Abstract
Suicidality is a life-threatening symptom in patients with bipolar disorder (BD). Impulsivity and mood instability are associated with suicidality in mood disorders. Evidence suggests that gray and white matter abnormalities are linked with impulsivity in mood disorders, but little is known about the association between corpus callosum (CC) and impulsivity in BD. We examined the relationship between CC areas, impulsivity and suicidality in BD patients. We studied 10 female BD patients with a history of suicide attempt (mean±sd age 36.2±10.1y), 10 female BD patients without suicide attempt history (44.2±12.5y) and 27 female healthy subjects (36.9±13.8y). Impulsivity was evaluated by the Barratt Impulsivity Scale (BIS). We traced MR images to measure the areas of the CC genu, anterior body, posterior body, isthmus and splenium. The genu was divided into anterior, middle and posterior regions. The suicidal and non-suicidal BD patients had significantly higher BIS total, attention and non-planning scores than the healthy subjects (Ps<0.01), and the suicidal BD patients had significantly higher BIS motor scores than the non-suicidal BD and healthy subjects (Ps<0.01). There were no significant differences among the three groups on any regional CC areas, although the suicidal BD patients had the smallest areas. The suicidal BD patients showed a significant inverse correlation between anterior genu area and the BIS total (r=−0.75, p=0.04), motor (r=−0.79, p=0.02) and non-planning scores (r=−0.79, p=0.02). These correlations were not found in the non-suicidal BD patients or healthy subjects. The results suggest that the anterior medial frontal region may be involved in the pathophysiology of impulsive and suicidal behaviors in BD.
Keywords: impulsivity, suicidality, bipolar disorder, corpus callosum, anterior medial frontal, genu
Thoughts of death, suicidal ideation and completed suicide are serious symptoms that shorten the life of persons with bipolar disorder (BD). The Epidemiologic Catchment Area Study demonstrated that the lifetime rate of suicide attempts was 29.2% for BD compared to 15.9% for major depressive disorder (MDD) and 4.2% for all other DSM-III Axis I disorders combined [11]. Twelve percent of all of death in BD patients results from suicide [15]. Clinically suicide attempters are characterized by aggressive/impulsivity traits, hopelessness or pessimistic traits, comorbid substance abuse or alcoholism, history of physical or sexual abuse during childhood, and history of head trauma or neurological disorders [23]. BD patients with a history of suicide attempts exhibit high impulsivity and aggression [33] and show higher trait impulsivity compared to healthy subjects [34].
Although the association between suicidal symptoms and impulsivity in BD patients is becoming widely recognized, the brain pathophysilogy underpinning this association is unknown. Growing evidence from neuroimaging studies suggests that the anterior medial frontal regions of the brain, including ventromedial, orbitofrontal (OFC) and anterior cingulate cortices (ACC), play a crucial role in impulsive, risky, and suicidal behaviors. Healthy subjects with high impulsivity had smaller left and right OFC volumes [24] compared to less impulsive subjects. MDD patients with a history of suicide attempt, but not those without such history, had smaller left and right OFC volumes compared to healthy subjects [26]. Remitted MDD patients with a history of suicide attempt still had exaggerated activation of OFC and ACC during emotional faces tasks compared to those without the history [19]. These implicated brain regions are part of the fronto-limbic circuit that is involved in the pathophysiology of BD [31].
In addition to these abnormal gray matter findings, white matter abnormalities also have been linked with suicidality in mood disorders [12, 29, 30]. The corpus callosum (CC) is the largest nerve fiber pathway of the brain, containing between 200 and 800 million axon fibers that connect intra- and inter-hemispheric subregions [6]. The genu and anterior CC connect the lateral and medial surfaces of the frontal lobes [32] that are thought to be relevant to mood regulation. In this study, we focused on the white matter of the anterior medial regions of the CC in BD. We examined the association between CC areas, impulsivity and suicidality in BD patients compared to healthy subjects. We hypothesized that BD patients with a history of suicide attempt (suicidal BD) would have higher impulsivity and smaller genu and anterior CC areas than BD patients without a history of suicide attempt (non-suicidal BD) and healthy comparison subjects. We also hypothesized that smaller anterior CC areas would predict higher impulsivity in the whole sample and the prediction would be strongest for the suicidal BD patients.
