Abstract
The survival and growth of individual cells in a tissue can be nonautonomously regulated by the properties of adjacent cells. In mosaic Drosophila imaginal discs, for example, wild-type cells induce the elimination of adjacent slow-growing Minute cells by apoptosis, while, conversely, certain types of faster-growing cells are able to eliminate adjacent wild-type cells. This process, known as cell competition, represents one example of a diverse group of phenomena in which short-range heterotypic interactions result in the selective elimination of one type of cell by another. The mechanisms that designate “winner” and “loser” genotypes in these processes are not known. Here we show that apoptosis is observed preferentially at boundaries that separate populations of cells that express different levels of the transmembrane protein Crumbs (Crb). Cells that express higher levels of Crb tend to be eliminated when they are near cells that express lower levels of Crb. We also observe distortions in the structure of epithelia on either side of boundaries between populations of cells that differ in Crb expression. Thus, while previous studies have focused mostly on the cell autonomous functions of Crb, we show that Crb can regulate cell survival and tissue morphology nonautonomously. Moreover, we find that the extracellular domain (ECD) of Crb, which seems to be dispensable for some of the other characterized functions of Crb, is required to elicit the nonautonomous effects on cell survival. The ECD can also regulate the subcellular localization of Hippo pathway components, and possibly other proteins, in adjacent cells and may therefore directly mediate these effects. Several genetic lesions alter Crb levels, including loss-of-function mutations in hyperplastic tumor suppressors in the Hippo-Salvador-Warts pathway and in neoplastic tumor suppressor genes, such as scribble. Thus, Crb may be part of a “surveillance mechanism” that is responsible for the cell death that is observed at the boundaries of mutant clones in these cases.
INTRODUCTION
The phenomenon of cell competition was uncovered in studies of Drosophila imaginal discs and illustrates a remarkable feature of cells - that cell survival is based, not only on the intrinsic properties of the cell or of surrounding cells, but on a comparison of the properties of a cell to those of its neighbors. A class of mutations, known as Minutes (M), that are mostly in genes encoding ribosomal proteins, dominantly and autonomously reduce cellular growth rates. Animals composed entirely of M/+ cells develop to the adult stage more slowly, but are of relatively normal size. However, clones of M/+ cells generated within wild-type imaginal discs are eliminated during development by apoptosis (Morata and Ripoll, 1975, Moreno et al., 2002). Conversely, wild-type cells can be eliminated when they are adjacent to faster-growing cells that overexpress Myc or have inactivating mutations in components of the Hippo-Salvador-Warts (HSW) pathway (known as “supercompetitors”) (de la Cova et al., 2004; Moreno and Basler, 2004; Tyler et al., 2007). This progressive and selective elimination of a certain cell type by another is thought to be caused by short-range interactions at the boundaries between the two populations. In addition to cell competition, there are several other known instances in which short-range interactions nonautonomously influence cell survival, independent of growth rates (Adachi-Yamada and O’Connor, 2002, Milán et al., 2002). None of these mechanisms are well understood.
A central question pertaining to all of these phenomena is: How do cells compare themselves with their neighbors? In one model for cell competition, “loser” cells die because they do not receive sufficient levels of Decapentaplegic (Dpp), a survival factor that is sequestered away by adjacent “winner” cells (Moreno and Basler, 2004). However, the most intense competition is thought to occur in the center of the wing pouch – the region with the highest level of Dpp signaling. Furthermore, the role of Dpp in some instances of cell competition has been disputed (de la Cova et al., 2004). There is evidence from tissue culture experiments that diffusible factors may play a role in cell-competition-like phenomena, but such factors have not yet been identified (Senoo-Matsuda and Johnston, 2007). While, several downstream factors have been implicated in inducing death in designated losers including the JNK pathway (Moreno et al, 2002), Hid (de la Cova et al, 2004) and Flower (Rhiner et al., 2010), the mechanism that initially designates winners and losers still remains enigmatic.
In order to identify factors that might nonautonomously regulate cell survival, we used a genetic approach in Drosophila to screen for mutations that reduce the survival of non-mutant cells in a mosaic eye. We identified multiple alleles of crumbs (crb), which encodes a transmembrane protein with a large extracellular domain (Crb-ECD) containing many EGF repeats (Tepass et al., 1990). Crb was initially characterized as a regulator of apicobasal polarity that promotes the establishment of the apical domain in the embryonic ectoderm and has more recently been shown to regulate cell proliferation and survival in a cell-autonomous manner. Here we identify non-cell autonomous functions for Crb in regulating cell survival by demonstrating that apoptosis is observed preferentially at boundaries that separate populations of cells that express different levels of Crb. As a result, cells that express higher levels of Crb tend to be eliminated when they are near cells that express lower levels of Crb. These non-cell-autonomous functions for Crb point to an important role for the ECD of Crb in imaginal disc cells.
MATERIALS AND METHODS
Fly Stocks & Husbandry
The following fly stocks were used in this study;
w eyFLP2; cl2L3 w+ FRT40A/CyO, y+
y w eyFLP2;; FRT82B w+ cl3R3/TM6B,y+
y w eyFLP2;; FRT82B w+ cl3R3 ubi-GFP/S-T
w;; FRT40A
w;; pten [MGH1] FRT40A/CyO
w;; ft [fd] FRT40A/CyO
w;; ex [11d3] FRT40A/CyO
w;;FRT82B
w;;FRT82B Tsc1[Q87X]
w;;FRT82B cic[Q474X]
w;;FRT82B wts [MGH1]
w;;FRT82B wts[X1]
w;;FRT82B sav[3]
w;;FRT82B crb[11A22]
w;;FRT82B crb[8F105]
w;;FRT82B crb[Q328X]
w;;FRT82B crb[W445X]
w;;FRT82B ubi-GFPnls RpS3P lac92
y w eyFLP;; FRT82B w+ ubi-GFP
y w hsFLP;; FRT82B w+ ubi-GFP
UAS-CrbWT
UAS-CrbExtraTMGFP
UAS-CrbIntraMyc2b
UAS-FasII
UAS-dMyc
UAS-GFP
w;; Act<CD2<Gal4, UAS-GFP
tubGal80TS
w;;FRT82B tubGal80
ptc-Gal4
y w hsFLP; Sp;+/S-T
y w eyFLP; Sp;Dr/S-T
Generally, experimental crosses were raised at 25° on food prepared according to the recipe from the Bloomington Stock Center. In experiments where timing was critical, eggs were collected at room temp for 4–6 hr on grape juice plates in the dark. Grape plates were then placed at 25°. After 24 hr, L1 larvae were picked and 40 larvae were placed in each vial with a dollop of yeast paste, and vials were placed at 25°. In experiments where timing was not as critical, eggs were collected directly into vials for 24 hr or less.
Immunohistochemistry
Discs were dissected in PBS, fixed in 4% paraformaldehyde in PBS, washed in 0.1% Triton in PBS, and mounted in Slow Fade Gold. The following primary antibodies were used:
AC3 (Rabbit, 1:200) from Cell Signaling
phalloidin-TRITC (1:500) from Sigma
Crb-ECD (Rat, 1:500) from U. Tepass (Tepass et al., 1990)
Crb-ICD (Rat, 1:500) from H. Bellen (Bhat et al., 1999)
Scribble (Guinea Pig, 1:500) (Bilder and Perrimon, 2000)
Expanded (Guinea Pig, 1:5000) from R. Fehon (Maitra et al., 2006)
Merlin (Guinea Pig, 1:7500) from R.Fehon (McCartney and Fehon, 1996)
Shotgun/E-Cadherin (Rat, 1:50) from DSHB
Armadillo (Mouse, 1:20) from DSHB
Diap1 (Mouse, 1:200) from B. Hay (Yoo et al., 2002)
Secondary Alexa-Fluor antibodies from Invitrogen were used at 1:1000.
Fluorescent images were taken on Leica TCS SL or Zeiss LSM700 confocal microscopes.
