individual variability. Mutagenesis. 2001;16(6):467–474. doi: 10.1093/mutage/16.6.467. [DOI] [PubMed] [Google Scholar]
- 19.Kuo HW, Chang SF, Wu KY, Wu FY. Chromium (VI) induced oxidative damage to DNA: increase of urinary 8-hydroxydeoxyguanosine concentrations (8-OHdG) among electroplating workers. Occupational and Environmental Medicine. 2003;60(8):590–594. doi: 10.1136/oem.60.8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vodicka P, Kumar R, Stetina R, et al. Genetic polymorphisms in DNA repair genes and possible links with DNA repair rates, chromosomal aberrations and single-strand breaks in DNA. Carcinogenesis. 2004;25(5):757–763. doi: 10.1093/carcin/bgh064. [DOI] [PubMed] [Google Scholar]
- 21.Kohno T, Shinmura K, Tosaka M, et al. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16(25):3219–3225. doi: 10.1038/sj.onc.1201872. [DOI] [PubMed] [Google Scholar]
- 22.Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000;61(3):154–166. doi: 10.1159/000028396. [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Cho SH, Park SK, et al. Combined effect of glutathione S-transferase M1 and T1 genotypes on bladder cancer risk. Cancer Letters. 2002;177(2):173–179. doi: 10.1016/s0304-3835(01)00820-5. [DOI] [PubMed] [Google Scholar]
- 24.Deng Z, Wei Y, Ma Y. Glutathione-S-transferase M1 genotype in patients with hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2001;23(6):477–479. [PubMed] [Google Scholar]
- 25.Sierra-Torres CH, Au WW, Arrastia CD, et al. Polymorphisms for chemical metabolizing genes and risk for cervical neoplasia. Environmental and Molecular Mutagenesis. 2003;41(1):69–76. doi: 10.1002/em.10132. [DOI] [PubMed] [Google Scholar]
- 26.Kumar M, Chauhan LKS, Paul BN, Agarwal SK, Goel SK. GSTM1, GSTT1, and GSTP1 polymorphism in North Indian population and its influence on the hydroquinone-induced in vitro genotoxicity. Toxicology Mechanisms and Methods. 2009;19(1):59–65. doi: 10.1080/15376510802399057. [DOI] [PubMed] [Google Scholar]
- 27.Kumar M, Tewari S, Sharma P, et al. Study of genetic polymorphism in solvent exposed population and its correlation to in vitro effect of trichloroethylene on lymphocytes. Journal of Environmental Biology. 2009;30(5):685–691. [PubMed] [Google Scholar]
- 28.CGWB. Groundwater Pollution Studies in Unnao-Kanpur Industrial Areas, Uttar Pradesh. Central Ground Water Board, Lucknow, India, 2000.
- 29.Schaffner IR, Singh RK, Lamb STR, Kirkland DN. Enhanced bioremediation pilot study of A Cr (VI)-impacted overburden groundwater system in Kanpur, Uttar Pradesh, India. Proceedings of the 23rd Annual International Conference on Soils Sediments and Water; 2010; pp. 1–18. [Google Scholar]
- 30.APHA. Standard Methods for the Examination of Water and Wastewater. 21st edition. Washington, DC, USA: 2005. [Google Scholar]
- 31.WMA. Ethical Principles for Medical Research Involving Human Subjects. 59th edition. Seoul, Korea: World Medical Association General Assembly; 2008. [Google Scholar]
- 32.Abdel-Rahman SZ, El-Zein RA, Anwar WA, Au WW. A multiplex PCR procedure for polymorphic analysis of GSTM1 and GSTT1 genes in population studies. Cancer Letters. 1996;107(2):229–233. doi: 10.1016/0304-3835(96)04832-x. [DOI] [PubMed] [Google Scholar]
- 33.Harth V, Donat S, Ko Y, Abel J, Vetter H, Brüning T. NAD(P)H quinone oxidoreductase 1 codon 609 polymorphism and its association to colorectal cancer. Archives of Toxicology. 2000;73(10-11):528–531. doi: 10.1007/s002040050004. [DOI] [PubMed] [Google Scholar]
- 34.Wang CL, Hsieh MC, Hsin SC, et al. The hOGG1 Ser326Cys gene polymorphism is associated with decreased insulin sensitivity in subjects with normal glucose tolerance. Journal of Human Genetics. 2006;51(2):124–128. doi: 10.1007/s10038-005-0335-8. [DOI] [PubMed] [Google Scholar]
