Abstract
Cell-based therapies with various lymphocyte subsets hold promise for the treatment of several diseases, including cancer and disease resulting from inflammation and infection. The ability to genetically engineer lymphocyte subsets has the potential to improve the natural immune response and correct impaired immunity. In this Review we focus on the lymphocyte subsets that have been modified genetically or by other means for therapeutic benefit, on the technologies used to engineer lymphocytes and on the latest progress and hurdles in translating these technologies to the clinic.
The adoptive transfer of various mature lymphocyte subsets into patients with the goal of treating a disease or correcting a congenital immunodeficiency is an old concept that has recently gained momentum in the clinic. Both allogeneic and autologous lymphocytes have been used over the years. Perhaps the most potent therapeutic benefit yet realized with unmodified lymphocytes is the ‘allogeneic effect’ — the tumoricidal activity that follows an infusion of allogeneic lymphocytes. It was appreciated only retrospectively that the powerful and durable antitumour effects of bone marrow transplantation can largely be ascribed to allogeneic T cell transfer. The interest in genetically modifying lymphocytes has increased dramatically in recent years, as several basic and translational scientists have concluded that the modification of autologous lymphocytes should enable the creation of pharmacologically enhanced immune cells that are more potent and have a larger therapeutic window than allogeneic T cell transfer1. In 1990, the first study using genetically modified T cell infusions in patients with cancer was reported. In this study, retrovirus-mediated insertion of the neomycin phosphotransferase gene into the genome of lymphocytes was used to track tumour infiltration following infusion2. The goal of these studies was to mark but not pharmacologically alter the function of the infused T cells. The study was a clinical success in that there was no significant toxicity; however, scientifically there was much room for improvement as at 1 week after the infusion barely 0.01% of the transferred cells remained in the circulation. Significant progress has been achieved since then, and in this Review we describe the increasing range of tools that are available to modify lymphocytes, the various lymphoid subsets and lymphoid progenitors that are suitable for use in immunotherapies, and the potential safety issues and clinical progress in the fields of immunotherapy for autoimmunity, cancer and infectious diseases.
Choosing the tools
Advances in basic science have presented numerous approaches to engineer lymphocytes at the genomic, RNA, epigenetic and protein levels, with the goal of pharmacologically enhancing the immune system.
Virus vector-based approaches
Recombinant adenoviruses and adeno-associated viruses have been the main vectors used for human gene transfer research that involves targeting cells that are not derived from haematopoietic cells. However, although adeno-associated viruses can mediate site-selective integration into the target cell genome under some conditions, it has not been successfully applied to T cells. An overview of viral vectors proposed for engineering the immune system is shown in Table 1. For lymphocyte-based therapies, chromosome-integrating vectors that are derived from gammaretroviruses or lentiviruses have been most useful for long-term gene expression because of their ability to integrate into the host genome, a feature that can result in permanent expression of the transgene, and for their low intrinsic immunogenicity3. The use of gammaretroviruses is somewhat cumbersome because it requires the induction of cell replication for vector integration and, as discussed below, there may be more safety concerns associated with gammaretroviruses than with lentiviruses. Lentivirus-derived vectors are more efficient for gene transfer, in part because of their ability to integrate into the genome of non-dividing cells4,5 and because, in some circumstances, they are less susceptible to gene silencing by host restriction factors6,7.
Table 1. Virus- and non-virus-based approaches for engineering lymphocytes.
| Approach | Advantages | Disadvantages | use in the clinic | Refs |
|---|---|---|---|---|
| Virus-based approaches | ||||
| Gammaretrovirus vectors |
|
|
Extensive clinical use | 2,6,151 |
| Lentivirus vectors |
|
|
Early stage clinical trials with engineered T cells using VSV-G pseudotyped lentivirus vectors | 4,152 |
| Spumavirus vectors or foamy virus vectors |
|
|
Not yet reached the clinic; foamy virus-mediated insertion of CD18 into HSCs corrected congenital CD18 deficiency in dogs | 8,11,153,154 |
| Ad5–F35 vectors |
|
|
Ongoing clinical trial with modified CD4+ T cells for HIV infection (clinicaltrials.gov indentifier NCT00842634). | 12,13,46, 155–157 |
| Lymphotropic herpesvirus (HHV6 and HHV7) vectors |
|
|
Proposed for use in gene transfer and vaccine therapy | 158,159,160 |
| Non-virus-based approaches | ||||
| Random integration with electroporation |
|
|
Clinical trial with a CD20-specific chimeric immune receptor was successful and resulted in engineered T cells with suboptimal engraftment and antitumour effects | 161,162 |
| Transposon-based integration |
|
|
Not yet entered clinical trials | 16,17 |
Ad, human adenovirus serotype; APC, antigen presenting cell; HHV, human herpes virus; HSC, haematopoietic stem cell; LTR, long terminal repeat; NK, natural killer; VSV-G, vesicular stomatitis virus G.
Foamy virus vectors are derived from the Spumavirus genus of retroviruses8 and may have advantages over gammaretroviruses and lentiviruses, of which the most important is that the wild-type foamy virus seems to be non-pathogenic in non-human primates and humans. These vectors will soon enter clinical testing and have promising integration properties that may prove to render them the safest of the integrating viral vectors9–11.
In contrast to recombinant viral vectors derived from human adenovirus serotype 5 (Ad5), viral vectors derived from both Ad5 and Ad35 (Ad5–35 vectors) were reported to mediate gene transfer in up to 10% of resting T cells and 30–45% of T cells after activation with phyto-haemagglutinin12. we found that Ad5–35 vectors resulted in gene transfer in more than 90% of T cells after activation by CD3- and CD28-specific antibodies13. Ad5–35 vectors have promise for adoptive transfer of engineered lymphocytes in clinical situations where expression of a transgene for less than a week is required. In contrast to retroviral vector-transduced cells, the Ad5-35 vector transgene will not persist because adenovirus-based vectors do not integrate into the host genome. It is not yet known if residual adenovirus proteins will render the Ad5-35-transduced T cells immunogenic and/or impair their persistence.
Non-virus-based approaches
The use of approaches to engineer lymphocytes that do not involve viral vectors has increasing promise (Table 1). electroporation can now efficiently introduce plasmid DNA into lymphocytes for transient expression of transgenes14. However, electroporation can occasionally result in genome integration, although this occurs at much lower frequencies than with virus-based approaches, resulting in stable expression and the accompanying potential for genotoxicity The first clinical trial testing the adoptive transfer of T cells engineered using electroporation was recently reported15, and although this approach was safe, the cells were short-lived after transfer, probably owing to the long-term culture of the cells that was necessary to achieve sufficient integration efficiency.
Transposon-based systems can integrate transgenes much more efficiently than plasmids that do not contain an integrating element16,17. Transposons are most commonly introduced into cells by electroporation or lipofection and they contain nuclear localization signals that, in the presence of transposase enzymes, integrate into the genome at 5′-TA-3′ sequences. various transposase-based systems are now entering clinical trials to test the safety and feasibility of this approach to engineer T cells18.
Non-viral vectors have several advantages over viral vectors in engineering cells, including lower financial costs and perceived safety benefits. clinical-grade plasmid DNA-based approaches are substantially less expensive than approaches using recombinant viral vectors. what is less certain is the safety profile of these approaches. Gammaretroviruses and lentiviruses have a proven safety record for use in human T cell engineering19,20, whereas the relative genotoxicity of transposons is unknown. The integration sites of lentivirus-based vectors are not random and do not seem to favour proto-oncogenes21. It remains unknown whether transposon-based systems will be more or less favourable in this regard and whether they can reach the efficiencies that are achieved by virus-based integration. Approaches to achieve site-specific integration and DNA editing are described in Box 1. These approaches are just entering clinical trials and, if found to have sufficient efficiency, should allay concerns regarding lymphocyte engineering fusing non-site-specific integration approaches.
Box 1. Targeted integration and genetic editing using zinc-finger nucleases.
Targeted genetic modification at a predetermined locus in embryonic stem cells has been possible for two decades using homologous recombination134, enabling the routine creation of genetically targeted mice. However, this approach has not been useful for human gene therapy because the low efficiency of recombination necessitates prolonged cell selection in culture. Zinc-finger nucleases (ZFNs) are chimeric proteins that contain zinc-finger DNA-binding proteins that enable site-specific DNA binding when fused to the catalytic domain of the type IIS restriction enzyme FokI. In essence, ZFNs are designer restriction endonucleases that can cleave DNA specifically at a predetermined genomic target site135. The FokI restriction endonuclease is not sequence specific: the target specificity is conferred by the zinc-finger proteins. Once a site-specific double-stranded break is made by FokI, the cell repairs the break using the non-homologous end-joining repair process, which can result in the knockout of the desired gene or the site-specific incorporation of transgene DNA. The potential applications of ZFN technology have been reviewed elsewhere136,137.