Quantification of Red Tissue in Adult Eyes
Pictures of adult eyes were taken on a Leica Z16 APO system with Montage software from Synoptics Ltd. Using Adobe Photoshop, the outline of the eye was selected using the polygonal lasso. To select red pixels, the image was converted to ‘grayscale’ and ‘shadows’ within the eye area were selected. Total eye and red tissue area were measured in pixels by using the ‘histogram’ dialogue for selected regions, and the ratio of the number of red pixels to total pixels in the eye was calculated.
Ethylmethanesulfonate (EMS) Mutagenesis
3–4 day old w;FRT82B males were starved, fed 25 mM EMS in 1% sucrose, and then crossed to “tester” virgins, y w eyFLP2; FRT82B w+ cl3R3/TM6B,y+. F1 progeny were screened for a visual reduction in the amount of red tissue.
Clone Induction Protocol
For the experiments in figures 2A–F and 3A–D, 7 min heat shocks were performed at the indicated time points at 37° in a circulating water bath and imaginal discs were dissected at 114 hours after egg deposition (hr AED). For the experiment in figure 2G–I, 15 min heat shocks were performed at 66 hr AED at 37° in a circulating water bath and imaginal discs were dissected at 90 hr AED.
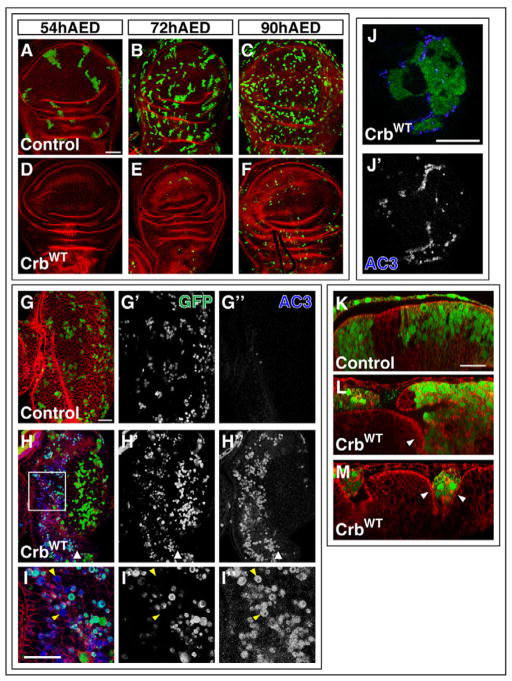
Figure 2. Crb-overexpressing cells are eliminated from wild-type imaginal discs.
(A–F) Wing imaginal discs containing GFP-positive FLP-out clones (green) induced at one of three times during larval development. In all cases, discs were dissected at 114hr AED and stained with phalloidin (red). (A, D) Induction at 54 hr AED of control clones (A) and Crb-overexpressing clones (D). (B, E) Induction at 72 hr AED of control clones (B) and Crb-overexpressing clones (E). (C, F) Induction at 90 hr AED of control clones (C) and Crb-overexpressing clones (F). (G–I) Eye-imaginal discs containing control (G) or Crb-overexpressing clones (H). The clones are marked by GFP (green) and shown separately in (G′) and (H′). AC3 staining (blue) is shown separately in (G″) and H″. White arrowhead in (H) indicates position of morphogenetic furrow. Higher magnification image of the boxed region in (H) is shown in (I). Most AC3-postive cells are GFP-positive. Yellow arrowheads point to AC3-positive, GFP-negative cells. (J) Eye-imaginal disc with large Crb-expressing FLP-out clone (green) stained for AC3 (blue). Large FLP-out clones were made using a temperature-sensitive Gal80 (see Methods). The AC3 staining is shown separately in (J′). (K–M) XZ images of control (K) or Crb-overexpressing (L, M) clones. In (L) an entirely extruded clone can be seen on the left and on the right the wild-type epithelium can be seen folding basally as another clone is apparently being extruded. (M) shows infolding of the normal epithelium on both sides of a Crb-expressing clone. In (K–M), apical is up for the disc proper.
Scale bars: 50μm in A–F, J and 20μm in G–I and K–M.
In the experiments that included the temperature-sensitive Gal80 (Fig. 2J,L,M, S3C,D, 3F), larvae were kept at the permissive temperature, 18°, for the majority of development. Heat shocks were at 37° in a circulating water bath and larvae were subsequently returned to 18°. At the appropriate time, larvae were shifted to the non-permissive temperature, 30°, to induce expression of Gal4-driven transgenes. Larvae were maintained at 30° until they were dissected as wandering 3rd instars. For the experiment in figure 2J, a 15 min heat shock was performed on day 4 and larvae were shifted to 30° on day 6. For the experiment in figure 2K–M, a 20 min heat shock was performed on day 2 and larvae were shifted on day 7.
Quantification of Cell Size
Z-stacks of Minute mosaic discs were analyzed in Image J. Groups of 20–100 wild-type or Minute cells were outlined with the polygon selection tool according to their apical profile, as visualized by Crb staining. The resulting area, in pixels, was divided by the number of cells to obtain the average cell area. A total of 500 cells were analyzed per genotype per disc for 3 mosaic discs.
Quantification of Crb Staining Intensity
To quantify Crb intensity independent of focal plane, we analyzed 0.75μm section Z-stacks, taken at 40x, which spanned the entire apical-to-basal distance where Crb was expressed in the field of view. For each image, 60–80 square selections of equal size (approximately 169 pixels) were drawn around random borders distributed evenly across the image. Selections were made with the GFP channel off so that the experimenter was blind to the location of the clone borders. The mean pixel intensity of each selection was calculated and the maximum value obtained among the sections was taken. Once all of the Crb staining intensities were recorded for all of the selections in an image, the GFP channel was overlaid and the selections were identified as either being clearly GFP positive, clearly GFP negative or ambiguous (i.e. near the clone boundary). Selections that could not be conclusively genotyped were discarded. The intensity values from selections that were clearly GFP-positive or clearly GFP-negative were averaged separately and the ratio of GFP-positive to GFP-negative was taken. Similar results were obtained whether the maximum value, the average value, or the average of the three highest values of all the Z-sections were analyzed (data not shown).
RESULTS
Identification of crb in a screen for nonautonomous regulators of cell survival
To identify factors that are required, nonautonomously, for cell survival, we designed a screen aimed at identifying mutations that make cells more capable of eliminating their non-mutant neighbors. To that end, we generated mosaic eyes that contain small patches of heterozygous tissue that are surrounded by mutant cells (Fig. 1A, B). Mosaic eyes generated by FLP/FRT- induced mitotic recombination are normally mostly composed of patches of homozygous mutant (white) and wild-type (red) cells in roughly equal proportions (Newsome et al., 2000). However, the eyes also contain small patches of heterozygous tissue (also red). The heterozygous patches include cells that have either not undergone mitotic recombination or have segregated their sister chromatids during anaphase to preserve their heterozygosity (Fig. 1A). Including a recessive cell-lethal mutation on the wild-type chromosome (Newsome et al., 2000) eliminates the wild-type twin spots, leaving the small patches of heterozygous tissue surrounded by mutant cells (Fig. 1A, middle). A mutation of interest would decrease the size of the patches of heterozygous tissue in the eye, without necessarily reducing overall eye size (Fig. 1A, right). We used conditions that eliminated the wild-type twin-spots using a recessive cell-lethal mutation and thus created a “competition” between mutant cells and the heterozygous cells. This sensitizes the assay for the nonautonomous effects of mutant tissue in two ways. First, because the patches of heterozygous cells are small, a greater proportion of the heterozygous cells are bordered by, and therefore potentially exposed to, short-range signals from mutant cells. Second, since the heterozygous tissue appears as small discrete patches, any change in the size of the patches is more obvious.
