Abstract
Skeletal loading in vertebrates controls modeling drifts, modulated remodeling rates, and affects growth trajectories. It is unclear whether the majority of the mechanical stimulus detected by bone cells originates from muscle contraction forces, or from gravitational forces associated with substrate impact. A number of clinical and basic science reports indicate that muscle forces play a dominant role in generating the mechanical stimulus in exercise-induced bone gain. While it is in most cases difficult to separate the effects of gravitational forces acting on body mass from muscle contractions, several well-conceived experiments offer considerable insight into the propensity of muscle-derived forces per se to drive the adaptive response in bone. Load-induced osteogenesis requires that mechanical signals come packaged with particular characteristics, all of which can be generated from either gravitational or muscle forces. Neither of these two sources has been demonstrated empirically to be the source of bone’s adaptive response, but a convincing body of data suggests that muscle contractions are present, significant, and capable of accounting for a large majority of the adaptive responses.
Keywords: mechanotransduction, loading, muscle, strength, bone mass
“Trauma excepted, muscles cause the largest loads and the largest bone strains, and these strains help to control the biological mechanisms that determine whole-bone strength.”
-Harold Frost, 2000
As indicated by Frost and echoed by others, one of the prevailing dogmas in the field of bone functional adaptation posits that bone morphology, structure, and strength are dominated by the forces generated by muscle contraction. While it is widely accepted that bone adapts to the mechanical demands to which it is subjected, the origin of the mechanical demands that provide the driving stimulus for the tissue, i.e., muscle contraction or substrate reaction forces, have come under closer scrutiny in recent years.(5) The argument over the “muscle forces or gravitational loads” is not simply an academic one; there are real and potentially therapeutic consequences of characterizing the specific roles of muscle forces and gravitational loads on bone adaptation.
Perhaps an example from the field of bone cell mechanobiology can highlight the importance and benefit of elucidating the origin of a physical stimulus: in light of the well-known adaptive nature of bone tissue described more that a century ago, (28) it has been known for many years that bone cells are sensitive to mechanical forces. It was commonly assumed that tissue deformation during loading (e.g., exercise) would stretch resident bone cells (e.g., osteocytes), and this stretching action would generate a cascade of signaling events that would eventually result in enhanced structural adaptation.(2, 21) In the mid-1980s and the years to follow, many experiments indicated that the degree to which bone cells need to be stretched, in order to elicit an osteogenic response, was greater than the surrounding mineralized tissue could bear.(3, 16, 29) These studies lead to the discovery that fluid shear forces, and not mechanical stretch, were the driving stimulus behind load-induced osteogenesis in whole living bones. Subsequent experiments showed that modulating the flow rate of extracellular fluid through the bone matrix, without deforming the tissue at all, could generate a robust osteogenic response, similar to the response generated from vigorous physical activity.(1, 22, 23, 30–32) With the identification of the precise nature of the physical stimulus driving the response, it became possible to manipulate the biology of the system with minimal superfluous stimulation. New therapies and exercise-related approaches to bone health can be streamlined to account for these discoveries. In a similar fashion, deciphering the primary stimulus for adaptive response on a more macroscopic level (muscle forces or gravitational loads) carries with it the potential to design physical activities and therapies aimed specifically at improving bone mass more effectively.
The remainder of this communication reviews some pertinent information implicating the role of muscle force as the primary driving stimulus in bone functional adaptation, with the goal of providing some clarification on what muscle contraction can and can not account for. It should be stated at the outset that muscle-derived forces and gravitational-derived forces are by no means mutually exclusive phenomena in the weight-bearing skeleton. Sorting out the individual contributions of muscle and gravitational forces is hampered by a lack of appropriate models that specifically address this question. However, as we piece together portions of the published literature, a paradigm emerges that indicates muscle-derived forces are present, significant, and capable of eliciting adaptive responses. Whether those forces are the primary stimuli is not settled, and physiologically, may not matter from the tissue’s perspective.