The development of ZFNs has now achieved sufficient efficiency to enable clinical approaches to site-specific genome modification138. We have recently reported that the transient expression of ZFNs encoded by plasmid DNA that bind to the HIV-1 co-receptor CC-chemokine receptor 5 (CCR5) gene disrupts the locus in approximately 50% of cells, creating cells that are resistant to HIV infection13. In collaboration with Sangamo Biosciences we have initiated the first protocol incorporating ZFN technology, testing the adoptive transfer of CCR5-disrupted CD4+ T cells for patients with HIV infection (clinicaltrials.gov indentifier NCT00842634). In this protocol, two ZFNs that bind specific sequences of CCR5 on opposite strands of DNA are introduced into CD4+ T cells. The cleavage domain of FokI is fused to the 3′ end of each ZFN, which allows dimerization of the FokI cleavage domains, in turn resulting in the generation of a double-stranded break. Repair of the double-stranded break in most cases results in the subsequent disruption of CCR5 by creating a null allele.
RNA engineering
For some applications, genomic alteration of adoptively transferred lymphocytes may not be required to achieve substantial therapeutic effects (Box 2). RNA-based electroporation of lymphocytes using in vitro transcribed mRNA mediates transient expression of proteins for approximately 1 week, and redirected T cells transduced with RNA encoding T cell receptors (TCRs) or chimeric antigen receptors have the expected gains of function22,23. This approach is attractive because RNA transduction efficiencies can approach 100% and the immediate toxicity is much lower than that of approaches using electroporation of plasmid DNA. other investigators have successfully modified T cell functions using RNA transduction of chemokine receptors and cytokines24,25. Furthermore, RNA-based approaches are expected to be less genotoxic than approaches that intentionally modify the genome. Transposase enzymes can be delivered as mRNA26, thereby avoiding the possibility of genomic integration and continued chromosome ‘hopping’ (REF. 27).
Box 2. Alternative approaches to modify lymphocytes.
Important examples of non-virus-based approaches to engineer lymphocytes that leave the genomic DNA sequence intact are listed here.
RnA transduction
mRNA and small interfering RNA (siRNA) can be efficiently introduced into lymphocytes and dendritic cells with low toxicity22. For example, the transfer of siRNA designed to downregulate FAS (also known as CD95) expression can render T cells resistant to the apoptotic effects of tumour cells that express FAS ligand (also known as CD95L)139. Rossi and colleagues have developed siRNA technology to allow the expression of multiple siRNAs in lymphocytes, so that various combinations of biological effects can be manipulated to generate HIV-resistant cells19.
DNA methyltransferase inhibitors
The addition of DNA methyltransferase inhibitors to cell culture medium can stabilize the expression and function of forkhead box P3 (FOXP3) in regulatory T (TReg) cells36.
Histone deacetylase (HDAC) inhibitors
There are multiple genes that result in hyperacetylation of histones, and multiple agents that inhibit HDACs39. Inhibition of HDAC9 enhances TRegcell function38.
Protein transduction and painting
The use of protein transduction domains enables the efficient introduction of biologically active cargo into lymphocytes, including proteins and siRNA42. Surface engineering of lymphocytes may be used to redirect and alter the function, using strategies that incorporate biotinylation140 or protein painting50,141.
In addition to mRNA engineering, various approaches using regulated expression of microRNAs might be used for pharmacological alteration of lymphocyte function, as first shown for microRNA-181 (REF. 28). The role of microRNAs in the regulation of lymphocyte function is reviewed in REF. 29. clinical trials using mRNA-transduced dendritic cells have been safely conducted30 and trials using mRNA-electroporated lymphocytes are being initiated by several groups.
Epigenetic engineering
In addition to the expression of master transcription genes31, recent studies have shown that several epigenetic events, including DNA methylation, histone modifications and changes in chromatin structure, can affect lymphocyte subset differentiation and function32. Epigenetic marks can be heritable but are inherently plastic because the DNA sequence remains unchanged, and in theory these marks provide an approach for epigenetically modified adoptive cell therapy, which is expected to be less durable than the use of genetically engineered cells but intrinsically safer. In mammalian cells, ∼5% of the deoxycytidine residues in DNA are present as 5-methyl-deoxycytidine residues33, which are primarily found in CpG motifs. methylation occurs immediately after DNA replication and involves the transfer of a methyl group in a reaction catalysed by DNA methyl-transferases (DNMTs). In general, methylation of DNA represses gene expression, whereas demethylation results in gene activation34. In early studies, the inhibition of DNA methylation with the incorporation of azacytidine in cell culture was shown to promote interleukin-2 (IL-2) secretion in transformed cell lines35, and recent studies indicate that epigenetic regulation of the regulatory T (TReg) cell-associated transcription factor forkhead box P3 (FOXP3) can be predictably controlled with dNmT inhibitors to generate functional, specific and seemingly stable TReg cells36. However, the use of DNMT inhibitors in vivo can also augment the effects of adoptively transferred conventional T cells37. The demethylation agent 5-aza-2′-deoxycytidine, decitabine (dacogen; eisai), has been approved by the uS Food and drug Administration for the treatment of patients with myelodysplastic syndromes and may be useful for various adoptive transfer strategies.
Histone deacetylase (HDAC) inhibitors have been shown to be potent inducers of immunosuppressive pathways. TReg cells express more HDAC9 than do conventional T cells, and systemic treatment with HDAC inhibitors stimulates TReg cells38, as does the incorporation of various HDAC inhibitors during in vitro cell culture (T. Akimova and w. w. Hancock, personal communication). However, systemic therapy with less specific HDAC inhibitors also mediates direct antitumour effects39 and augments the antitumour effects of adoptively transferred conventional T cells40. Thus, the incorporation of various DNMT inhibitors and HDAC inhibitors in cell cultures as an approach to engineer lymphocyte subsets has the potential to augment T cell function and maintain fidelity in vivo. Indeed, it is conceivable that the effects of inhibitors on lymphocyte function during in vitro engineering may be more pronounced and selective because in vivo therapies with these inhibitors can have opposing effects owing to indirect effects on the tumour, the tumour microenvironment or antigen presentation that are distinct from the direct effects on lymphocyte subsets. Further studies are required to clarify the pleiotropic and tissue-specific expression of DNMT and HDAC inhibitors41 in order to guide their use for optimally augmenting or suppressing lymphocyte functions. However, it is already clear that the intentional epigenetic remodelling of lymphocytes has the potential to enhance or suppress lymphocyte effector functions.
Protein transduction
Advances in fusion protein technology allow high transduction efficiencies for many mammalian cell types. Proteins exceeding 100 kDa can be introduced into cells by incorporation of small protein transduction domains (PTDs) derived from the HIV protein Tat, the antennapedia homeodomain from Drosophila melanogaster or the VP22 protein from herpes simplex virus (HSV)42. Recent advances of the technology allow the delivery of packaged synthetic small interfering RNA (siRNA) duplexes into cells43. The addition of recombinant proteins and siRNA complexes to cell culture approaches has several potential uses for engineering lymphocytes. First, the HIV Tat PTD has been shown to efficiently introduce peptides into the mHc class I pathway for presentation by antigen-presenting cells (APCs), and the antigen-loaded APCs then efficiently prime cytotoxic T lymphocyte (CTL) responses44. Because human T cells, unlike mouse T cells, can efficiently function as APCs to prime CTL responses45,46, protein transduction technology could be used to load T cells in vitro with desired antigens for an adoptive transfer-based vaccine approach. Because proteins fused to PTDs can pass through cell membranes and retain biological function, they also have the potential to modify lymphocyte function in a cell-autonomous manner. In recent studies, the cytoplasmic domain of cytotoxic T lymphocyte antigen 4 (CTLA4) fused to a PTd has been introduced into T cells and shown to specifically inhibit TCR signalling47, and the systemic administration of CTLA4-PTD ameliorated collagen-induced arthritis in mice48. Recent studies indicate that bispecific antibodies targeting CD3 and CD19 can mediate substantial antitumour effects that are presumably MHC independent in patients with lymphoma49, and thus the use of protein-based transduction and a related strategy, known as protein painting50, has significant opportunity to engineer enhanced lymphocyte functions without the risks of gene transfer approaches.
General considerations
The ultimate choice of the technology used to engineer cellular immunity should be based on several scientific and practical considerations. Scientific considerations include the size of the transgene, the number of transgenes, the requirement for permanent or transient genetic modification, the transgene expression level (high or low) and whether constitutive or inducible transgene expression is desired. For therapy with engineered lymphocytes using integrating vectors, a key issue facing the field is whether the risk of oncogenic transformation is greater with retroviral vectors than with lentiviral vectors. None of the clinical trials carried out so far using T cells genetically modified by gammaretrovirus vectors has reported adverse events due to insertional mutagenesis. cyclin-dependent kinase inhibitor 2A (CDKN2A) is a tumour suppressor protein and, in tumour-prone mice engrafted with CDKN2A-deficient bone marrow, a gammaretrovirus vector was found to accelerate tumorigenesis, whereas a lentivirus vector, present at higher copy number, did not51,52. For approaches that require integrated transgenes, targeted integration is preferable to the integration patterns of retroviruses. However, at present, only zinc-finger nucleases (ZFNs) can mediate targeted integration, and the safety and feasibility of this approach is still unknown (Box 1). Some strains of adeno-associated virus can mediate targeted integration; however, to date this has not been possible with T cells.
Practical considerations that guide the type of cellular engineering used include the cost of products for cell culture and the availability of clinical-grade vector systems. These considerations should be based on the target product profile of the engineered lymphocytes. In some instances only a brief persistence of adoptively transferred lymphocytes may be required, whereas in others lifelong persistence seems desirable. For example, therapy with engineered lymphocytes is ongoing for patients with advanced cancer53 and persistant therapy has been proposed for patients with type 1 diabetes54 or multiple sclerosis55. ethical considerations would dictate that a much higher level of safety would be required for the engineered lymphocytes used for type 1 diabetes, a chronic disease for which life-sustaining but non-curative therapy is available, than those used for patients with cancer who do not have long-term therapeutic options.