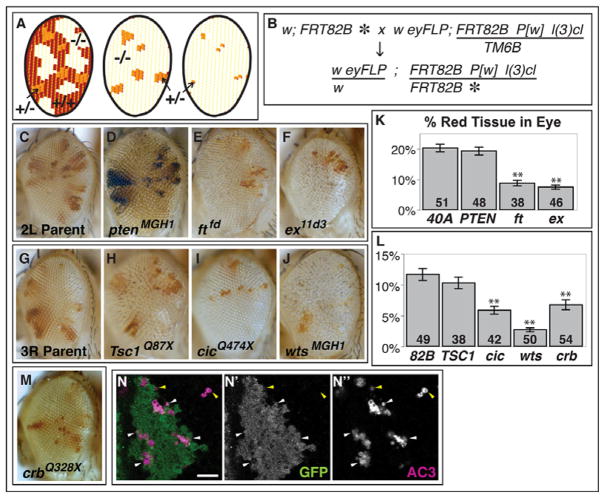
Figure 1. A screen for mutations affecting cell competition identifies crb.
(A) FLP/FRT-mediated mitotic recombination of heterozygous eye precursor cells normally results in adult eyes that are composed predominantly of homozygous mutant and homozygous wild-type tissue, but small patches of heterozygous tissue are also present (left). The addition of a recessive cell lethal mutation on the wild-type chromosome results in an adult eye that is composed of the small patches of heterozygous tissue surrounded by homozygous mutant cells (middle). If the mutation confers a competitive advantage to the mutant cells a reduction in size of the heterozygous patches will be observed (right). (B) Cross scheme for identifying mutations on chromosome arm 3R that make cells supercompetitors. The asterisk indicates a mutagenized chromosome. (C–F) Adult eyes from mutants on 2L that were tested in this assay; (G–J) Adult eyes from mutants on 3R that were tested in this assay; (K) quantification of the area of red tissue in adult eyes mosaic for mutations on 2L; (L) quantification of the area of red tissue in eyes mosaic for mutations on 3R. Error bars show SE. ** indicates < 0.001 significance in the Mann-Whitney Z test. (M) Adult eye phenotype of crbQ328X. (N) Clusters of AC3-positive wild-type cells (white arrowheads) are found near the border of crbQ328X clones in mosaic eye discs. Wild-type cells are GFP-positive and mutant cells lack GFP expression. Occasionally AC3-positive staining is observed within mutant clones (yellow arrowheads).
Scale bars: 10μm in N.
First, we used this assay to test mutations that had previously been shown to cause, or not cause, cell competition (Fig. 1C–L). Cells mutant for fat (ft), expanded (ex), hippo (hpo), salvador (sav) and warts (wts), all components of the HSW pathway, were previously shown to be supercompetitors (Tyler et al., 2007) and, accordingly, scored positive in this assay as assessed by a decrease in the amount of red tissue remaining in the eye (Fig. 1E, F, J, K, L and data not shown). Consistent with a previous report that cells overexpressing PI3K activity do not become supercompetitors, (de la Cova et al., 2004) we found that cells that are mutant for pten, which encodes a negative regulator of PI3K, do not reduce the representation of adjacent wild-type cells (Fig. 1D, K). We also tested capicua (cic) (Jimenez et al., 2000) a negative regulator of growth downstream of the RTK/Ras pathway (Tseng et al., 2007), which scored positive (Fig. 1I, L) and Tsc1 (Fig. 1H, L), an inhibitor of growth through the target of rapamycin pathway (reviewed in (Kwiatkowski and Manning, 2005)), which scored negative. Thus, different growth-promoting pathways vary in their apparent ability to make cells supercompetitors in this assay.
We then screened 18,000 flies that had mosaic eyes containing clones homozygous for a mutagenized chromosome arm, specifically 3R (Fig. 1B), and retained 20 mutants where the red patches were smaller than those in the eyes of control flies. These included 3 alleles of wts and 3 alleles of sav. We also recovered a lethal complementation group composed of four members that did not correspond to any previously described regulators of cell competition on 3R. All four alleles genetically mapped to the 95F region.
Several lines of evidence indicate that a mutation in crb is responsible for the clonal phenotype of these mutants. First, the independently derived crb11A22 allele (Tepass et al., 1990), which is reported to be null, failed to complement the lethality of our four alleles and also elicited a similar phenotype (Fig. S1D). Second, sequencing the coding region of the crb gene in two of the four alleles revealed nonsense mutations that would result in highly truncated proteins that would most likely be nonfunctional (Fig. S1A). Finally, an anti-Crb antibody raised against Crb-ECD (Pellikka et al., 2002) fails to detect any protein in mutant clones in the imaginal discs (data not shown). Taken together, these findings indicate that crb clones reduce the amount of heterozygous tissue in the adult eye.
The reduction in the size of the patches of heterozygous tissue adjacent to crb mutant clones might be caused by a mechanism other than cell death. For example, it may be caused by an increased rate of growth of crb cells in conjunction with a mechanism for the maintenance of overall eye size, which would prematurely terminate the growth of the heterozygous tissue. However, crb mosaic eyes are slightly larger than wild-type eyes. In addition, crb heterozygous animals with mosaic eyes do not show any significant differences from wild type in their developmental timing to pupation (Fig. S1E). The time to 50% pupation was 126.7+/− 1.0 hr (n= total of 410 flies from 3 independent experiments) for control and 129.9+/−2.6 hr (n= total of 285 flies from 3 independent experiments) for crb heterozygous animals with mosaic eyes. Thus, we see no indication of an organ size checkpoint mechanism that might terminate the growth of the heterozygous cells prematurely. Overgrowth alone is insufficient to reduce the size of wild-type patches, since pten cells cause a greater increase in the overall size of the eye than crb cells, indicating a stronger overgrowth phenotype, but do not cause a comparable reduction in size of the patches of heterozygous tissue (compare Fig. 1D to 1M).
Alternatively, crb cells may eliminate their wild-type neighbors through apoptosis, as occurs during cell competition. To test this hypothesis, we examined eye-imaginal discs from third-instar larvae using an antibody to activated caspase 3 (AC3) (Fig. 1N and quantified in Supplementary Table 1). Twelve discs of each genotype were examined and the discs were scored without the experimenter being aware of the genotype, so as to prevent any scoring bias. The total number of AC3-positive cells in 12 discs containing crb clones (total = 273 AC3-positive cells) was much higher than in 12 discs containing clones of a FRT82B chromosome (total = 70 AC3-positive cells). Of the 273 AC3-positive cells in discs containing crb clones, 188 (69%) were also GFP-positive indicating that they were either wild-type cells or heterozygous cells. Of these 188 cells that were positive for both GFP and AC3, 150 (80%) were immediately adjacent to crb mutant cells. By comparison, Li and Baker found that in discs containing Minute Rps18/+ clones, 94% of AC3-positive cells were M/+ cells and of those, 71% were adjacent to wild-type cells (Li and Baker, 2007). Thus, the pattern of cell death in mosaic discs containing crb clones indicates that crb cells can function in a nonautonomous manner to promote the death of wild-type cells at the clonal boundaries and suggests that cell competition is occurring at those boundaries.
The ability of mutant cells to induce the death of adjacent wild-type cells was first described for Myc (de la Cova et al., 2004; Moreno and Basler, 2004) and subsequently for the HSW pathway (Tyler et al., 2007). We therefore examined mosaic imaginal discs containing crb clones using reporters of Myc and HSW pathway activity. We did not observe changes in levels of Myc protein or in the Myc reporter, Fibrillarin (Grewal et al., 2005), between crb and wild-type clones (data not shown) suggesting that the apoptosis that is observed in mosaic discs does not arise from non-uniform Myc activity. To look for changes in the activity of the HSW pathway, we stained crb mosaic discs with antibodies to DIAP1, since DIAP1 levels are increased when signaling via this pathway is reduced (Tapon et al., 2002). We observed a slight increase in the levels of the DIAP1 protein (Fig. S2A) as also reported by others (Chen et al., 2010; Grzeschik et al., 2010; Ling et al., 2010; Robinson et al., 2010). However, the levels of two other HSW pathway reporters, Merlin and Cyclin E, were unchanged (data not shown). This might mean that DIAP1 is a more sensitive reporter of HSW pathway activity or that Crb only regulates a subset of HSW target genes. Moreover, in wild-type cells Ex is localized apically, while in crb mutant cells we found Ex in puncta that were not restricted to the apical domain (Fig. S2B–E). This mislocalization of Ex in crb clones has also been described by several other groups (Chen et al., 2010; Grzeschik et al., 2010; Ling et al., 2010; Robinson et al., 2010). This, together with the observation by one group that Ex binds directly to the intracellular domain of Crb (Crb-ICD) (Ling et al., 2010) suggests that the localization of Ex to the apical membrane is dependent upon Crb and that mislocalization of Ex results in increased expression of genes that are normally repressed by HSW signaling. In contrast, even though wts clones express elevated levels of Ex, the apical localization of Ex is maintained (Fig. S2F). Since Yki-dependent transcription is elevated in crb clones as well as in cells mutant for several components of the HSW pathway, the mechanism by which these cells become supercompetitors could be related.