Muscle strength and bone mass: lessons from the upper limb
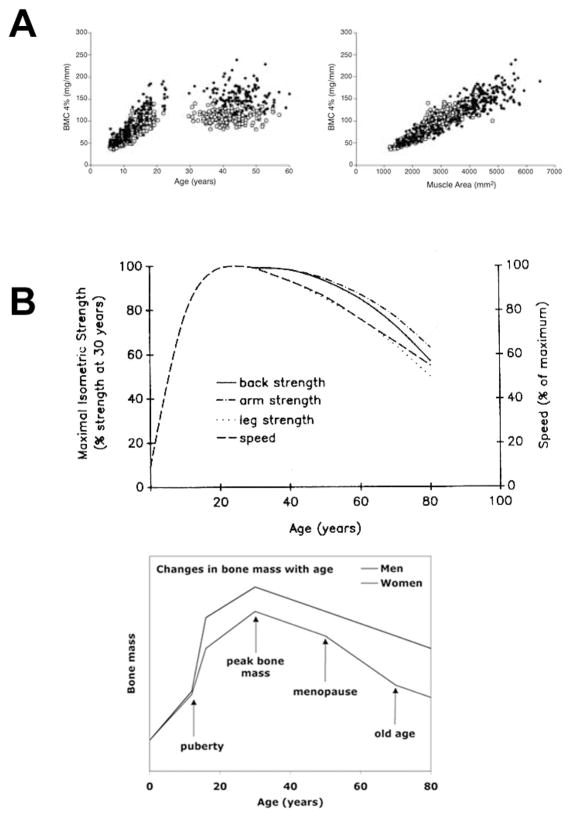
Separating the effects of gravitational loads from those of muscle loads is a difficult undertaking, but perhaps the most logical place to begin is the upper limb. By virtue of the bipedal posture of humans, the upper limb harbors substantial muscle mass but does not transmit body weight to the ground, and is therefore not subject to ground reaction forces. A brief survey of the multitude of studies looking at the relation between muscle strength/mass and bone mass at various sites in the upper limb reveals a consistent, significant, positive association between the two.(9) It is also evident that the gain and decline of bone mass mirrors the gain and decline of muscle strength throughout life, even in the upper limb (Fig. 1). Those results could be interpreted several ways: (1) stronger muscles pull with greater force on the skeleton, and consequently the bone must adapt; (2) larger, heavier bones require greater muscle force to move them, and consequently, the muscle must adapt to the greater bone mass; or (3) muscle mass and bone mass are controlled independently by the genetic program and/or the physical environment, and the associations between the two are spurious. Although the aforementioned results do not prove a cause–effect relationship (it is reasonable to assume that larger individuals might harbor genes for both large muscles and large bones (20)), more elegant studies have probed this relation to look for the underlying muscle mass effects. For example, Daly et al.(6) measured muscle and bone size in the racket and non-racket arm of subadult competitive tennis players using magnetic resonance imaging. Their data confirmed earlier studies showing an increase in muscle and bone mass in the playing vs. non-playing arm, but further probing of their results generated the novel finding that the side-to-side differences in muscle area accounted for a statistically significant amount (20%) of the variation in the side-to-side differences in humeral rigidity. Those analyses overcome the confounding effects of body size, genetics, and self selection on the muscle size–bone size relation, but conversely, they also highlight that there is a significant portion of the variation in bone size not explained by muscle mass.
Figure 1.
(A) Bone mineral content in the distal radius across a wide age range of subjects demonstrates the poor association with age (left panel), compared to the close association with forearm muscle area (right panel). From Schönau, 2005.(19) (B) Isometric muscle strength measurements over a wide age range shows a very similar profile to bone mass over the same range. From Brooks and Faulkner.(4)
The associations between muscle/strength and bone in children is strong enough that clinical techniques for disease diagnosis can be founded upon them. For example, it is well know that DXA- and QCT-derived measurements are an inadequate diagnostic tool in children, for the simple fact that BMD and BMC are a partial function of stature (i.e., shorter kids will have less BMC than taller kids at the same age). The normal range of variation in stature, even for a given age and developmental stage, is too wide to facilitate the use of DXA-derived measurements in assessing bone mass and potential disease. Schönau’s solution to this problem was to develop the “functional muscle-bone unit” as a means for assessing whether too much or too little bone exists in the growing skeleton, based on strength (i.e., muscle mass) indices.(19) In healthy children and adolescents, upper limb bone strength, or a surrogate thereof (e.g. second moments of area), is matched to height-adjusted muscle mass. Deficiencies in the amount of bone per unit muscle strength versus deficiencies in both factors allows for classification of diagnoses into primary (true, or intrinsic) and secondary (physiologic) bone disorders. It is interesting to note, and perhaps telling with respect to the influence of muscle forces on bone mass, that in most of the disease states studied by Schönau, bone mass is typically not overadapted for muscle mass. In other words, when the muscle goes, so goes the bone, but not vice versa. The potential uncoupling of upper limb muscle strength to bone strength in certain disease states, and the strong association under normal circumstances, argues for a strong influence of muscle forces as a key determinant of bone mass and strength.