Choosing the delivery service
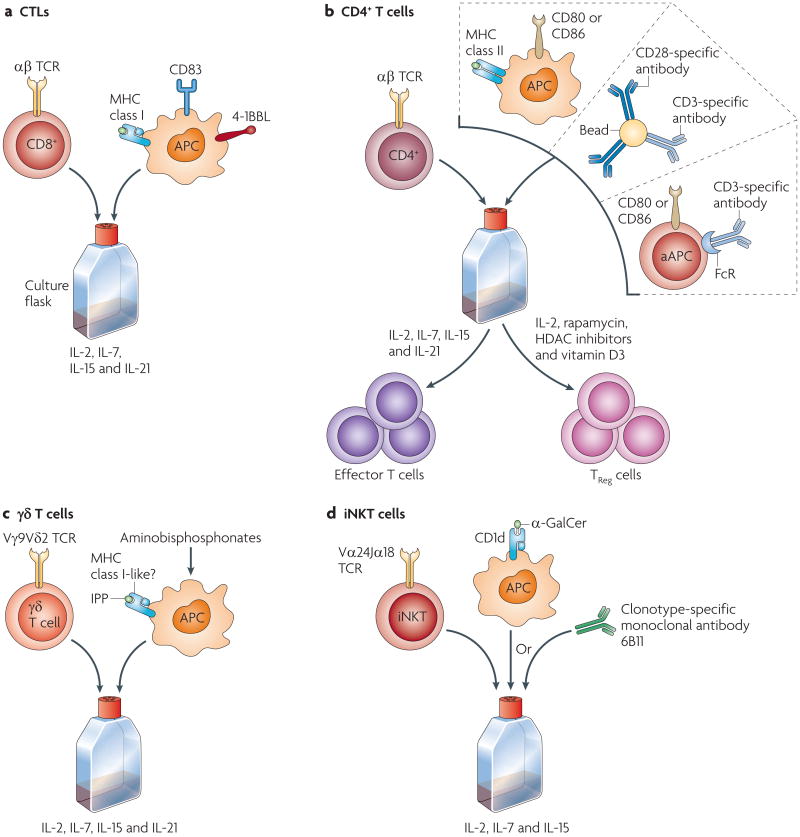
Mature T cells are among the most suitable cells for modification, and stable modification has been achieved using several approaches. The efficiency of lymphocyte modification has been consistently higher than with haematopoietic stem cells (HSCs)19, such that with current technologies transgene delivery and expression in T cells is not limiting. An overview of cell culture approaches used for various lymphocyte subsets that have been tested in human trials is shown in Fig. 1.
Figure 1. Cell culture approaches for adoptive transfer of lymphocyte subsets.
Culture conditions required for distinct lymphocyte subsets vary, depending on the activation and co-stimulatory requirements. a | Cytotoxic T lymphocytes (CTLs) express αβ T cell receptors (TCRs) and are stimulated by antigen-presenting cells (APCs) that express MHC class I molecules. CTLs require 4-1BB ligand (4-1BBL; also known as CD137L) stimulation for clonal expansion and have been expanded in vitro by the addition of feeder cells or artificial APCs that express the co-stimulatory ligands147–149. b | CD4+ T cells are stimulated by APCs that express peptide-loaded MHC class II complexes. The main co-stimulatory molecule expressed by CD4+ T cells is CD28, which binds to CD80 or CD86 expressed on APCs. Effector CD4+ T cells can be stimulated by artificial APCs (aAPCs) or beads that bear CD3-specific antibody in the presence of various cytokines76. Regulatory CD4+ T (TReg) cells require culturing in interleukin-2 (IL-2), and the addition of several reagents to the culture may enhance the suppressive functions of ex vivo expanded TReg cells68. c| Cells expressing γδ TCRs, such as the Vγ9Vδ2 TCR expressed by T cells in the blood, are stimulated by APCs that present exogenous isopentenyl pyrophosphate (IPP) or other endogenous ligands stimulated by aminobisphosphonates such as zoledronate86,89. d| CD1d-restricted Vα24Jα 18+ invaria1nt natural killer T (iNKT) cells are stimulated by α-galactosylceramide (α-GalCer)99 or the 6B11 clonotype-specific monoclonal antibody150. FcR, Fc receptor; HDAC, histone deacetylase.
Conventional T cell subsets
Immunotherapy with engineered T cells is attractive for several reasons, including the long-lived persistence that has been shown in humans following adoptive transfer56. A major advantage of adoptive cell therapy is that the therapeutic effects can be augmented by isolating the lymphocytes that have desired effector or regulatory properties while removing the cells that may have antagonistic effects. clinical studies with effector T cells are the most advanced, and have progressed to a Phase III clinical trial testing the efficacy of T cells that are transduced to express an HSV thymidine kinase (HSV-TK) conditional ‘safety switch’ in the setting of haploidentical stem cell transplantation for high-risk acute leukaemia in remission57. In this approach, allogeneic T cells can mediate antitumour effects in the context of allogeneic stem cell transplantation, and in the event of significant graft-versus-host disease (GVHD) the cells can be ablated by administration of the antiviral drug ganciclovir; the cells expressing HSV-TK are selectively killed because HSV-TK mono-phosphorylates ganciclovir (a guanosine analogue), which is eventually converted to the toxic triphosphate form and incorporated into replicating DNA where it causes chain termination and cell death.
At present, there is considerable enthusiasm for the approaches involving the adoptive transfer of engineered CD8+ CTLs. The transfer of MHC class I-restricted TCRs can ‘convert’ a population of polyclonal CD8+ T cells to CTLs of monoclonal TCR specificity58. This approach is attractive because high-affinity CTLs of appropriate specificity are generally lacking in patients with advanced cancer or chronic infections. Thus, the introduction of TCRs with high affinity or even a supraphysiological affinity has the potential to increase the recognition and killing of tumour cells that have low expression of cognate peptide-MHC class I complexes. In the case of HIV infection, high-affinity TCRs seem to have an improved ability to control viral infection and to delay the appearance of virus escape mutants59. clinical trials testing the adoptive transfer of engineered TCRs for cancer have been reported with promising clinical results and serious but generally reversible tissue-specific ‘on target’ toxicity60.
Since being first reported in mice more than two decades ago61, the transfer of CD8+ T cells engineered to express MHC-unrestricted chimeric antigen receptors is now rapidly advancing in human trials. chimeric antigen receptors have the potential to serve as an ‘off the shelf ’ reagent to redirect T cells with cytotoxic or regulatory functions to desired cell surface ligands of various tumour, stromal and viral targets62. Recently, the first report to show clinical antitumour effects with chimeric antigen receptors investigated the effects of adoptively transferred autologous T cells, expressing a chimeric antigen receptor that targeted the diasialoganglioside GD2, in patients with advanced neuroblastoma63. This trial used a first-generation chimeric antigen receptor comprised of a TCRζ domain, and improved methods of cell culture were shown to contribute to enhanced persistence of the adoptively transferred chimeric antigen receptor modified T cells. In particular, the subset of T cells with antigen-specific chimeric antigen receptors specific for epstein-Barr virus had improved in vivo survival, probably because they received appropriate co-stimulation from professional APCs expressing epstein-Barr virus antigens. more advanced chimeric receptor and vector designs incorporating multiple co-stimulatory domains and lentiviral vector technology are now entering clinical trials at several centres64,65.
The adoptive transfer of engineered CD4+ T cells has promise for adoptive therapy of cancer and HIV (reviewed in REFS 19,66). A recent intriguing study found that in vitro-polarized T helper 17 (TH17) cells were more effective in mediating regression of B16 melanoma than unpolarized TH0 cells or TH1 cells67. The adoptive transfer of TReg cells has the potential to prevent GvHd and solid organ transplant rejection and to prevent or treat autoimmunity68. engineered TReg cells have been proposed for the treatment of autoimmune disease based on results from preclinical models. In one model, genetic modification of polyclonal TReg cells with a chimeric antigen receptor consisting of a myelin basic protein epitope bound to the extracellular and transmembrane domains of an mHc class II molecule linked to the cytoplasmic domain of the TCRζ chain resulted in functional TReg cell activation following recognition of these modified TReg cells by myelin basic protein-specific T cells, thus preventing or reducing the lethality of experimental autoimmune encephalomyelitis69. others have shown that TReg cells engineered to express chimeric antigen receptors have potential for the treatment of colitis70. one issue with redirected T cells has been whether sufficient survival of T cells expressing chimeric antigen receptors will occur to mediate long-term effects. It has been shown that endogenous cytotoxic CD4+ T cells specific for varicella zoster virus (VZV) can be engineered to express tumour-specific chimeric antigen receptors. The VSV-specific T cells can be expanded in vivo by stimulation of their native TCR by administration of VZV vaccine, but they retain the ability to recognize and lyse tumour targets in an MHC-independent manner through the VZV-specific chimeric antigen receptor71. Together, these results are important because target cells in patients with cancer and chronic viral infections often have decreased expression of antigen-loaded MHC molecules.