Crb-overexpressing cells are eliminated from wild-type imaginal discs
If crb clones can influence the survival of adjacent wild-type cells, then similar interactions might occur between wild-type cells and cells that overexpress Crb. Using the FLP-out method (Pignoni and Zipursky, 1997; Struhl and Basler, 1993), we generated clones of cells overexpressing the full-length Crb protein in wild-type wing imaginal discs at different times of development and compared them at 114 hr AED to control clones that overexpressed GFP alone (Fig. 2A–F). As expected, control clones induced at earlier times (54 hr AED) were larger than those induced at later times (72 hr or 90 hr AED) (Fig. 2A–C). In marked contrast, no Crb-overexpressing clones were observed at 114 hr AED when they were induced at 54 hr AED, and only a few clones were observed when induced at 72 hr AED. While Crb-overexpressing clones were most consistently observed when they were induced at 90 hr AED, they were still significantly smaller than control clones induced at the same time (Fig. 2D–F). Thus, Crb-overexpressing clones are eliminated from wild-type imaginal discs over time. Moreover, the overexpression of Crb alone does not appear to be sufficient to cause cells to die. When Crb was previously overexpressed in the entire posterior compartment of the wing disc, the cells were viable and neoplastic overgrowth of the tissue was observed (Lu and Bilder, 2005). Similarly, as described below, overexpressing Crb in the anterior compartment with a patched (ptc) -Gal4 driver did not visibly reduce the size of the compartment (Fig. S3C,D). Therefore, a heterotypic interaction with wild-type cells in the same compartment appears to be required for the elimination of Crb-overexpressing cells.
To better understand how Crb-overexpressing cells are eliminated from wild-type imaginal discs, we stained discs containing Crb-overexpressing clones with the AC3 antibody. Only small clones could be recovered, and most of these few surviving Crb-overexpressing cells were positive for AC3, indicating that they were undergoing apoptosis (Fig. 2G–I). In Minute- and Myc-induced cell competition, losers are eliminated first from the pouch region of the wing imaginal disc (Simpson, 1979, Moreno and Basler, 2004; Moreno et al., 2002). Similarly, Crb-overexpressing cells in the wing imaginal disc were eliminated first from the pouch (Fig. 2D–F). In the eye-imaginal disc (Fig. 2H) cell death is most conspicuous anterior to the morphogenetic furrow, where cells are mitotically active.
If Crb-overexpressing cells undergo apoptosis in response to heterotypic interactions with adjacent wild-type cells, as occurs in cell competition, then Crb-overexpressing cells in contact with wild-type cells should be more susceptible to apoptosis, while Crb-overexpressing cells surrounded by other Crb-overexpressing cells should not. To investigate this possibility, we created large Crb-overexpressing clones by using a temperature-sensitive form of Gal80 (Gal80-TS) (McGuire et al., 2003) in conjunction with the flip-out method of clone induction. The Gal80-TS allowed us to inhibit Crb expression until the clones had grown to a large size (Fig. 2J). Clones were induced at 48 hr AED, but Crb expression was kept off (through incubation at 18°C) until shortly before dissection when the culture was shifted to 30°C (see methods). Upon staining these discs for AC3, we observed much higher levels of AC3 at the borders of these large clones, than in the center of the clones.
Additionally, when compared to wild-type cells in the wing imaginal disc, which are columnar and have an accumulation of actin near their apical membrane, the Crb-overexpressing cells were more rounded and had high levels of actin near the entire outline of the cell. Several of the Crb-overexpressing clones were no longer a part of the disc proper and had been extruded apically. Cells in these extruded clones continued to express GFP that localized to seemingly-intact nuclei, but large numbers of AC3 positive cells and cell debris surrounded the intact cells (Fig. 2L, M). Thus, death-promoting pathways related to cell extrusion (e.g. anoikis) (Frisch and Francis, 1994) could also contribute to their death.
Nonautonomous effects of Crb-overexpressing cells on adjacent wild-type cells
Interestingly, the Crb-overexpressing cells also appeared to have some nonautonomous effects on the survival and morphology of adjacent wild-type cells. The wild-type epithelium surrounding Crb-overexpressing clones was often folded basally in what appeared to be a precursory step to the extrusion of the Crb-overexpressing cells (white arrowheads in Fig. 2L,M). Furthermore, in addition to the large number of AC3-positive GFP-positive (Crb-overexpressing) cells, we clearly observed some GFP-negative (wild-type) cells that were positive for AC3 (yellow arrowheads in Fig. 2I). While we cannot exclude the possibility that some dying cells cease to express GFP, a more likely explanation is that some of the wild-type cells adjacent to Crb-overexpressing cells also undergo apoptosis.
We further explored the properties of this nonautonomous apoptosis by overexpressing Crb with ptc-Gal4. This driver is expressed in the anterior compartment of the wing imaginal disc in a decreasing gradient away from the compartment boundary, with the highest level of expression in a stripe of cells immediately anterior to the boundary (Fig. S3A). As previously reported, Myc-overexpressing cells cannot induce apoptosis in wild-type cells that are on the opposite side of a compartment boundary (de la Cova et al., 2004, Fig. S3B). To compare, we examined the pattern of cell death in ptc>Crb discs. Again, we inhibited developmental expression of Crb with Gal80TS. Just eight hours after the temperature shift, to induce Crb overexpression, we observed high levels of apoptosis in the ptc>Crb discs (Fig. S3C,D). Most of the AC3-positive cells were in extruded clumps (red arrowheads in Fig. S3C,D, and Fig. 3F). However, there were also high numbers of AC3-positive cells within the disc proper (yellow arrowheads in Fig. S3C,D) on both sides of and close to the compartment boundary. The proximity of these cells to the compartment boundary suggests that a global size regulatory mechanism (Mesquita et al., 2010) is not responsible for these cell deaths. ptc-Gal4-driven expression of Crb also caused morphological changes analogous to those observed with clonal overexpression of Crb (described below, compare Fig. 2L,M with 3F). Thus, in contrast to Myc-overexpressing cells, Crb-overexpressing cells appear to induce nonautonmous effects on survival and morphology across the compartment boundary.
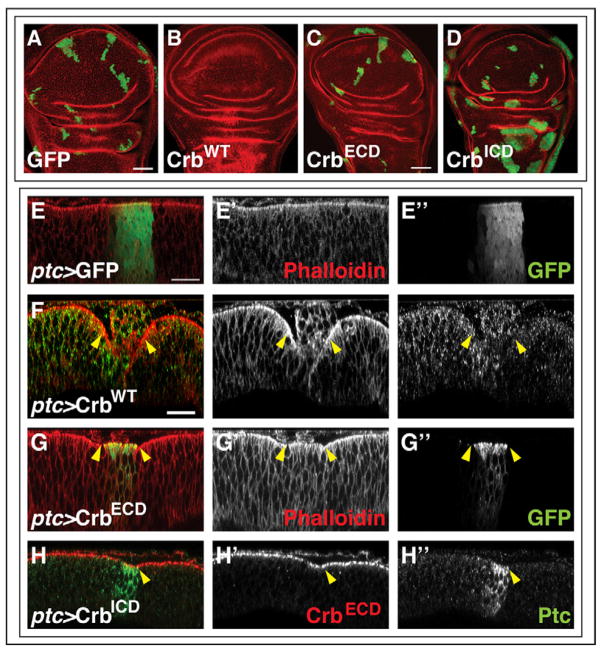
Figure 3. Role of Crb domains in clone elimination and epithelial morphology.