A third and equally informative observation concerning the role of muscle forces on bone mass is the clinical finding that newborns suffering from intrauterine onset neuromuscular paralysis exhibit normal bone length but severely reduced cortical thickness and mass.(17, 18) These infants are frequently born with multiple fractures (e.g., humerus, radius, femur) that occurred prior to delivery. Considering that normal and paralyzed fetuses are both in a nearly “weightless” aqueous environment (bathed in amniotic fluid), it is unlikely that significant ground reaction forces are generated during the gestational period. Yet it is only when the muscle contractile forces are lost that the bones become pencil thin and fracture easily. These observations would argue for muscle-derived forces and against ground reaction/gravitational force as the primary stimulus driving the adaptive modeling response, at least in the developing skeleton.
Muscle strength and bone mass: lessons from the lower limb
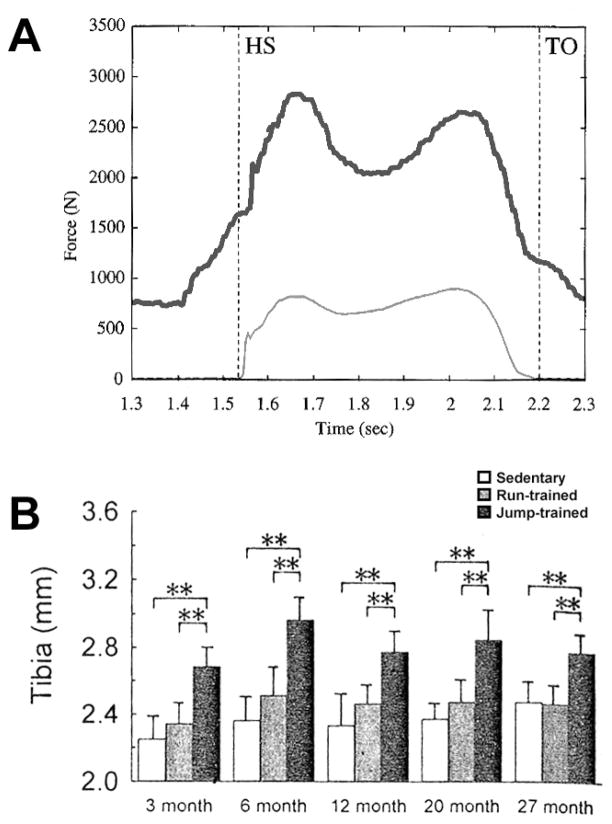
Studies examining portions of the skeleton that experience significant components of both muscle-derived forces and gravitational/ground reaction forces have yielded data implicating the primary role of muscle force in bone mass regulation. In a study conducted in patients receiving partial hip replacements, Lu et al. outfitted the prostheses with telemetrically monitored force transducers located inside the shafts of the prostheses.(12) These subjects were asked to walk along a force plate (among other activities) while the ground reaction forces were collected from the force plate and axial force was telemetrically collected from the transducers within the prosthesis. Greater than 70% of the forces generated within the femur during a normal gait cycle were found to result from muscle forces (which also were monitored via EMG), leaving less than 30% derived from body weight (Fig. 2). These data, while informative, are limited in that the proportion of forces in the long bones resulting from muscle contraction might change, even dramatically, in a high-load (exercise) scenario, which is when bone formation is primarily stimulated.
Figure 2.