Studies indicate that, on a per-cell basis, the adoptive transfer of T cells with extensive replicative capacity have improved engraftment and antitumour effects than transfer of terminally differentiated effector T cells that have a more potent cytotoxic effector function72. This might be because central memory (TCM) T cells can self renew and differentiate into effector T cells in vivo, whereas terminal effector memory T (TEM) cells have lost this plasticity73,74. A controversy has arisen in the field as to how best to generate T cells with good replicative capacity and the desired function. One approach is to isolate TCM cells with the desired specificity in vitro by sorting or other means of physical separation, engineer the desired antigen specificity, expand and then infuse the TCM cells75. we have proposed that manipulation of bulk T cell culture conditions can enrich and maintain TCM cells, and thereby remove the need for cell sorting procedures. our group and others have found that cell culture conditions that augment co-stimulation through CD28 and 4-1BB (also known as CD137) promote the maintenance of TCM cells in vitro76-78and in vivo79-81. The use of memory stem cells (that is, T cells programmed for the most extensive self renewal) has significant potential82.
γδ T cells
Recent advances in our understanding of the biology of γδ T cells suggest that these cells have promise as a molecularly targeted immunotherapy, with several properties that αβ T cells lack. There is evidence that both αβ T cells and γδ T cells have a role in tumour immunosurveillance83,84, although the relevance of γδ T cells to tumour immunosurveillance for certain tumours has been questioned85. Human γδ T cells can kill various primary carcinomas86, and a recent study indicates that they are cytotoxic to cancer-initiating cells87. Another feature of human γδ T cells is that they can prime αβ T cells with an efficiency similar to that of dendritic cells88, and thus adoptive transfer of γδ T cells could serve to augment cytotoxic T cell effector functions. unlike αβ T cells, γδ T cells are activated by various non-peptide endogenous ligands that are expressed on stressed cells. clinical grade synthetic ligands are now available that allow the efficient expansion of the major subset of Vγ9Vδ2+ T cells in peripheral blood, or cells may be treated with aminobisphosphonates such as zoledronate (Zometa/Aclasta/Reclast; Novartis)89, which are agents that increase the expression of endogenous ligands such as isopentenyl pyrophosphate, a steroid intermediate that promotes the expansion of γδ T cells (see Supplementary information S1, (figure)). Human γδ T cells in the blood seem to be more terminally differentiated, whereas those in tissues are less differentiated. It is not known how the population of vγ9vδ2+ T cells in the blood are maintained, as at present they do not have an extensive proliferative capacity in vitro, unlike αβ T cells86. It is possible that optimal conditions for in vitro propagation have not yet been identified and, consistent with this, a recent report showed that inclusion of IL-2 and IL-21 in the culture medium with the synthetic ligand isopentenyl pyrophosphate generated γδ T cells with more potent cytotoxicity and higher TH1-type cytokine secretion than if only IL-2 is used90.
Adoptively transferred γδ T cells can eradicate human tumour xenografts in various SCID mouse models91, and two recent clinical studies have shown the safety and feasibility of adoptive transfer of peripheral blood Vγ9Vδ2+ T cells stimulated with the synthetic ligand 2-methyl-3-butenyl-1-pyrophosphate and IL-2 (REF. 92) or isopentenyl pyrophosphate and IL-2 (REF. 93).
Human γδ T cells can be engineered to express non-MHC-restricted chimeric antigen receptors94 and αβ TCRs95. The expression of additional TCR chains in T cells can lead to the generation of T cells with unknown specificity owing to the formation of mixed dimers between the endogenous and introduced TCR chains96. The use of γδ T cells may provide a significant safety feature over the use of TCR-ENGINEERED αβ T cells, taking advantage of the finding that α and β TCR chains cannot pair with γ and δ TCR CHAINS. Nevertheless, it remains unclear whether the adoptively transferred γδ T cells will home to tumours or sites of inflammation and, if so, what is the best source of γδ T cells. Blood-derived vγ9vδ2+ T cells are the most convenient cells to harvest and have cytotoxic effector functions against various tumours86, but vγ9vδ2− T cells derived from tissues may be preferable as they may have longer persistence and improved homing capacity, especially for patients with tumours located in the skin and intestines, sites that are preferentially targeted by γδ T cells.
Natural killer T cells
Natural killer T (NKT) cells are functionally related to γδ T cells in that they also bridge innate and adaptive immune responses and can enhance or suppress immunity97. The best characterized human NKT cell subpopulation, referred to as invariant NKT (iNKT) cells, expresses CD161 and an invariant Vα24Jα18 TCR that recognizes α-galactosylceramide (α-GalCer) presented by the MHC class I-like molecule CD1d. After activation, iNKT cells have MHC-independent cytotoxic activity for various tumours and secrete high levels of interferon-γ (IFNγ), although this function becomes impaired in patients with cancer98. compared with mouse NKT cells, human NKT cells are rare (<1% of total lymphocytes). However, human NKT cells, unlike mouse NKT cells, can undergo substantial expansion in vitro after repeated stimulation with α-Galcer-pulsed APCs, similar to conventional αβ T cells stimulated with peptide-loaded APCs99. The first adoptive transfer studies of iNKT cells have been conducted by adapting this method to a clinical-scale process. expanded iNKT cells were administered intravenously to patients with lung cancer100 and by intra-arterial injection to patients with squamous cell carcinoma of the head and neck101. The results were promising, demonstrating safety, feasibility and some evidence of antitumour efficacy. In mice, iNKT cells have also been shown to have immunosuppressive effects97, so caution must be taken for their use in patients with cancer, but this may mean that they have potential for the treatment of autoimmunity.
Future considerations for engineered lymphocyte therapy
Robust clinical-scale culture technologies have now been developed for αβ T cells, TReg cells, γδ T cells and iNKT cells, allowing for the first time adoptive transfer approaches with diverse subsets of engineered lymphocytes. For clinical scenarios in which long-term engraftment of the adoptively transferred T cells is desired αβ T cells are preferable, and the extensive proliferative capacity that iNKT cells display in vitro indicates that they may have similar enhanced persistence. However, for some applications, short-term engraftment provided by γδ T cells may be sufficient or even desired for situations in which long-term engraftment might cause toxicity. In this case, short-term persistence of the engineered cells would replace the need to include conditional safety switches. Another attractive feature of γδ T cells is the possibility of expressing αβ TCRS in these cells without the risk of generating mixed heterodimeric TCRs. In addition, the distinct homing properties and intrinsic high level cytotoxicity of engineered γδ T cells and iNKT cells together may have additional or even synergistic therapeutic effects over the use of only αβ T cells. Finally, various approaches using lymphocytes as vehicles to deliver cargo to tumours such as oncolytic viral vectors have been proposed102, and redirected γδ T cells and iNKT cells may be preferred for these approaches to increase the delivery of oncolytic vectors and take advantage of the ability of these cellular carriers to protect from systemic viral neutralization.
when our understanding of lymphocyte development improves, another area of opportunity will be the adoptive transfer of engineered precursor cells that are programmed to have the functions of effector lymphocytes. examples include the adoptive transfer of engineered common lymphoid progenitor cells103, memory stem cells82 and ‘retrogenic T cells’, which are embryonic stem cells or HSCs engineered to express transgenic TCRs or chimeric antigen receptors after they differentiate into T cells in vivo104–107.
Genotoxicity and other safety considerations
Lymphocyte engineering procedures can take place in vitro or can be done in vivo using various targeting strategies108-110. To date, only strategies using in vitro engineering and integrating vectors have reached clinical trials, in part owing to the inherent safety of in vitro approaches. A general feature of gene transfer strategies using in vitro engineered lymphocytes is that the potential toxicity is diminished and more predictable than after in vivo gene transfer approaches.
There are several potential safety concerns with engineered lymphocytes. The safety profile of autologous non-modified lymphocyte adoptive transfers is well established (reviewed in REF. 111) and the only common adverse events have been attributed to cytokine release, which is well tolerated with premedication, and manifestations of autoimmunity such as vitiligo and thyroiditis. Recently, we have observed colitis and skin rashes resembling GVHD in patients given non-modified autologous T cells under conditions that promote homeostatic expansion of the transferred cells112. In addition to these adverse effects of unmodified lymphocytes, the toxicity due to transfer of engineered T cells is expected to include various ‘on-target’ and ‘off-target’ toxicities from transgenes, as well as vector-specific toxicity.
Transgene-specific toxicity
Toxicity attributable to the specificity of the transgene has been observed and for future trials the expected toxicities should be considered on a case by case basis, depending on the particular modification. on-target toxicity can occur as a consequence of the modified lymphocyte reacting to the structure targeted. This was reported in the first trial of gene-modified T cells expressing an HLA-A2-restricted TCR specific for MART1 (melanoma antigen recognized by autologous T cells 1; also known as MLANA) antigen113: both antitumour effects (on-target efficacy) and toxicity at non-tumour sites that also express MART1, such as the skin, ears and eyes, (on-target, off-organ toxicity) were observed. The most extreme experience with on-target, off-organ toxicity occurred in a clinical trial in which patients were given T cells engineered to express a chimeric antigen receptor that was specific for carbonic anhydrase IX (CAIX), a transmembrane protein that is overexpressed by cancerous kidney cells. In three of three patients, severe liver toxicity ensued within 1 week following infusion of the gene-modified T cells114,115. Subsequent investigation revealed that the CAIX protein was also expressed in the biliary tract of the liver, illustrating the need to carefully pick the target as well as the potent effects of engineered lymphocytes.