(A–D) Wing discs dissected at 114 hr AED containing clones induced at 54 hr AED that overexpress GFP (A), full-length Crb (B), Crb-ECD (C), or Crb-ICD (D). Note that Crb-ICD clones in the notum region are rounder and more overgrown. (E–H) X–Z sections through wing imaginal discs expressing GFP (E), full-length Crb (F), Crb-ECD (G) or Crb-ICD (H) with ptc-Gal4. The most common disc morphology is shown for each genotype (quantified in Supplementary Table S2). Disc morphology is visualized using phalloidin staining (E, F, G) or anti-Crb-ECD antibody staining (red) (H). The ptc domain is visualized in green through GFP expression (E), anti-Ptc antibody staining (F, H) or the GFP-tag on the Crb-ECD construct (G). In (E–H) apical is up for the disc proper.
Scale bars: 20μm.
Crb-ECD is required for the elimination of Crb-overexpressing cells
If cell survival decisions are based on some kind of comparison between adjacent cells, this requires that each cell is able to assess either some property on the surface of its neighbor or the levels of an extracellular factor that reflects some property of its neighbor. Yet most situations where cell competition has been studied to date result from changes in the levels of intracellular proteins. In contrast, Crb is a transmembrane protein with a large ECD containing many EGF repeats that can mediate protein-protein interactions. This raises the possibility that nonautonomous effects involving Crb could be mediated by the direct interaction of Crb-ECD with proteins on adjacent cells. However, no proteins have been shown to interact with the ECD and, at least under conditions of overexpression, the ECD appears unnecessary for the rescue of the cell polarity defect in crb embryos (Wodarz et al., 1995).
In order to determine the region of the Crb protein that was required for the elimination of Crb-overexpressing cells and adjacent wild-type cells, we made clones that overexpressed either a membrane-bound, GFP-tagged ECD (Pellikka et al., 2002) or a membrane-bound, Myc-tagged ICD (Wodarz et al., 1995) (Fig. 3A–D). The size and frequency of clones overexpressing the ECD were not noticeably different from GFP-expressing control clones (Fig. 3A, C). Similarly clones overexpressing the ICD were not reduced in size or eliminated (Fig. 3D). Instead the ICD-expressing clones were larger and had smoother outlines than control clones, and this effect was exaggerated in the notum. This is consistent with increased Yki-mediated transcription elicited by overexpression of this domain (Chen et al., 2010; Grzeschik et al., 2010; Ling et al., 2010; Robinson et al., 2010). Thus, both the ECD and ICD appear to be necessary for the elimination of cells that overexpress Crb.
We also observed a similar relationship between the domains of Crb in their effect on the curvature of the imaginal disc epithelium as viewed along the apicobasal axis. As described earlier, clones overexpressing Crb using the FLP-out system are consistently extruded from the apical surface of the disc proper. In contrast, with clones overexpressing Crb-ECD or Crb-ICD we did not find any pronounced changes in the clone morphology (data not shown). For an alternative mode of expression, we used the ptc-Gal4 driver (Fig. 3E–H). As with clones generated by the FLP-out system, overexpression of full-length Crb results in the extrusion of the Crb-expressing cells while the immediately adjacent epithelium, including the wild-type epithelium posterior to the compartment boundary and the more anterior epithelium which expressed much lower levels of full-length Crb, were folded basally under this extruded mass of cells. Interestingly, with this driver we also observed consistent morphological changes in the epithelium with expression of either Crb-ECD or Crb-ICD (Fig. 3G, H and quantified in Supplementary Table 2). The apical surface of the epithelium in the region expressing the highest level of Crb-ECD appeared convex and the border with the wild-type cells was kinked (Fig. 3G). In contrast, a slight concavity was observed when the ICD was overexpressed, also resulting in a kink in the apical surface at the anterior-posterior (A–P) compartment boundary (Fig. 3H). That the morphological changes were more apparent when Crb-ECD and Crb-ICD were overexpressed in the region of ptc expression but not in clones, may indicate that this region is a structurally sensitive area where slight changes in cell morphology or adhesion can lead to visible changes in the epithelial structure. Moreover, whether from physical force or as a result of signaling, the changes in morphology that we observe in regions overexpressing the various forms of Crb appear to be transmitted to the adjacent wild-type cells. These morphological effects could conceivably have a role in sensitizing cells to pro-apoptotic signals at the clonal boundary and, for example, account for the death that we see in wild-type cells adjacent to Crb-overexpressing clones.
Crb exerts nonautonomous effects on protein localization in adjacent cells
We next investigated whether the Crb-ECD could mediate any nonautonomous effects by altering the localization of molecules on adjacent cells. Consistent with previous reports, we observed that the localization of Crb protein in wild-type cells adjacent to crb clones is altered (Fig. 4A) (Pellikka et al., 2002, Chen et al., 2010). Specifically, the level of Crb protein is greatly reduced on the surface of the wild-type cells that is immediately adjacent to mutant cells, resulting in an asymmetric distribution of Crb in these wild-type cells. The preferential localization of Crb on membranes adjacent to Crb-expressing cells suggests that Crb molecules on adjacent cells interact, either directly or indirectly. We observe an analogous effect at borders between cells that express a known homophilic adhesion molecule, Fas II, and adjacent cells that do not (Fig. S4A).
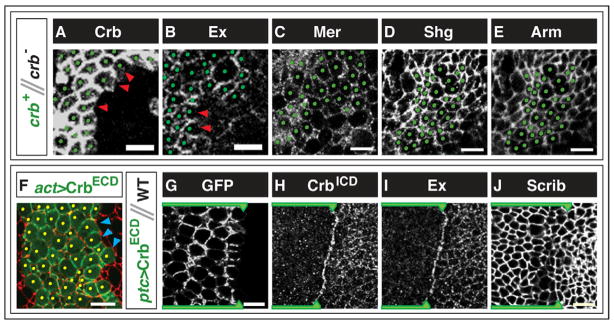
Figure 4. Changes in protein localization at borders between cells expressing different levels of Crb.
(A–E) Regions where crb mutant clones abut non-mutant clones (heterozygous for crb) in mosaic eye imaginal discs. Non-mutant cells are marked with green dots. Stains shown are Crb (A), Expanded (Ex) (B), Merlin (Mer) (C), Shotgun (Shg)/E-cadherin (D), or Armadillo (Arm) (E). (D) and (E) show the same clone. In non-mutant cells, only Crb and Ex are depleted from the portion of the membrane that is in contact with the crb mutant cells (red arrowheads). (F) Border of a clone overexpressing the Crb-ECD protein visualized by its GFP-tag (green). Blue arrowheads show its exclusion from membranes that abut wild-type cells. Phalloidin is shown in red. (G–J) Border between the anterior compartment, expressing Crb-ECD (marked with green line and arrowhead), and posterior compartment, containing wild-type cells in wing imaginal discs. (H) and (I) show the same disc. Panel (G) shows a more basal section than (H), (I) or (J). The ECD construct in the Crb-ECD-expressing cells is visualized through its GFP-tag in (F, G). Antibody stains shown are anti-Crb-ICD (H), Ex (I) and Scrib (J).
Scale bars: 5μm.