MATERIALS AND METHODS
Subjects
Ten women with BD and positive history of suicide attempt, 10 women with BD and negative history of suicide attempt, and 27 healthy women were studied. The participants were recruited at hospitals and clinics and through advertisements broadcast in the community. All of the patients met DSM-IV criteria for BD by the Structured Clinical Interview (SCID) [14]. We screened 24 BD patients. Four patients were excluded, two because they had current alcohol dependence and two because they had incomplete demographic data. Healthy subjects were screened for DSM-IV axis I disorders by the SCID nonpatient version [13]. None of patients had a history of electroconvulsive therapy or a substance use disorder within 6 months preceding the study. None of healthy subjects had current or past axis I DSM-IV psychiatric disorders or any first-degree relatives with any Axis I psychiatric disorder.
Subject Assessment
All participants also received laboratory tests and a physical examination to rule out physical illnesses. All participants were evaluated for handedness by the Edinburgh inventory [27]. We excluded any participant with current endocrinological disease, history of head trauma with loss of consciousness, current or previous neurological disease, family history of hereditary neurological disorders, or any current medical condition such as active liver disease, kidney problems, or respiratory problems. A history of suicide attempts was assessed by a suicide history interview. We classified subjects as having positive suicidal history if they reported one or more self-injurious acts committed with at least some intent to die. Current mood state of the patients was evaluated using the 21-item Hamilton Rating Scale for Depression (HAM-D) [16] and the Young Mania Rating Scale (YMRS) [40]. The Barratt Impulsiveness Scale version 11A (BIS) [4, 28] was used to evaluate trait impulsivity. The BIS is a self-report questionnaire containing 30 4-point Likert-type items. Scoring yields a total score and three subscale scores derived by factor analysis: attention (rapid shifts and impatience with complexity), motor (impetuous action) and non-planning (lack of future orientation) [4, 28]. Higher scores indicate higher impulsivity. The Institutional Review Board of The University of Texas Health Science Center at San Antonio approved the study. Written informed consent was obtained from all the participants after a description of the study was provided.
MRI Acquisition
Brain images were collected on a Philips 1.5 T MR system (Philips Medical System, Andover, MA). Images were collected using an axial 3-dimensional T1 weighted field, fast echo sequence (field of view 256 mm, view matrix 256 × 256, repetition time 24 msec, echo time 5 msec, flip angle 40 degrees, slice thickness 1 mm). All MRI scans were reviewed to rule out clinically significant abnormalities. Anatomical measurements were conducted on a PC workstation (Dell Computers, Austin, TX). Anatomical boundaries of sub-regions including genu, anterior body, posterior body, isthmus and splenium were delineated in the sagittal plane according to standard brain atlases [18, 41] and methods [38]. Genu was divided into anterior, middle and posterior sub-regions [20]. CC areas were quantified in square centimeters. The inter-rater reliability (intra-class correlation coefficient [ICC]) was calculated using 10 training scans traced independently by two evaluators (N.N. and M.A.N.). The ICCs were 0.97 for total genu, 0.97 for anterior body, 0.90 for posterior body, 0.97 for isthmus, 0.98 for splenium, 0.99 for anterior genu, 0.92 for middle genu and 0.96 for posterior genu. Total brain volumes (TBV) were manually measured by a well-trained evaluator (M.A.N.). The tracing method used for CC areas was described previously [22].
Statistical analyses
We compared BD patients with and without a history of suicide attempt and healthy control subjects using analysis of covariance (ANCOVA) with TBV and age [20] as covariates, focusing on anterior regions of interest (ROI); i.e. the anterior, middle and posterior genu regions and anterior body. We also analyzed the other CC ROIs, i.e., posterior body, isthmus, and splenium, but we had no a priori hypotheses for these. We examined the correlation between the ROI areas and BIS total scores using partial correlations with TBV and age as control variables in each of the three groups. For the regions that revealed significant correlations with the BIS total score, we also computed the partial correlations between the ROI areas and the three BIS subscale scores to assess impulsivity more specifically. For the BD patients, Spearman’s rank order correlation was also used to test the association between the CC areas and four clinical variables; HAMD, YMRS, the age of illness onset and the length of illness. All statistical analyses were conducted using SPSS for Windows statistical software, version 15.0 (SPSS, Inc., Chicago, IL). Two-tailed statistical significance was considered at p<0.05.