For ethical and both scientific and lay perception issues, genetic engineering is held to a higher safety standard than many other experimental therapies. To date, there is promising efficacy following the infusion of gene-modified T cells in various clinical trials, and no serious adverse events that are a result of the engineering process have been reported. mature lymphocytes are intrinsically resistant to transformation; however, the specific context of the genetic engineering can increase the risk of transformation. For example, a serious concern has recently been reported following the introduction of a vector that encoded IL-15 into human CTLs116. The cells exhibited logarithmic in vitro growth in the absence of exogenous cytokine support for more than 1 year after transduction with a gam-maretrovirus-based vector encoding IL-15. The clone exhibited constitutive telomerase activity, and the presence of an autocrine loop was suggested by impaired cell proliferation following knockdown of IL-15 receptor α-subunit expression. The authors concluded that although the cells have the theoretical promise to generate T cells with enhanced effector qualities, this particular approach would have unacceptable risk for genotoxicity in the context of a clinical application. However, the beneficial effects of cytokine gene transfer in T cells can be substantial and the addition of a conditional suicide gene has the potential to ensure the safety of the approach.
The increasing interest in the use of lymphocytes engineered to express transgenic TCRs has raised concerns for new forms of off-target toxicities that may occur117. For example, the transgenic TCRs may exhibit imperfect allelic exclusion and mispairing can occur with the endogenous and transgene TCR chains, leading to the generation of TCRs with new specificities that have not been selected for tolerance in the thymus. Toxicity from ‘mixed TCR dimers’ has not yet occurred in clinical trials; however, it is premature to conclude that this will not occur, as there is at present insufficient clinical experience with T cells expressing transduced TCRs. Finally, using phage display technology to engineer TCRs and lentiviral vectors118, our group has recently reported that T cells engineered to express TCRs with enhanced affinity to their cognate antigen have greater antiviral activity than the parental TCR-modified T cells59. It is possible that picomolar affinity variants of TCRs may have off-target specificities through the development of crossreactivity to other mHc proteins or degenerate peptide recognition.
Vector-specific toxicity
Lymphocytes modified by genome-integrating vectors can be expected to have various toxicities (Box 3). The most serious effect reported to date is cell transformation. It is well documented that gammaretroviruses can cause insertional activation of proto-oncogenes in animals, resulting in cancer119. As the human genome comprises approximately 3 × 109 bases, if transgene integration into cellular alleles with an average target size of 10 kb is a random process, then integrations into each gene would be expected to occur at a frequency of 1 in 300,000 integrations120. As 107 to 109 lymphocytes are typically transduced in current clinical trials, each gene would be expected to be disrupted or mutated often, with the most frequent outcome being the generation of a null allele. Recent studies indicate that the mouse mammary tumour virus, a gammaret-rovirus, has a nearly random dispersion of integration sites121. Gammaretroviruses integrate preferentially near transcription start sites, and may be particularly prone to cause insertional activation of proto-oncogenes. By contrast, lentiviruses preferentially target transcription units, and analysis of the integration-site positions in the human genome revealed that the HIV-1 integration sites are clustered122. Initial characterization of the integration sites in patients with HIV or AIDS receiving adoptively transferred lentivirus-engineered CD4+ T cells indicates that the pattern is similar to that of wild-type HIV-1 integration sites20. In particular, no integrations were identified within 50 kb of proto-oncogenes or known tumour suppressor genes. These empirical data, in conjunction with natural history data from patients with HIV or AIDS who have not developed T cell leukaemia, are consistent with a favourable profile for genotoxicity of lentivirus-engineered lymphocytes used in clinical trials. whether this will prove to be true for lentivirus-engineered HSCs and progenitor cells remains to be established.
Box 3. Vector-specific toxicities due to genetic modification.
Gene modification in lymphocytes is most commonly accomplished using gammaretrovirus vectors or lentivirus vectors. The infusion of gene-modified lymphocytes may be associated with the following adverse effects:
Insertional mutagenesis due to proviral DNA integration, which could lead to clonal proliferation and leukaemia. All forms of genome modification that result in DNA breaks have some risk of oncogenesis. In addition, vector-specific elements such as retroviral long terminal repeats can have genotoxicity124. Insertional mutagenesis that resulted in leukaemia has occurred after gene modification of haematopoietic stem cells142 but has not been reported following modification of mature lymphocytes. Details of the mechanisms of insertional activation have been reviewed elsewhere120.
Generation of replication-competent lentiviruses and retroviruses. The retroviral and lentiviral vectors are designed to support only a single round of infection. Early-generation retroviral vectors were susceptible to contamination with helper viruses that produced infectious retroviruses, and these replication-competent retroviruses resulted in T cell lymphoma in rhesus monkeys143. Replication-competent lentiviruses can be generated in vitro during vector production by recombination of vector plasmids or in vivo by mobilization of the vector proviral DNA by infectious lentiviruses such as HIV. Conditional replication of a lentiviral vector that was self limiting has occurred during a T cell gene therapy trial144; however, the long-term safety of this approach remains to be shown. Under some circumstances, controlled conditional replication of vectors could be of therapeutic benefit145.
Germline transmission of the transgene or germline alteration, which could result in vertical transmission to offspring.
Generation of infectious virus that could be transmitted to other people. This potentially catastrophic event was the subject of a post-apocalyptic book146 and a recent movie (I Am Legend, 2007) about a virologist who uses a recombinant measles virus to cure cancer.
Are engineered T cells ‘safer’ than engineered HSCs?
The development of retroviral vectors provided the first means to engineer lymphocytes at efficiencies that would support their use in clinical trials. of historical interest, bone marrow-derived HSCs were proposed for the first clinical trials involving gene transfer therapy commencing with the development of efficient gamma retrovirus-based vectors in the early 1980s123. However, the promise of engineered HSCs that could generate a permanently engineered immune system — an immunological ‘holy grail’ — has not yet been realized for various reasons. These include poor engraftment and low transgene expression in the progeny of engineered HScs and the later development of leukaemia in some patients after infusion of engineered HSCs124. By contrast, engineered lymphocytes have not suffered from these technical limitations, and investigators have turned increasingly to lymphocyte engineering. Thus, an issue is whether it is safer to use lymphocytes or HSCs for gene transfer therapy. In elegant studies of SCID-X1-immunodeficient patients using gammaretroviral vectors to restore IL-2 receptor γ-chain gene expression in HSCs, investigators showed strong therapeutic efficacy: nine of ten patients were successfully treated, (although, four of the nine developed T cell leukaemia 31-68 months after gene transfer)125. At present, no malignancies have been reported in humans following adoptive transfer of genetically engineered T cells. of note is the long incubation period observed before the development of overt leukaemia. we are aware of more than 200 patients receiving treatment as part of various T cell-based trials using gammaretroviral-engineered T cells for cancer, HIV and congenital adenosine deaminase deficiency, in which the patients routinely have detectable engraftment of the engineered T cells for 5 years or longer56,126,127. There have been no incidences of transformation reported, and thus as these patients have been engrafted for periods of time that exceed the ‘incubation’ time required to observe overt transformation in the case of SCID-X1 correction with HSCs, the combined clinical data with engineered T cells supports the notion that gammaretrovirus-based gene transfer therapy with T cells is safe. However, the safety profile could change as the field increasingly turns to the use of engineered lymphocytes that have a more extensive self-renewal capacity.
To experimentally address this issue, von Laer's group128 transduced mature T cells or HSCs with gammaretroviral vectors expressing the T cell oncogenes LMO2, TCL1 and TRKA and then transferred the cells into recombinase-activating gene 1 (RAG1)- deficient mice. The animals were followed for at least 1 year and no animals given T cell infusions developed T cell malignancies. By contrast, mice that received an engineered HSC transplant developed T cell lymphoma or leukaemia at a high frequency, leading to the conclusion that polyclonal mature T cells in vivo are less susceptible to transformation by known T cell proto-oncogenes than progenitor cells. Studies in mice indicate that there is an inverse relation between clonal frequency and survival of adoptively transferred T cells129, suggesting that intraclonal competition is a mechanism to maintain a diverse repertoire of T cells and an optimal environment for the generation of long-lived memory cells. One mechanism that may control transformation of engineered T cells is that clonal competition may prevent the emergence of overt leukaemia in mature T cell populations.
Conclusions and perspectives
cell transfer therapy with engineered cells is perhaps the ultimate example of personalized medicine. To become widely available, genetically engineered cells will have to be shown to be clinically effective, scalable, reproducibly manufactured and appropriately priced and marketed. At present, there are formidable challenges in the logistics and costs of goods that present obstacles to the implementation of therapy with modified cells. However, expensive therapies become economically justified if sufficiently effective. Given that the costs of non-curative antibody therapies and protein replacement therapies frequently exceed US$100,000 per year130-133, a one-time treatment with engineered cells that may have long-term or even indefinite persistence could be cost effective. Advances in the understanding of lymphocyte biology and cell culture technology, coupled with improved safety and efficiency of genetic modification strategies, have created enthusiasm for several novel adoptive transfer strategies that are now poised for translation into clinical trials.