Nonautonomous effects on localization are also seen in downstream molecules that interact with Crb. Ex protein levels are greatly reduced in the portion of the membrane where wild-type cells abut crb mutant cells (Fig. 4B). This is consistent with the finding that Ex physically interacts with the ICD of Crb (Ling et al., 2010). In contrast, we observed no differences in the level of Merlin at the interface between wild-type cells and crb cells, pairs of crb cells, or pairs of wild-type cells (Fig. 4C). E-cadherin and Armadillo protein levels are similarly unaltered at these junctions (Fig. 4D,E). Thus, there is not a general lack of adhesion between cells at the boundaries of crb clones.
We wondered whether the interaction between Crb molecules could also cause additional Crb molecules to be drawn to a border where the adjacent cell expressed higher levels of Crb. To test this, we generated FLP-out clones that overexpressed the Crb-ECD protein (Fig. 4F, individual channels shown in Fig. S4B). Since this protein is fused to GFP, we were able to visualize its localization by examining the membrane-associated GFP. Additionally, these cells also expressed a cytoplasmic GFP from a UAS-GFP transgene. In the cells at the periphery of the GFP-expressing clone, the levels of the membrane-associated GFP was greatly reduced on the membrane that is apposed to wild-type cells when compared to surfaces that contact cells that also overexpress the Crb-ECD.
We also expressed the Crb-ECD protein in the region immediately anterior to the A–P compartment boundary of the wing imaginal disc using ptc-Gal4 (Fig. 4G–J, S3C,D). In cells abutting the compartment boundary, the Crb-ECD, visualized through its GFP tag, was mostly excluded from the posterior border shared with wild-type cells (Fig. 4G). We were also able to assess the localization of endogenous Crb in the ptc>Crb-ECD discs with an antibody that recognizes the ICD of Crb (Fig. 4H). In doing so, we observed a striking increase in the levels of endogenous Crb along the border between the wild-type and Crb-overexpressing cells. This is consistent with the endogenous Crb in the wild-type cells being drawn toward the Crb-ECD-overexpressing cell. Ex levels were also higher at this border, mimicking the pattern of Crb localization (Fig. 4I). These changes in protein localization are not a unique property of the compartment boundary, as similar changes are found at the borders of flip-out clones (Fig. S4E–H). It is nevertheless notable that these interactions can occur across the compartment boundary which represents a barrier for cell competition involving Minutes or Myc. Again, we observe that the distribution of some proteins, including E-cadherin (Fig. S3E–H), remains normal, demonstrating that the nonautonomous effect on protein localization elicited by Crb-ECD affects some, but not all, proteins.
This pattern of Crb localization is consistent with the ECD of Crb binding, either directly or indirectly, to Crb molecules on adjacent cells and altering the localization of Ex and possibly other intracellular proteins in those cells. Since asymmetric localization of Crb within individual cells would be predicted to occur at the boundaries between cells expressing different levels of Crb, we wondered whether this asymmetry would be sufficient to induce apoptosis. We drove expression of the Crb-ECD in flip-out clones (data not shown) and using the ptc-Gal4 driver and stained wing imaginal discs with the AC3 antibody (Fig. S3E). There was no obvious increase in the number of AC3-positive wild-type cells adjacent to Crb-ECD-overexpressing cells. These findings are inconsistent with a simple model in which a planar asymmetry in Crb localization alone is sufficient to induce the elimination of cells (see Discussion). It might indicate that other proteins that associate specifically with full-length Crb are necessary for such an interaction or that Crb may participate in “inside-out” signaling, as has been observed for integrins (Ginsberg et al., 1992). While overexpression of Crb-ICD with ptc-Gal4 did cause an increase in apoptosis in the wing disc (Fig. S3F), these AC3-positive cells were distributed throughout the tissue rather than being closely associated with the compartment boundary and stripe of ptc expression, especially in the anterior compartment. This implicates a tissue-wide or systemic regulatory mechanism that is likely to be distinct from the short-range signaling mechanism that induces apoptosis at the borders of full-length Crb-overexpressing clones.
Are Crb levels altered in Minute- or Myc- mediated cell competition?
We next investigated whether differences in Crb levels may play a role in the two best-studied examples of cell competition – the elimination of Minute cells by wild-type cells and the elimination of wild-type cells by Myc-overexpressing cells. We visualized Crb levels by antibody staining in wing discs containing Minute clones or overexpressing Myc in the region of ptc expression. As previously reported, Myc-overexpressing cells were larger than wild-type cells (Johnston et al 1999). However, we did not find any consistent differences in the intensity of Crb staining along the membrane of Myc-overexpressing cells.
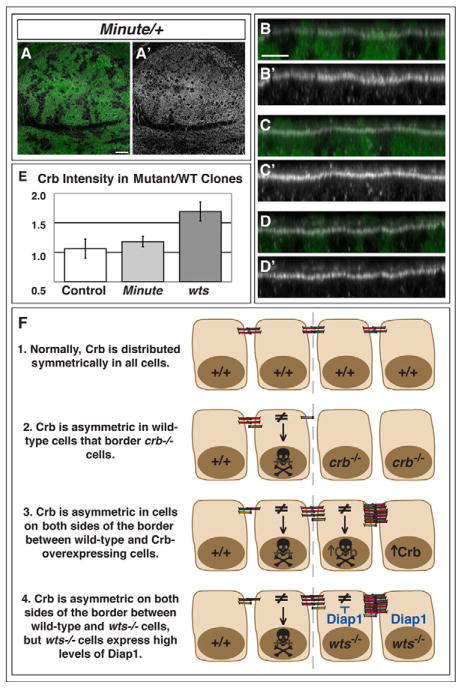
While, by eye, the regions of mosaic discs containing Minute cells appeared to have slightly higher levels of Crb than adjacent wild-type regions (Fig. 5A–D), we did not find any significant differences when we quantified the intensity of Crb staining along the membrane (Fig. 5E, see Methods). Differences in Minute cell size or morphology may explain why Minute cells appear to have elevated Crb levels. The apical profile of Minute cells was reduced, occupying, on average, approximately 80+/−7% of the area of wild-type cells in the same disc (n = average area of 500 cells per genotype per disc for 3 discs). As Crb staining is more intense at cell junctions, a greater number of cells, and therefore a greater number of junctions per area, may give the appearance of higher staining intensity. Furthermore, the intensity of Crb staining was highly dependent on the focal plane and position in the disc. Crb is restricted to a narrow plane of expression above the adherens junction, and its expression is higher in cells near the Dorsal-Ventral compartment boundary. In XZ views, we found that Minute patches frequently had a convex curvature along the apicobasal axis (Fig. 5B–D). This phenotype, however, was not as consistent as when Crb constructs were overexpressed in the ptc expression region (Fig. 3). Thus, while we did not find any conclusive differences in cell surface Crb concentration, there appear to be differences in cell size and epithelial morphology between Minute and wild-type cells that may influence the efficacy of binding of cell-surface molecules, such as Crb, across clone boundaries.
Figure 5. The role of Crb in cell survival.
(A–D) Minute mosaic wing disc stained with Anti-Crb antibody. GFP-negative wild-type clones are in a GFP-positive Minute+/− background. Anti-Crb is in white and shown separately in A′-D′ (B–D) Three different XZ slices through the disc in (A). (E) Quantification of average Crb staining intensity along the membrane in control, Minute, and wts mosaic wing discs. Presented as a ratio of the intensity in mutant over wild-type regions (see Methods). Error bars show SE. (F) Model for the regulation of cell survival by the planar distribution of Crb protein within a cell. Grey dashed line represents the border between two populations of cells. Crb is normally distributed symmetrically along the entire apical domain of epithelial cells (1). Cells with an asymmetric distribution of Crb (≠) will undergo apoptosis (represented by skull). This occurs in wild-type cells that border crb−/− mutant cells (2). Juxtaposition of wild-type cells and Crb-overexpressing cells (3) or cells mutant for components of the Hippo-Salvador-Warts pathway (4) results in asymmetric distribution of Crb protein in both types of cells at the border. However, cells mutant for components of the Hippo-Salvador-Warts pathway have high levels of Diap1, which inhibits apoptosis in those cells.
Scale bars: 20μm in A and 10μm in B–D.