- 35.Kumar M, Agarwal SK, Goel SK. Lung cancer risk in North Indian population: role of genetic polymorphisms and smoking. Molecular and Cellular Biochemistry. 2009;322(1-2):73–79. doi: 10.1007/s11010-008-9941-z. [DOI] [PubMed] [Google Scholar]
- 36.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. Journal of Investigative Dermatology. 2006;126(12):2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 37.Kerb R, Brockmöller J, Reum T, Roots I. Deficiency of glutathione S-transferases T1 and M1 as heritable factors of increased cutaneous UV sensitivity. Journal of Investigative Dermatology. 1997;108(2):229–232. doi: 10.1111/1523-1747.ep12335337. [DOI] [PubMed] [Google Scholar]
- 38.Lear JT, Smith AG, Strange RC, Fryer AA. Detoxifying enzyme genotypes and susceptibility to cutaneous malignancy. British Journal of Dermatology. 2000;142(1):8–15. doi: 10.1046/j.1365-2133.2000.03339.x. [DOI] [PubMed] [Google Scholar]
- 39.Tsai PC, Huang WY, Lee YC, Chan SH, Guo YL. Genetic polymorphisms in CYP1A1 and GSTM1 predispose humans to PCBs/PCDFs-induced skin lesions. Chemosphere. 2006;63(8):1410–1418. doi: 10.1016/j.chemosphere.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Breton CV, Kile ML, Catalano PJ, et al. GSTM1 and APE1 genotypes affect arsenic-induced oxidative stress: a repeated measures study. Environmental Health. 2007;6, article 39 doi: 10.1186/1476-069X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fryer AA, Ramsay HM, Lovatt TJ, et al. Polymorphisms in glutathione S-transferases and non-melanoma skin cancer risk in Australian renal transplant recipients. Carcinogenesis. 2005;26(1):185–191. doi: 10.1093/carcin/bgh291. [DOI] [PubMed] [Google Scholar]
- 42.Thompson CM, Proctor DM, Suh M, et al. Comparison of the effects of hexavalent chromium in the alimentary canal of F344 rats and B6C3F1 mice following exposure in drinking water: implications for carcinogenic modes of action. The Journal of Toxicological Sciences. 2011;125(1):79–90. doi: 10.1093/toxsci/kfr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Flora S, Wetterhahn KE. Mechanisms of chromium metabolism and genotoxicity. Life Chemistry Reports. 1989;7(3):169–244. [Google Scholar]
- 44.Wu FY, Tsai FJ, Kuo HW, et al. Cytogenetic study of workers exposed to chromium compounds. Mutation Research. 2000;464(2):289–296. doi: 10.1016/s1383-5718(99)00206-5. [DOI] [PubMed] [Google Scholar]
- 45.Cotton SC, Sharp L, Little J, Brockton N. Glutathione S-transferase polymorphisms and colorectal cancer: a huge review. American Journal of Epidemiology. 2000;151(1):7–32. doi: 10.1093/oxfordjournals.aje.a010124. [DOI] [PubMed] [Google Scholar]
- 46.Wong RH, Kuo CY, Hsu ML, et al. Increased levels of 8-hydroxy-2′-deoxyguanosine attributable to carcinogenic metal exposure among schoolchildren. Environmental Health Perspectives. 2005;113(10):1386–1390. doi: 10.1289/ehp.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vavilin VA, Safronova OG, Lyapunova AA, et al. Interaction of GSTM1, GSTT1, and GSTP1 genotypes in determination of predisposition to atopic dermatitis. Bulletin of Experimental Biology and Medicine. 2003;136(4):388–391. doi: 10.1023/b:bebm.0000010960.06583.20. [DOI] [PubMed] [Google Scholar]
- 48.Wang B, Shiao J, Chen CJ, Lee YC, Guo YL. Tumour necrotizing factor-α promoter and GST-T1 genotype predict skin allergy to chromate in cement workers in Taiwan. Contact Dermatitis. 2007;57(5):309–315. doi: 10.1111/j.1600-0536.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 49.Freiman A, Bird G, Metelitsa AI, Barankin B, Lauzon GJ. Cutaneous effects of smoking. Journal of Cutaneous Medicine and Surgery. 2004;8(6):415–423. doi: 10.1007/s10227-005-0020-8. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Graziano JH, Parvez F, et al. Modification of risk of arsenic-induced skin lesions by sunlight exposure, smoking, and occupational exposures in Bangladesh. Epidemiology. 2006;17(4):459–467. doi: 10.1097/01.ede.0000220554.50837.7f. [DOI] [PubMed] [Google Scholar]