Supplementary Material
Acknowledgments
C.H.J. is grateful for support by the US National Institutes of Health (NIH) (grants 5R01CA105216, 1R01CA120409, 5P01CA066726 and 1U19AI082628) and the Alliance for Cancer Gene Therapy. B.R.B. acknowledges direct support of this work by the US NIH (grants 2R01HL56067, R01AI34495, R01CA72669, P01CA142106 and P01AI056299) as well as a Leukemia and Lymphoma Translational Research Award. J.L.R. receives support from the US NIH (grants P30AI045008, R01AI057838, R01CA113783, R41CA130547, U19AI066290, U19AI082628 and P01AI080192) as well as from the JDRF Center on Cord Blood Therapies for Type 1 Diabetes.
Glossary
- Adoptive transfer
A form of immunotherapy in which effector lymphocytes are transfused. Allogeneic adoptive transfer is usually referred to as donor lymphocyte infusion and autologous lymphocyte adoptive transfer is referred to as adoptive transfer therapy.
- Therapeutic window
The range of concentrations of a drug, or numbers of immune cells, that will achieve therapeutic effects in most patients with adverse effects in only a small percentage. In cell-based therapies, the condition of the host and the dose and schedule of administration can alter the therapeutic window.
- Adeno-associated virus (AAV)
A replication-defective non-enveloped virus that is a member of the Parvovirus family. Vectors derived from AAV are attractive because AAV does not cause disease and they can mediate long-term gene transfer in both dividing and non-dividing cells.
- Gammaretrovirus
A genus of the retroviridae family. Vectors using gammaretroviruses were the first integrating vectors to be used clinically. Most vectors are derived from endogenous retroviruses isolated from mice such as the murine leukaemia virus.
- Transposon
Mobile DNA elements that can relocate within the genome of their hosts. Transposons can be used for various applications, including insertional mutagenesis, gene identification, gene tagging and DNA sequencing.
- Chimeric antigen receptor
Also known as chimeric immune receptor. Non-MHC-restricted chimeric antigen receptors combine antigen specificity and T cell-activating properties in a single fusion molecule. Most chimeric antigen receptors use an antibody-derived antigen-binding motif to recognize surface-expressed targets, in contrast to T cell receptors, which usually recognize peptide antigens presented by MHC molecules.
- MicroRNAs
Single-stranded RNA molecules approximately 21-23 nucleotides in length that are thought to regulate the expression of other genes.
- Epigenetic marks
Chemical modifications of chromatin that retain an intact DNA sequence and can modify gene expression. Examples are methylation of cytosine residues and histone modification by acetylation.
- Regulatory T (TReg) cell
A specialized type of CD4+ T cell that can suppress the responses of other T cells. TReg cells provide a crucial mechanism for the maintenance of peripheral self tolerance and subset of these cells is characterized by the expression of CD25 and FOXP3.
- Protein transduction domains (PTDs)
Peptides derived from several viruses, such as the HIV Tat PTD, that can enhance cellular uptake of proteins or polynucleotides. In general, the PTD must be covalently attached to the protein.
- Small interfering RNA (siRNA)
Synthetic RNA molecules of 19-23 nucleotides that are used to ‘knock down’ (that is, silence the expression of) a specific gene. This approach is known as RNA interference (RNAi) and is mediated by the sequence-specific degradation of mRNA.
- Insertional mutagenesis
Genotoxicity from DNA-based engineering that can result in cellular transformation through various mechanisms. DNA insertion can result in mutations that lead to the activation of oncogenes or to the inactivation of tumour suppressor genes.
- Zinc-finger nucleases (ZFNs)
Chimeric proteins comprised of engineered zinc-finger proteins fused to the catalytic domain of a restriction endonuclease that can bind and cleave DNA specifically at a unique and predetermined site in the human genome.
- Target product profile
A prospective and dynamic summary of the ideal characteristics of a drug or biological product to ensure that the desired quality and hence the safety and efficacy of a drug product is achieved The target product profile forms the basis of design for the development of the product.
- T helper 17 (TH17) cells
A subset of CD4+ T helper cells that produce interleukin-17 (IL-17) and that are thought to be important in inflammatory and autoimmune diseases. Their generation involves IL-23 and IL-21, as well as the transcription factors RORγt (retinoic-acid-receptor-related orphan receptor-γt) and STAT3 (signal transducer and activator of transcription 3).
- Experimental autoimmune encephalomyelitis
An experimental model of multiple sclerosis that is induced by immunization of susceptible animals with myelin-derived antigens, such as myelin basic protein, proteolipid protein or myelin oligodendrocyte glycoprotein.
- Central memory T (TCM) cells
Antigen-experienced T cells that express cell surface receptors required for homing to secondary lymphoid organs. These cells are generally thought to be long-lived and can serve as the precursors to effector T cells for recall responses.
- Effector memory T (TEM) cells
Terminally differentiated T cells that lack lymph node-homing receptors but express receptors that enable them to home to inflamed tissues. Effector memory T cells can exert immediate effector functions without the need for further differentiation.
- Vγ9Vδ2+ T cells
Vγ9Vδ2+ T cell receptors are expressed by γδ T cells. Vγ9Vδ2+ T cells are unique to humans and primates and are a minor fraction of the leukocyte population in peripheral blood (0.5–5%).
- SCID mouse
A naturally occurring mouse mutant with severe combined immune deficiency due to an inability to rearrange antigen receptor chain genes.
- Natural killer T (NKT) cells
A subpopulation of T cells that expresses both NK cell- and T cell- markers. In the C57BL/6 mouse strain, NKT cells express the NK1.1 (NKRP1C) molecule and the T cell receptor (TCR). Some NKT cells recognize CD1d-associated lipid antigens and express a restricted repertoire of TCRs. After TCR stimulation of naive mice, NKT cells rapidly produce interleukin-4 and interferon-γ.
- Replication-competent lentivirus (RCL)
A lentivirus vector that produces infectious virions, RCLs are designed to support only one infections cycle however mutations can induce replication competency.
References
- 1.Weiden PL, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. This was the first study to indicate the potent antitumour effects of human T cells. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, et al. Gene transfer into humans — immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. This was the first human gene transfer study. [DOI] [PubMed] [Google Scholar]
- 3.Uchida N, Cone RD, Freeman GJ, Mulligan RC, Cantor H. High efficiency gene transfer into murine T cell clones using a retroviral vector. J Immunol. 1986;136:1876–1879. [PubMed] [Google Scholar]
- 4.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 5.Amado RG, Chen IS. Lentiviral vectors — the promise of gene therapy within reach? Science. 1999;285:674–676. doi: 10.1126/science.285.5428.674. [DOI] [PubMed] [Google Scholar]
- 6.Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- 7.Wolf D, Goff SP. Host restriction factors blocking retroviral replication. Annu Rev Genet. 2008;42:143–163. doi: 10.1146/annurev.genet.42.110807.091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell DW, Miller AD. Foamy virus vectors. J Virol. 1996;70:217–222. doi: 10.1128/jvi.70.1.217-222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beard BC, et al. Unique integration profiles in a canine model of long-term repopulating cells transduced with gammaretro virus, lentivirus, or foamy virus. Hum Gene Ther. 2007;18:423–434. doi: 10.1089/hum.2007.011. [DOI] [PubMed] [Google Scholar]
- 10.Rethwilm A. Foamy virus vectors: an awaited alternative to gammaretro- and lentiviral vectors. Curr Gene Ther. 2007;7:261–271. doi: 10.2174/156652307781369092. [DOI] [PubMed] [Google Scholar]
- 11.Trobridge GD, et al. Foamy virus vector integration sites in normal human cells. Proc Natl Acad Sci USA. 2006;103:1498–1503. doi: 10.1073/pnas.0510046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroers R, et al. Gene transfer into human T lymphocytes and natural killer cells by Ad5/F35 chimeric adenoviral vectors. Exp Hematol. 2004;32:536–546. doi: 10.1016/j.exphem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Perez EE, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nature Biotech. 2008;26:808–816. doi: 10.1038/nbt1410. The first study to show that site-specific modification of cells can be accomplished at levels sufficient for therapeutic efficacy in an animal model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fratantoni JC, Dzekunov S, Singh JC, Liu LN. A non-viral gene delivery system designed for clinical use. Cytotherapy. 2003;5:208–210. doi: 10.1080/14653240310001479. [DOI] [PubMed] [Google Scholar]
- 15.Park JR, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 16.Dupuy AJ, Akagi K, Largaespada DA, Copeland NG, Jenkins NA. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature. 2005;436:221–226. doi: 10.1038/nature03691. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, et al. Stable gene transfer and expression in human primary T-cells by the Sleeping Beauty transposon system. Blood. 2006;107:483–491. doi: 10.1182/blood-2005-05-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh H, et al. Redirecting specificity of T-cell populations for CD 19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. References 16, 17 and 18 show the potential of transposon-based systems to engineer lymphocyte functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi JJ, June CH, Kohn DB. Genetic therapies for HIV/AIDS. Nature Biotech. 2007;25:1444–1454. doi: 10.1038/nbt1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang GP, et al. Analysis of lentiviral vector integration in HIV+ study subjects receiving autologous infusions of gene modified CD4+ T cells. Mol Ther. 2009;17:844–850. doi: 10.1038/mt.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushman FD. Retroviral integration and human gene therapy. J Clin Invest. 2007;117:2083–2086. doi: 10.1172/JCI32949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon SH, et al. Adoptive immunotherapy using human peripheral blood lymphocytes transferred with RNA encoding Her-2/neu-specific chimeric immune receptor in ovarian cancer xenograft model. Cancer Gene Ther. 2008;16:489–497. doi: 10.1038/cgt.2008.98. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell DA, et al. Selective modification of antigen-specific T cells by RNA electroporation. Hum Gene Ther. 2008;19:511–521. doi: 10.1089/hum.2007.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowley J, Monie A, Hung CF, Wu TC. Expression of IL-15RA or an IL-15/IL-15RA fusion on CD8+ T cells modifies adoptively transferred T-cell function in cis. Eur J Immunol. 2009;39:491–506. doi: 10.1002/eji.200838594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilber A, et al. Messenger RNA as a source of transposase for sleeping beauty transposon-mediated correction of hereditary tyrosinemia type I. Mol Ther. 2007;15:1280–1287. doi: 10.1038/sj.mt.6300160. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, et al. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:9290–9295. doi: 10.1073/pnas.0801017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li QJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. This study was the first to indicate the potential of engineering microRNA to alter T cell function. [DOI] [PubMed] [Google Scholar]