DISCUSSION
Previous work has shown that in genetically mosaic tissues certain types of cells survive at the expense of others. Yet, what is being compared between cells of different genotypes and how it determines survival is not known. In a genetic screen for nonautonomous regulators of cell survival we identified crb, which encodes a cell surface protein. Here we examined tissues where groups of cells with different Crb levels were juxtaposed and observed high levels of cell death, specifically near the boundaries between the two populations. Based on the requirement of the ECD in these effects and indications that Crb molecules may interact across cell junctions, we propose that Crb itself could function as a comparison factor to directly regulate cell survival nonautonomously.
We refer to the nonautonomous cell death caused by differences in Crb levels as an example, albeit atypical, of cell competition because: (1) We observe apoptosis at the boundaries between cells that express different levels of Crb, (2) Crb is known to be a regulator of growth (Richardson and Pichaud, 2010), and (3) Crb can modulate pathways that have been implicated in cell competition (Chen et al., 2010, Grzeschik et al., 2010, Ling et al., 2010, Robinson et al., 2010). However, we note that the low levels of apoptosis in wild-type “winners” adjacent to Crb-overexpressing clones and the ability of Crb-overexpressing cells to induce cell death across the A–P compartment boundary distinguishes Crb-mediated competition from some other instances of cell competition. “Cell competition” is currently a very loosely defined term and is known to encompass, at least, two independent mechanisms e.g. Myc-dependent and Myc-independent (Vincent et al, 2011). Future comparisons of all instances of cell competition may establish criteria for distinguishing the phenomena in a more mechanistically meaningful way.
Interactions between Crb proteins on adjacent cells
We observe that Crb-ECD is required for the elimination of Crb overexpressing cells from wild-type tissues. The ECD of transmembrane proteins often mediates cell-cell interactions, but there were no reported binding partners for the Crb-ECD. Through a series of experiments we, and others (Chen et al., 2010), corroborated a previous observation (Pellikka et al., 2002) suggesting that Crb molecules on adjacent cells might interact with each other through their ECD. The interaction of Crb molecules, either directly or indirectly across cell borders has at least three consequences. First, changes in Crb levels can influence the properties of an adjacent cell, specifically the localization of at least two, but potentially many, proteins in that cell. Second, the interaction of Crb molecules could result in the stabilization of Crb at that border, either by preventing it from being internalized or diffusing away laterally. Third, if the two cells express different levels of Crb, then the cell that expresses higher levels of Crb would be predicted to have more “free” Crb i.e. Crb that is not interacting with Crb on an adjacent cell. We considered different models for how a comparison of Crb levels might regulate cell survival.
If Crb is indeed capable of interacting directly with Crb on adjacent cells, then the juxtaposition of two cells that express different levels of Crb would result in the presence of unbound Crb in the cell with higher levels of Crb. The binding of Crb to Crb on adjacent cells could be an epithelial surveillance mechanism that inhibits apoptosis; thus, unbound Crb could activate apoptosis. This model can explain the death of wild-type cells adjacent to Crb mutant cells and the death of Crb-overexpressing cells adjacent to wild-type cells, as well as the death of scrib, lgl, and dlg mutant cells adjacent to wild-type cells. At least in the embryo, scrib, lgl, and dlg mutant cells have been shown to express higher levels of Crb (Bilder and Perrimon, 2000), adjacent to wild-type cells. However, cells with mutations in the HSW pathway express higher levels of Crb than wild type (Genevet et al., 2009; Hamaratoglu et al., 2009), yet they are also supercompetitors (Tyler et al., 2007). Furthermore, this model cannot account for the apoptosis seen in some wild-type cells adjacent to Crb-overexpressing cells. Thus, the relative levels of Crb alone cannot determine the directionality of competition, which led us to propose another model.
We next considered a model where differences in Crb levels still trigger competition, but the outcome is determined by other factors (Fig. 5F). In this model, when two populations of cells that express different levels of Crb abut each other, cells on both sides of the boundary are expected to have an asymmetric planar intracellular distribution of Crb and the proteins that it binds to, such as Ex, along their membrane. The asymmetric distribution of one of these Crb-binding molecules could promote apoptosis. Under normal conditions, both pro-apoptotic and anti-apoptotic proteins might be distributed uniformly throughout the cell with an overall excess of anti-apoptotic proteins. The asymmetric localization of a membrane-associated pro-apoptotic protein could result in its local concentration exceeding that of anti-apoptotic proteins and result in the activation of pro-apototic signaling cascades. Indeed, work by other groups has indicated that asymmetries in components of the HSW pathway such as changes in the cadherins, Ft and Dachsous (Ds), might regulate a variety of biological processes including cell polarity and cell proliferation (Rogulja et al., 2008; Willecke et al., 2006; Yang et al., 2002).
This model would predict that cells on both sides of the border would be predisposed to death, and the propensity of each population to die would depend on its levels of anti-apoptotic proteins. Thus, it explains why death occurs preferentially in wild-type cells adjacent to crb clones (crb cells have a slight increase in DIAP1 levels) but on both sides, albeit at different levels, of clones that overexpress full-length Crb. This mechanism can also explain the apparent paradox that cells with mutations in the HSW pathway components ex, hpo, sav and wts mutant cells, behave as supercompetitors even though they express higher levels of Crb. Since the mutant cells also express increased DIAP1 levels, apoptosis would mostly be confined to wild-type cells.
Also consistent with this model is the elimination by cell competition of clones of cells that are mutant for scrib or dlg (Brumby and Richardson, 2003; Woods and Bryant, 1991), which express higher levels of Crb (Bilder and Perrimon, 2000). Indeed scrib clones have been shown to induce JNK activity in mutant cells as well as in adjacent wild-type cells (Igaki et al., 2009).
The observation that overexpression of the Crb-ECD alone, which induces planar asymmetry of Crb and Ex in adjacent wild-type cells, does not promote apoptosis of those cells argues that inducing planar asymmetry of Crb is, in itself, insufficient to activate apoptosis. One caveat may be that other proteins that associate specifically with full-length Crb may be required for the intercellular signaling that activates apoptosis. Alternatively, as has been observed for integrins (Ginsberg et al., 1992), only full-length Crb may be capable of “inside-out” signaling. Such signaling may be necessary for the death of adjacent cells.
The observation that death occurs on both sides of a boundary where a heterotypic interaction occurs is also not unprecedented. Dpp signaling-deficient clones are thought to trigger a homeostatic response by causing discontinuities in the slope of the gradient of Dpp signaling that also leads to their elimination from wild-type imaginal discs. In this process, known as “morphogenetic apoptosis,” death on both sides of the border is thought to re-establish the slope of the gradient (Adachi-Yamada and O’Connor, 2002; Adachi-Yamada and O’Connor, 2004).
Links between apicobasal polarity and cell survival
Until recently, the most well characterized role of Crb was in regulating apicobasal polarity in the embryonic epithelium. The loss of Crb in embryos causes epithelial cells to have a diminished apical membrane (Tepass, 1996), and groups of cells are often extruded as hollow cysts with their small apical domains facing inwards. Conversely, overexpression of Crb generates cysts with their apical domains facing outward. In the embryo, this apicalization is phenocopied by overexpression of Crb-ICD, while overexpression of Crb-ECD has no apparent effect (Wodarz et al., 1995).
Does this function of Crb extend to the imaginal discs and could changes in polarity have any impact on cell survival? We found that overexpression of full-length Crb in the imaginal disc produces clear defects in epithelial polarity and morphology. Crb-WT overexpressing clones are often extruded apically and form cyst-like structures, as in the embryo. Interestingly, there also appear to be nonautonomous effects on the morphology of adjacent wild-type cells that fold underneath these extruding clones. The forces that generate the extrusion and kinks we observe could conceivably contribute to the death of cells at the clonal boundaries. Indeed, mechanical forces have previously been proposed to regulate the extent of cell proliferation in imaginal discs (Hufnagel et al., 2007, Aegerter-Wilmsen et al., 2007) and direct measurements have demonstrated correlations between forces and proliferation in endothelial cells in culture (Nelson et al., 2005). Alternatively, the morphological changes induced by alterations in Crb levels may result in changes in the relative amount of apical membrane. This could influence molecular interactions between proteins on the surface of these cells with those expressed on their wild-type neighbors resulting in changes in proliferation and survival.