(A) Representative force vs. time traces recorded from internal (instrumented femoral prosthesis) and external (force plate) sources, indicating that more than 70% (difference between the two traces) of the force transmitted through the femur during normal gait originates from muscle contraction. From Lu et al.(12) (B) Changes in the external diameter of the tibia after 8 wks of jumping exercise (or treadmill running) in rats of different age groups. The rats were trained to jump up to a platform, which precluded any impact loading (see text). Muscle-modulated jumping significantly enhanced periosteal growth. From Umemura et al.(24)
Other studies using animal models point to the effects of lower limb muscle contraction in osteogenesis, even in the complete absence of impact loading from ground reaction forces. These conditions were made possible in a clever set of experiments designed by Umemura and colleagues, in which they trained rats to jump up to an elevated platform, located half a meter above them.(24) The rats would propel themselves upward from the floor to the platform via explosive muscle contraction, but the impact of landing was precluded by training them to “catch the top edge of the box [i.e., platform]” with their front paws and climb up the remainder of the way.(15) A handful of experiments from this group, using the modified jumping model, demonstrate significant increases in bone fat-free dry weight, mechanical properties, and structural properties as a result of several variations on the “jump up” protocol.(25–27) Thus, a purely muscle-contraction mode of loading is capable of driving a robust osteogenic response, similar to results found in studies employing both muscle contraction and impact.
Muscle strength and bone mass: lessons from genetic mouse models
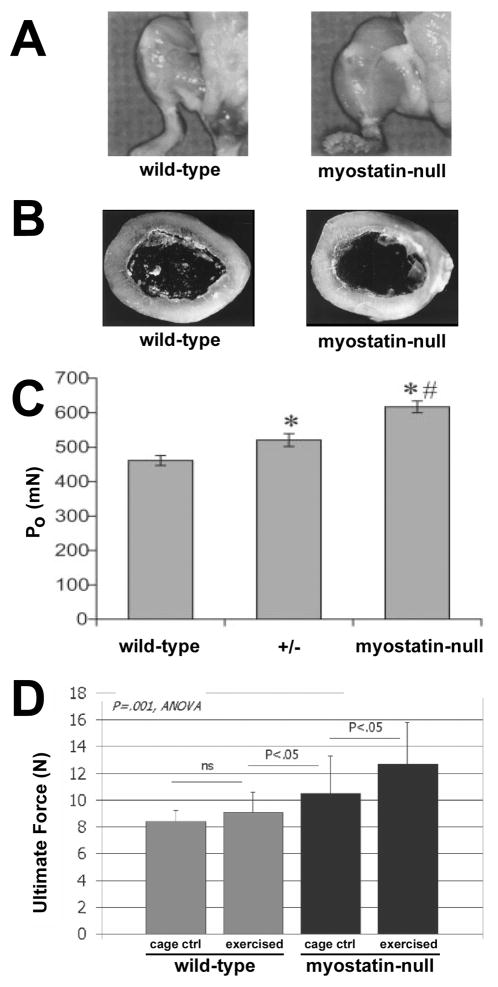
The age of genetic engineering is now upon us, and with it comes a host of tools that can provide seemingly limitless insight into the biological regulation of cells, tissues, and organs. It is now possible to build large muscles (7) or large bones (11) independently and in the absence of significant mechanical loading via targeted genetic manipulation. Several genetic mouse models have been generated that elucidate the effects of muscle mass/strength on bone mass/strength. For example, targeted deletion of the myostatin gene (GDF-8; a negative regulator of skeletal muscle growth) or muscle-specific overexpression of follistatin (an inhibitor of myostatin)—produce a dramatic increase in skeletal muscle mass.(10, 13)
The most well-studied of these mice, the myostatin knockout, was reported to exhibit normal femoral size, shape, and mass (with the exception of expanded trochanters and tubercles for muscle attachment) despite individual muscles reaching up to 3 times the mass of non-mutant mice.(7) Larger muscles would be expected to generate greater forces, but the similarity in bone mass could be explained by the observation that both mutant and wild-type mice were roughly equally inactive in their cages, and both genotypes exhibited identical body weight. To assess whether the larger muscles in the myostatin null mice were concomitantly more powerful, Mendias et al.(14) looked at muscle contractile properties of the myostatin mutants and found them to be significantly enhanced (~30% increase over wild-type), and remained proportional to muscle size. Given the greater muscle mass and increased contractile propensity in the myostatin nulls, the next obvious experiment would be to challenge the mice with an exercise regimen to ascertain whether the increased muscle mass and strength, if exerted, would result in increased bone mass beyond that seen in wild-type mice. Hamrick et al.(8) conducted that experiment by exercising these mice on a treadmill for 30 minutes/day, 5 days/week, for 4 weeks, and measured mechanical properties in the radius. Exercised myostatin-deficient mice exhibited an increase in whole bone structural properties of 30% compared with non-exercised caged controls, whereas exercised wild-type mice did exhibited only a small, non-significant increase compared with non-exercised cage controls. Thus, when put to use, bigger, stronger muscles translate into bigger, stronger bones.