- 29.Merkenschlager M, Wilson CB. RNAi and chromatin in T cell development and function. Curr Opin Immunol. 2008;20:131–138. doi: 10.1016/j.coi.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Su Z, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 31.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nature Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 32.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nature Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 33.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 34.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. [PubMed] [Google Scholar]
- 35.Ballas ZK. The use of 5-azacytidine to establish constitutive interleukin 2-producing clones of the EL4 thymoma. J Immunol. 1984;133:7–9. This was the first study to show that epigenetic modification could alter T cell function. [PubMed] [Google Scholar]
- 36.Polansky JK, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 37.Guo ZS, et al. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res. 2006;66:1105–1113. doi: 10.1158/0008-5472.CAN-05-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nature Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 39.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nature Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 40.Murakami T, et al. Transcriptional modulation using HDACi depsipeptide promotes immune cell-mediated tumor destruction of murine B16 melanoma. J Invest Dermatol. 2008;128:1506–1516. doi: 10.1038/sj.jid.5701216. [DOI] [PubMed] [Google Scholar]
- 41.Blackwell L, Norris J, Suto CM, Janzen WP. The use of diversity profiling to characterize chemical modulators of the histone deacetylases. Life Sci. 2008;82:1050–1058. doi: 10.1016/j.lfs.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 43.Eguchi A, et al. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nature Biotech. 2009;27:567–571. doi: 10.1038/nbt.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka Y, Dowdy SF, Linehan DC, Eberlein TJ, Goedegebuure PS. Induction of antigen-specific CTL by recombinant HIV trans-activating fusion protein-pulsed human monocyte-derived dendritic cells. J Immunol. 2003;170:1291–1298. doi: 10.4049/jimmunol.170.3.1291. [DOI] [PubMed] [Google Scholar]
- 45.Grube M, Melenhorst JJ, Barrett AJ. An APC for every occasion: induction and expansion of human Ag-specific CD4 and CD8 T cells using cellular and non-cellular APC. Cytotherapy. 2004;6:440–449. doi: 10.1080/14653240410005230. [DOI] [PubMed] [Google Scholar]
- 46.Melenhorst JJ, et al. Robust expansion of viral antigen-specific CD4+ and CD8+ T cells for adoptive T cell therapy using gene-modified activated T cells as antigen presenting cells. J Immunother. 2006;29:436–443. doi: 10.1097/01.cji.0000211302.52503.93. [DOI] [PubMed] [Google Scholar]
- 47.Choi JM, et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nature Med. 2006;12:574–579. doi: 10.1038/nm1385. [DOI] [PubMed] [Google Scholar]
- 48.Choi JM, et al. Transduction of the cytoplasmic domain of CTLA-4 inhibits TCR-SPECIFIC activation signals and prevents collagen-induced arthritis. Proc Natl Acad Sci USA. 2008;105:19875–19880. doi: 10.1073/pnas.0805198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bargou R, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 50.Chen A, Zheng G, Tykocinski ML. Hierarchical costimulator thresholds for distinct immune responses: application of a novel two-step Fc fusion protein transfer method. J Immunol. 2000;164:705–711. doi: 10.4049/jimmunol.164.2.705. [DOI] [PubMed] [Google Scholar]
- 51.Montini E, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nature Biotech. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 52.Montini E, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009;119:964–975. doi: 10.1172/JCI37630. References 51 and 52 describe potential genotoxicity of gammaretroviral and lentiviral vectors expressed in HSCs in tumour-prone mouse models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 54.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nature Rev Immunol. 2007;7:650–654. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- 55.Moisini I, Nguyen P, Fugger L, Geiger TL. Redirecting therapeutic T cells against myelin-specific T lymphocytes using a humanized myelin basic protein-HLA-DR2-ζ chimeric receptor. J Immunol. 2008;180:3601–3611. doi: 10.4049/jimmunol.180.5.3601. [DOI] [PubMed] [Google Scholar]
- 56.Muul LM, et al. Persistence and expression of the adenosine deaminase gene for 12 years and immune reaction to gene transfer components: long-term results of the first clinical gene therapy trial. Blood. 2003;101:2563–2569. doi: 10.1182/blood-2002-09-2800. [DOI] [PubMed] [Google Scholar]
- 57.Ciceri F, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 58.Cooper LJ, Kalos M, Lewinsohn DA, Riddell SR, Greenberg PD. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J Virol. 2000;74:8207–8212. doi: 10.1128/jvi.74.17.8207-8212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varela-Rohena A, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nature Med. 2008;14:1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson LA, et al. Gene therapy with human and mouse T cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. References 58- 60 show the potential efficacy and toxicity of adoptive therapy with transgenic TCRs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. The first study to show the activity of chimeric antigen receptors in T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pule MA, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. This study describes the first antitumour effects following the adoptive transfer of T cells modified to express chimeric antigen receptors in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCR ζ/CD28 receptor. Nature Biotech. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 65.Carpenito C, et al. Control of large established tumor xenografts with genetically re-targeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4+ T cells. Curr Opin Immunol. 2009;21:200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muranski P, et al. Tumor-specific Th 17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mekala DJ, Geiger TL. Immunotherapy of autoimmune encephalomyelitis with redirected CD4 + CD25+ T lymphocytes. Blood. 2005;105:2090–2092. doi: 10.1182/blood-2004-09-3579. [DOI] [PubMed] [Google Scholar]
- 70.Eran E, Nitzan A, Tova W, Eshhar Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology. 2009;136:1721–1731. doi: 10.1053/j.gastro.2009.01.049. [DOI] [PubMed] [Google Scholar]
- 71.Landmeier S, et al. Gene-engineered varicella-zoster virus reactive CD4+ cytotoxic T cells exert tumor-specific effector function. Cancer Res. 2007;67:8335–8343. doi: 10.1158/0008-5472.CAN-06-4426. [DOI] [PubMed] [Google Scholar]
- 72.Zhou J, et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature Rev Immunol. 2008;211:58–66. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 75.Berger C, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levine BL, et al. Effects of CD28 costimulation on long term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 77.Maus MV, et al. Extensive replicative capacity of human central memory T cells. J Immunol. 2004;172:6675–6683. doi: 10.4049/jimmunol.172.11.6675. [DOI] [PubMed] [Google Scholar]
- 78.Bondanza A, et al. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- 79.Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179:4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu Y, Zhu G, Luo L, Flies AS, Chen L. CD 137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood. 2007;109:4882–4889. doi: 10.1182/blood-2006-10-043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nature Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 83.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human γδ T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol. 2002;23:14–18. doi: 10.1016/s1471-4906(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 84.Lopez RD. Human γδ-T cells in adoptive immunotherapy of malignant and infectious diseases. Immunol Res. 2002;26:207–221. doi: 10.1385/IR:26:1-3:207. [DOI] [PubMed] [Google Scholar]
- 85.Inman BA, et al. Questionable relevance of Γδ T lymphocytes in renal cell carcinoma. J Immunol. 2008;180:3578–3584. doi: 10.4049/jimmunol.180.5.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thedrez A, et al. Self/non-self discrimination by human γδ T cells: simple solutions for a complex issue? Immunol Rev. 2007;215:123–135. doi: 10.1111/j.1600-065X.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 87.Todaro M, et al. Efficient killing of human colon cancer stem cells by γδ T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 88.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 89.Kondo M, et al. Zoledronate facilitates large-scale ex vivo expansion of functional γδ T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10:842–856. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- 90.Thedrez A, et al. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human Vγ9Vδ2 T cells for adoptive immunotherapy. J Immunol. 2009;182:3423–3431. doi: 10.4049/jimmunol.0803068. [DOI] [PubMed] [Google Scholar]
- 91.Kabelitz D, Wesch D, Pitters E, Zoller M. Characterization of tumor reactivity of human Vγ9Vδ2 γδ T cells in vitro and in SCID mice in vivo. J Immunol. 2004;173:6767–6776. doi: 10.4049/jimmunol.173.11.6767. [DOI] [PubMed] [Google Scholar]
- 92.Kobayashi H, et al. Safety profile and anti-tumor effects of adoptive immunotherapy using γδ T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bennouna J, et al. Phase-I study of Innacell γδ, an autologous cell-therapy product highly enriched in Vγ9Vδ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rischer M, et al. Human γδ T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br J Haematol. 2004;126:583–592. doi: 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- 95.van der Veeken LT, et al. αβ T-cell receptor engineered γδ T cells mediate effective antileukemic reactivity. Cancer Res. 2006;66:3331–3337. doi: 10.1158/0008-5472.CAN-05-4190. [DOI] [PubMed] [Google Scholar]