Many different examples of cell competition involve two populations of cells that differ in Crb expression. For example, cells mutant for scrib, dlg or lgl are eliminated in the presence of wild-type cells (Woods and Bryant, 1991, Brumby et al., 2003, Froldi et al., 2010, Menéndez et al., 2010, Ohsawa et al., 2011), and each of these mutations results in an expansion of the apical Crb-expressing domain in the embryo (Bilder and Perrimon, 2000). Similarly, mutations in components of the HSW pathway make cells supercompetitors (e.g. hpo, sav, wts) and also result in increased levels of Crb (Fig. 5E, Genevet et al., 2009; Hamaratoglu et al., 2009). Our studies suggest that Crb could function in a “comparison mechanism” in at least some cases of cell competition. Yet it is also possible that some of these same properties of Crb could have more general functions. For instance, the effects on proliferation and survival elicited by changes in the interactions between Crb molecules on adjacent cells could operate as part of a size-sensing mechanism during normal development. Mechanical forces generated by organ growth could alter cell geometry and hence Crb-Crb interactions. Thus cell competition, morphogenetic apoptosis, and other related phenomena, may have evolved as a consequence of a more general surveillance mechanism used in organ size control.
Supplementary Material
Highlights.
Cells mutant for Crumbs can promote apoptosis of adjacent wild-type cells.
Cells overexpressing full-length Crumbs die when next to wild-type cells.
Crumbs may interact with Crumbs on adjacent cells via its extracellular domain.
Several examples of cell competition involve differences in Crumbs levels.
Crumbs could function in a comparison mechanism that mediates cell competition.
Acknowledgments
We thank Ulrich Tepass, Elizabeth Knust, Rick Fehon, Bruce Hay, David Bilder, the Bloomington Stock Center and the Developmental Studies Hybridoma Bank for fly stocks and antibodies. We thank David Bilder, Nipam Patel, Todd Nystul, and members of the Hariharan laboratory for advice, helpful discussions, and comments on the manuscript. IKH was funded in part by grant 5RO1 GM61672, YH by training grant 5T32CA009041 (both from the NIH) and JAB by a predoctoral fellowship from the NSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi-Yamada T, O’Connor MB. Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev Biol. 2002;251:74–90. doi: 10.1006/dbio.2002.0821. [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T, O’Connor MB. Mechanisms for removal of developmentally abnormal cells: cell competition and morphogenetic apoptosis. J Biochem. 2004;136:13–7. doi: 10.1093/jb/mvh099. [DOI] [PubMed] [Google Scholar]
- Aegerter-Wilmsen T, Aegerter CM, Hafen E, Basler K. Model for the regulation of size in the wing imaginal disc of Drosophila. Mech Dev. 2007;124:318–26. doi: 10.1016/j.mod.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Izaddoost S, Lu Y, Cho KO, Choi KW, Bellen HJ. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–45. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–80. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–79. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-L, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci USA. 2010;107:15810–5. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–16. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froldi F, Ziosi M, Garoia F, Pession A, Grzeschik NA, Bellosta P, Strand D, Richardson HE, Grifoni D. The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC Biol. 2010;8:33. doi: 10.1186/1741-7007-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Polesello C, Blight K, Robertson F, Collinson LM, Pichaud F, Tapon N. The Hippo pathway regulates apical-domain size independently of its growth-control function. J Cell Sci. 2009;122:2360–70. doi: 10.1242/jcs.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MH, Du X, Plow EF. Inside-out integrin signalling. Curr Opin Cell Biol. 1992;4:766–71. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Li L, Orian A, Eisenman RN, Edgar BA. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat Cell Biol. 2005;7:295–302. doi: 10.1038/ncb1223. [DOI] [PubMed] [Google Scholar]
- Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20:573–81. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Gajewski K, Sansores-Garcia L, Morrison C, Tao C, Halder G. The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. J Cell Sci. 2009;122:2351–9. doi: 10.1242/jcs.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel L, Teleman AA, Rouault H, Cohen SM, Shraiman BI. On the mechanism of wing size determination in fly development. Proc Natl Acad Sci USA. 2007;104:3835–40. doi: 10.1073/pnas.0607134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Developmental Cell. 2009;16:458–65. doi: 10.1016/j.devcel.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G, Guichet A, Ephrussi A, Casanova J. Relief of gene repression by torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning. Genes Dev. 2000;14:224–31. [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–90. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14(Spec No 2):R251–8. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- Li W, Baker NE. Engulfment is required for cell competition. Cell. 2007;129:1215–25. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–9. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16:702–9. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- McCartney BM, Fehon RG. Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J Cell Biol. 1996;133:843–52. doi: 10.1083/jcb.133.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–8. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Menéndez J, Pérez-Garijo A, Calleja M, Morata G. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc Natl Acad Sci USA. 2010;107:14651–6. doi: 10.1073/pnas.1009376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita D, Dekanty A, Milan M. A dp53-dependent mechanism involved in coordinating tissue growth in Drosophila. PLoS Biol. 2010;8:e1000566. doi: 10.1371/journal.pbio.1000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milán M, Pérez L, Cohen SM. Short-range cell interactions and cell survival in the Drosophila wing. Developmental Cell. 2002;2:797–805. doi: 10.1016/s1534-5807(02)00169-7. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–21. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–29. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–9. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102:11594–9. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–60. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Sugimura K, Takino K, Xu T, Miyawaki A, Igaki T. Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Developmental Cell. 2011;20:315–28. doi: 10.1016/j.devcel.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–9. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by decapentaplegic. Development. 1997;124:271–8. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Rhiner C, López-Gay JM, Soldini D, Casas-Tinto S, Martín FA, Lombardía L, Moreno E. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Developmental Cell. 2010;18:985–98. doi: 10.1016/j.devcel.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Richardson ECN, Pichaud F. Crumbs is required to achieve proper organ size control during Drosophila head development. Development. 2010;137:641–50. doi: 10.1242/dev.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein expanded. Curr Biol. 2010;20:582–90. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Developmental Cell. 2008;15:309–21. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo-Matsuda N, Johnston LA. Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proc Natl Acad Sci USA. 2007;104:18543–8. doi: 10.1073/pnas.0709021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. Parameters of cell competition in the compartments of the wing disc of Drosophila. Dev Biol. 1979;69:182–93. doi: 10.1016/0012-1606(79)90284-7. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–40. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DCR, Schiripo TA, Haber DA, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–78. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Tepass U. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev Biol. 1996;177:217–25. doi: 10.1006/dbio.1996.0157. [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–99. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- Tseng A-SK, Tapon N, Kanda H, Cigizoglu S, Edelmann L, Pellock B, White K, Hariharan IK. Capicua regulates cell proliferation downstream of the receptor tyrosine kinase/ras signaling pathway. Curr Biol. 2007;17:728–33. doi: 10.1016/j.cub.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler DM, Li W, Zhuo N, Pellock B, Baker NE. Genes affecting cell competition in Drosophila. Genetics. 2007;175:643–57. doi: 10.1534/genetics.106.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JP, Kolahgar G, Gagliardi M, Piddini E. Steep differences in wingless signaling trigger Myc-independent competitive cell interactions. Dev Cell. 21:366–74. doi: 10.1016/j.devcel.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen C-L, Tao C, Zhang X, Halder G. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–64. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Yang C-h, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–88. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Yoo SJ, Huh JR, Muro I, Yu H, Wang L, Wang SL, Feldman RMR, Clem RJ, Müller H-AJ, Hay BA. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat Cell Biol. 2002;4:416–24. doi: 10.1038/ncb793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.