Conclusions
It is clear that muscle forces per se are capable of providing a sufficient stimulus to drive bone adaptation. It is also clear that muscle forces normally provide a significant amount of force, and consequently strain, the axial bones. Whether muscle forces are the primary stimulus remains to be demonstrated empirically. It is likely that the origin of the primary mechanical stimulus is less important to the bone tissue than the “spectral package” in which the stimulus is delivered. Mechanical signals must be of sufficient magnitude, be imposed at significant rates, and be dynamic in application in order for bone adaptation to occur. If physical activity generates ground reaction forces that meet those criteria, it is likely that the ground reaction forces will stimulate osteogenesis. Likewise, if muscular activity deforms the bone tissue in such a manner that those criteria are met, osteogenesis will occur. Perhaps in the coming years, the field will be able to build on some of the animal and human exercise models that have provided clues thus far, to ultimately clarify which mode—muscular or gravitational—provides the lion’s share of the driving force for bone adaptation.
Figure 3.
Myostatin deficiency results in skeletal muscle overgrowth (A), but the long bones of inactive mice are not affected by the double-muscle phenotype (B). Maximal tenatic force (PO) was increased significantly by the mutation. When challenged with exercise, the myostatin null mice gained significantly more bone than wild-type mice, as revealed by the 30% increase in bone strength (untimate force) among mutants. From McPherron et al., (13) Hamrick et al., (7) Mendias et al., (14) and Hamrick et al.(8)
References
- 1.Bergula AP, Huang W, Frangos JA. Femoral vein ligation increases bone mass in the hindlimb suspended rat. Bone. 1999;24:171–7. doi: 10.1016/s8756-3282(98)00165-3. [DOI] [PubMed] [Google Scholar]
- 2.Binderman I, Shimshoni Z, Somjen D. Biochemical pathways involved in the translation of physical stimulus into biological message. Calcif Tissue Int. 1984;36 (Suppl 1):S82–5. doi: 10.1007/BF02406139. [DOI] [PubMed] [Google Scholar]
- 3.Brand RA, Stanford CM, Nicolella DP. Primary adult human bone cells do not respond to tissue (continuum) level strains. J Orthop Sci. 2001;6:295–301. doi: 10.1007/s007760100051. [DOI] [PubMed] [Google Scholar]
- 4.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26:432–9. [PubMed] [Google Scholar]
- 5.Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12:1547–51. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- 6.Daly RM, Saxon L, Turner CH, Robling AG, Bass SL. The relationship between muscle size and bone geometry during growth and in response to exercise. Bone. 2004;34:281–7. doi: 10.1016/j.bone.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Hamrick MW, McPherron AC, Lovejoy CO, Hudson J. Femoral morphology and cross-sectional geometry of adult myostatin-deficient mice. Bone. 2000;27:343–9. doi: 10.1016/s8756-3282(00)00339-2. [DOI] [PubMed] [Google Scholar]
- 8.Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2006;21:477–83. doi: 10.1359/JBMR.051203. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa Y, Schneider P, Reiners C. Age, sex, and grip strength determine architectural bone parameters assessed by peripheral quantitative computed tomography (pQCT) at the human radius. J Biomech. 2001;34:497–503. doi: 10.1016/s0021-9290(00)00211-6. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE. 2007;2:e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–9. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 12.Lu TW, Taylor SJ, O’Connor JJ, Walker PS. Influence of muscle activity on the forces in the femur: an in vivo study. J Biomech. 1997;30:1101–6. doi: 10.1016/s0021-9290(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 13.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 14.Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol. 2006;101:898–905. doi: 10.1152/japplphysiol.00126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagasawa S, Honda A, Sogo N, Umemura Y. Effects of low-repetition jump exercise on osteogenic response in rats. J Bone Miner Metab. 2008;26:226–30. doi: 10.1007/s00774-007-0812-6. [DOI] [PubMed] [Google Scholar]