- 96.Schumacher TNM, Restifo NP. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2009;21:187–189. doi: 10.1016/j.coi.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2004;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 98.Tahir SM, et al. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 99.Rogers PR, et al. Expansion of human Vα24+ NKT cells by repeated stimulation with KRN7000. J Immunol Methods. 2004;285:197–214. doi: 10.1016/j.jim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Motohashi S, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 101.Kunii N, et al. Combination therapy of in vitro- expanded natural killer T cells and a-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harrington K, Vile R. Virus smuggling, tax evasion and tumor assassination. Nature Med. 2006;12:507–509. doi: 10.1038/nm0506-507. [DOI] [PubMed] [Google Scholar]
- 103.Zakrzewski JL, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nature Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 104.de Pooter RF, Cho SK, Carlyle JP, Zúñiga-Pflücker JC. In vitro generation of T lymphocytes from embryonic stem cell-derived prehematopoietic progenitors. Blood. 2003;102:1649–1653. doi: 10.1182/blood-2003-01-0224. [DOI] [PubMed] [Google Scholar]
- 105.Yang L, Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102:4518–4523. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Lent AU, et al. Functional human antigen-specific T cells produced in vitro using retroviral T cell receptor transfer into hematopoietic progenitors. J Immunol. 2007;179:4959–4968. doi: 10.4049/jimmunol.179.8.4959. [DOI] [PubMed] [Google Scholar]
- 107.Papapetrou EP, Kovalovsky D, Beloeil L, SantAngelo D, Sadelain M. Harnessing endogenous miR-181a to segregate transgenic antigen receptor expression in developing versus post-thymic T cells in murine hematopoietic chimeras. J Clin Invest. 2009;119:157–168. doi: 10.1172/JCI37216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kay MA, et al. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- 109.Zufferey R, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang L, Bailey L, Baltimore D, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci USA. 2006;103:11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rapoport AP, et al. Rapid immune recovery and GVHD-like engraftment syndrome following adoptive transfer of costimulated autologous T cells. Clin Cancer Res. 2009;15:4499–4507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lamers CH, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 115.Lamers CH, et al. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother. 2007;56:1875–1883. doi: 10.1007/s00262-007-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hsu C, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109:5168–5177. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schumacher TN. T-cell-receptor gene therapy. Nature Rev Immunol. 2002;2:512–519. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 118.Li Y, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nature Biotech. 2005;23:349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 119.Varmus H. Retroviruses. Science. 1988;240:1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- 120.Baum C, Kustikova O, Modlich U, Li Z, Fehse B. Mutagenesis oncogenesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther. 2006;17:253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- 121.Faschinger A, et al. Mouse mammary tumor virus integration site selection in human and mouse genomes. J Virol. 2008;82:1360–1367. doi: 10.1128/JVI.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lewinski MK, et al. Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription. J Virol. 2005;79:6610–6619. doi: 10.1128/JVI.79.11.6610-6619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Friedmann T. A brief history of gene therapy. Nature Genet. 1992;2:93–98. doi: 10.1038/ng1092-93. [DOI] [PubMed] [Google Scholar]
- 124.Chang AH, Sadelain M. The genetic engineering of hematopoietic stem cells: the rise of lentiviral vectors, the conundrum of the LTR, and the promise of lineage-restricted vectors. Mol Ther. 2007;15:445–456. doi: 10.1038/sj.mt.6300060. [DOI] [PubMed] [Google Scholar]
- 125.Hacein-Bey-Abina S, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heslop HE, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 127.Deeks S, et al. A phase II randomized study of HIV specific T-cell gene therapy in subjects with undetectable plasma viremia on combination anti-retroviral therapy. Mol Ther. 2002;5:788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 128.Newrzela S, et al. Resistance of mature T cells to oncogene transformation. Blood. 2008;112:2278–2286. doi: 10.1182/blood-2007-12-128751. This study compares the relative susceptibility of HSCs and T cells to genotoxicity after transduction with gammaretroviral vectors. [DOI] [PubMed] [Google Scholar]
- 129.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 130.Goldman DP, Clarke AE, Garber AM. Creating the costliest orphan. The Orphan Drug Act in the development of Ceredase. Int J Technol Assess Health Care. 1992;8:583–597. doi: 10.1017/s0266462300002294. [DOI] [PubMed] [Google Scholar]
- 131.Smiddy WE. Economic implications of current age-related macular degeneration treatments. Ophthalmology. 2009;116:481–487. doi: 10.1016/j.ophtha.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 132.Dedes KJ, et al. Bevacizumab in combination with paclitaxel for HER-2 negative metastatic breast cancer: an economic evaluation. Eur J Cancer. 2009;45:1397–1406. doi: 10.1016/j.ejca.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 133.Chamberlain MC. Bevacizumab plus irinotecan in recurrent glioblastoma. J Clin Oncol. 2008;26:1012–1013. doi: 10.1200/JCO.2007.15.1605. [DOI] [PubMed] [Google Scholar]
- 134.Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- 135.Tan S, et al. Zinc-finger protein-targeted gene regulation: genomewide single-gene specificity. Proc Natl Acad Sci USA. 2003;100:11997–12002. doi: 10.1073/pnas.2035056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Porteus MH, Carroll D. Gene targeting using zinc finger nucleases. Nature Biotech. 2005;23:967–973. doi: 10.1038/nbt1125. [DOI] [PubMed] [Google Scholar]
- 137.Santiago Y, et al. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc Natl Acad Sci USA. 2008;105:5809–5814. doi: 10.1073/pnas.0800940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Urnov FD, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 139.Dotti G, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–4684. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guttinger M, et al. Adoptive immunotherapy by avidin-driven cytotoxic T lymphocyte-tumor bridging. Cancer Res. 2000;60:4211–4215. [PubMed] [Google Scholar]
- 141.Tykocinski ML, Chen A, Huang JH, Weber MC, Zheng G. New designs for cancer vaccine and artificial veto cells: an emerging palette of protein paints. Immunol Res. 2003;27:565–574. doi: 10.1385/IR:27:2-3:565. [DOI] [PubMed] [Google Scholar]
- 142.Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 143.Donahue RE, et al. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J Exp Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Levine BL, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dropulic B, Hermankova M, Pitha PM. A conditionally replicating HIV-1 vector interferes with wild-type HIV-1 replication and spread. Proc Natl Acad Sci USA. 1996;93:11103–11108. doi: 10.1073/pnas.93.20.11103. References 144 and 145 describe the development of the first lentiviral vector tested in humans, given as an adoptive transfer of engineered CD4+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matheson R. I Am Legend. Walker; New York: 1954. [Google Scholar]
- 147.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 148.Oelke M, et al. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig coated artificial antigen presenting cells. Nature Med. 2003;9:619–625. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 149.Maus MV, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T cell receptor, CD28 and 4-1BB. Nature Biotech. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 150.Exley MA, et al. Selective activation, expansion, and monitoring of human iNKT cells with a monoclonal antibody specific for the TCR α-chain CDR3 loop. Eur J Immunol. 2008;38:1756–1766. doi: 10.1002/eji.200737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 152.Dull T, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Switzer WM, et al. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J Virol. 2004;78:2780–2789. doi: 10.1128/JVI.78.6.2780-2789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bauer TR, Jr, et al. Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors. Nature Med. 2008;14:93–97. doi: 10.1038/nm1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stevenson SC, Rollence M, Marshall-Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–4790. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takayama K, et al. A mosaic adenovirus possessing serotype Ad5 and serotype Ad3 knobs exhibits expanded tropism. Virology. 2003;309:282–293. doi: 10.1016/s0042-6822(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 157.Stecher H, Shayakhmetov DM, Stamatoyannopoulos G, Lieber A. A capsid-modified adenovirus vector devoid of all viral genes: assessment of transduction and toxicity in human hematopoietic cells. Mol Ther. 2001;4:36–44. doi: 10.1006/mthe.2000.0410. [DOI] [PubMed] [Google Scholar]
- 158.Mori Y. Recent topics related to human herpesvirus 6 cell tropism. Cell Microbiol. 2009;11:1001–1006. doi: 10.1111/j.1462-5822.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- 159.Romi H, Singer O, Rapaport D, Frenkel N. Tamplicon-7, a novel T-lymphotropic vector derived from human herpesvirus 7. J Virol. 1999;73:7001–7007. doi: 10.1128/jvi.73.8.7001-7007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Borenstein R, Singer O, Moseri A, Frenkel N. Use of amplicon-6 vectors derived from human herpesvirus 6 for efficient expression of membrane-associated and -secreted proteins in T cells. J Virol. 2004;78:4730–4743. doi: 10.1128/JVI.78.9.4730-4743.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Finney HM, Akbar AN, Lawson ADG. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD 134, and CD 137 in series with signals from the TCRζ chain. J Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 162.Till BG, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.