- 16.Owan I, Burr DB, Turner CH, Qiu J, Tu Y, et al. Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Am J Physiol. 1997;273:C810–5. doi: 10.1152/ajpcell.1997.273.3.C810. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez JI, Garcia-Alix A, Palacios J, Paniagua R. Changes in the long bones due to fetal immobility caused by neuromuscular disease. A radiographic and histological study. J Bone Joint Surg Am. 1988;70:1052–60. [PubMed] [Google Scholar]
- 18.Rodriguez JI, Palacios J, Garcia-Alix A, Pastor I, Paniagua R. Effects of immobilization on fetal bone development. A morphometric study in newborns with congenital neuromuscular diseases with intrauterine onset. Calcif Tissue Int. 1988;43:335–9. doi: 10.1007/BF02553275. [DOI] [PubMed] [Google Scholar]
- 19.Schoenau E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J Musculoskelet Neuronal Interact. 2005;5:232–8. [PubMed] [Google Scholar]
- 20.Seeman E, Hopper JL, Young NR, Formica C, Goss P, et al. Do genetic factors explain associations between muscle strength, lean mass, and bone density? A twin study. Am J Physiol. 1996;270:E320–7. doi: 10.1152/ajpendo.1996.270.2.E320. [DOI] [PubMed] [Google Scholar]
- 21.Somjen D, Binderman I, Berger E, Harell A. Bone remodelling induced by physical stress is prostaglandin E2 mediated. Biochim Biophys Acta. 1980;627:91–100. doi: 10.1016/0304-4165(80)90126-9. [DOI] [PubMed] [Google Scholar]
- 22.Stevens HY, Meays DR, Frangos JA. Pressure gradients and transport in the murine femur upon hindlimb suspension. Bone. 2006;39:565–72. doi: 10.1016/j.bone.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Su M, Jiang H, Zhang P, Liu Y, Wang E, et al. Knee-loading modality drives molecular transport in mouse femur. Ann Biomed Eng. 2006;34:1600–6. doi: 10.1007/s10439-006-9171-z. [DOI] [PubMed] [Google Scholar]
- 24.Umemura Y, Ishiko T, Tsujimoto H, Miura H, Mokushi N, et al. Effects of jump training on bone hypertrophy in young and old rats. Int J Sports Med. 1995;16:364–7. doi: 10.1055/s-2007-973021. [DOI] [PubMed] [Google Scholar]
- 25.Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res. 1997;12:1480–5. doi: 10.1359/jbmr.1997.12.9.1480. [DOI] [PubMed] [Google Scholar]
- 26.Umemura Y, Nagasawa S, Sogo N, Honda A. Effects of jump training on bone are preserved after detraining, regardless of estrogen secretion state in rats. J Appl Physiol. 2008;104:1116–20. doi: 10.1152/japplphysiol.00937.2007. [DOI] [PubMed] [Google Scholar]
- 27.Umemura Y, Sogo N, Honda A. Effects of intervals between jumps or bouts on osteogenic response to loading. J Appl Physiol. 2002;93:1345–8. doi: 10.1152/japplphysiol.00358.2002. [DOI] [PubMed] [Google Scholar]
- 28.Wolff J. Das Gesetz der Transformen der Knochen. Berlin: A. Hirschwald; 1892. [Google Scholar]
- 29.You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, et al. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng. 2000;122:387–93. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Su M, Liu Y, Hsu A, Yokota H. Knee loading dynamically alters intramedullary pressure in mouse femora. Bone. 2007;40:538–43. doi: 10.1016/j.bone.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Su M, Tanaka SM, Yokota H. Knee loading stimulates cortical bone formation in murine femurs. BMC Musculoskelet Disord. 2006;7:73. doi: 10.1186/1471-2474-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang P, Tanaka SM, Jiang H, Su M, Yokota H. Diaphyseal bone formation in murine tibiae in response to knee loading. J Appl Physiol. 2006;100:1452–9. doi: 10.1152/japplphysiol.00997.2005. [DOI] [PubMed] [Google Scholar]