RESULTS
Demographics and Impulsivity
There were no significant differences among the suicidal BD, the non-suicidal BD and healthy subjects for age, ethnicity, education or TBV (Table 1). There were 10 patients with a history of substance abuse, 4 patients with a history of mixed state and 4 patients with rapid cycling. Twelve subjects were medicated and 8 were ummedicated at the time of scanning. Medications included antidepressants, mood stabilizers and atypical antipsychotics. No significant difference between the suicidal and non-suicidal BD patients was found for the age at onset of illness, HAM-D or YMRS. The suicidal BD patients had significantly longer length of illness than the non-suicidal BD patients. ANOVA for the BIS scores demonstrated a significant difference among the three groups for total (F2,44=21.1, p<0.01), motor (F2,44=19.4, p<0.01), attention (F2,44=12.7, p<0.01) and non-planning (F2,44=16.3, p<0.01) impulsivity subtypes. Post-hoc pairwise contrasts with Sidak adjustments for multiple comparison revealed that the suicidal and non-suicidal BD patients had significantly higher BIS total, attention and non-planning scores than the healthy subjects (all Ps<0.01) and that the suicidal BD patients had significantly higher BIS motor scores than the non-suicidal BD and healthy subjects (Ps<0.01).
Table 1.
Demographics of the participants
| BD patients
|
Healthy subjects (n = 27) | Statistics | ||
|---|---|---|---|---|
| Suicidal (n = 10) | Non-suicidal (n = 10) | |||
| Age, mean±SD (y) | 36.2±10.1 | 44.2±12.5 | 36.9±13.8 | F(2,44)=1.94, p=0.16 |
| Ethnicity (n) | ||||
| Caucasian | 5 | 7 | 11 | χ2=12.0, p=0.15 |
| Hispanic | 2 | 3 | 13 | |
| Asian | 2 | 0 | 2 | |
| Afro-American | 0 | 0 | 2 | |
| Others | 1 | 0 | 0 | |
| Education level, median± SD | 5.0±1.4 | 4.0±1.6 | 4.0±1.6 | χ2=7.2, p=0.85 |
| Age onset of illness, mean±SD (y) | 17.1±6.4 | 13.6±8.3 | NA | t=−1.0, p=0.32 |
| Length of illness, mean±SD (y) | 22.4±15.7 | 27.2±14.0 | NA | t=2.20, p=0.05 |
| YMRS | 2.7±4.2 | 5.5±8.6 | NA | t=−0.93, p=0.37 |
| HAM-D | 16.3±10.4 | 14.4±9.4 | NA | t=−0.43, p=0.67 |
| Total brain volumes (cm3) | 1143.3±91.0 | 1170.2±69.2 | 1152.4±112.5 | F(2,44)=0.19, p=0.16 |
|
| ||||
| Corpus callosum area (cm2) | ||||
| Anterior genu | 0.99±0.20 | 1.00±0.23 | 1.01±0.19 | F(2,44)=0.07, p=0.93 |
| Middle genu | 0.40±0.08 | 0.40±0.16 | 0.41±0.09 | F(2,44)=0.02, p=0.98 |
| Posterior genu | 0.35±0.09 | 0.37±0.11 | 0.38±0.07 | F(2,44)=0.49, p=0.61 |
| Anterior body | 0.50±0.09 | 0.53±0.08 | 0.55±0.08 | F(2,44)=1.24, p=0.30 |
| Posterior body | 0.44±0.10 | 0.48±0.09 | 0.49±0.07 | F(2,44)=1.26, p=0.29 |
| Isthmus | 0.37±0.09 | 0.38±0.09 | 0.41±0.08 | F(2,44)=1.28, p=0.29 |
| Splenium | 1.46±0.23 | 1.51±0.22 | 1.51±0.26 | F(2,44)=0.14, p=0.87 |
YMRS, Young Mania Rating Scale; HAM-D, Hamilton Rating Scale for Depression
Corpus callosum areas
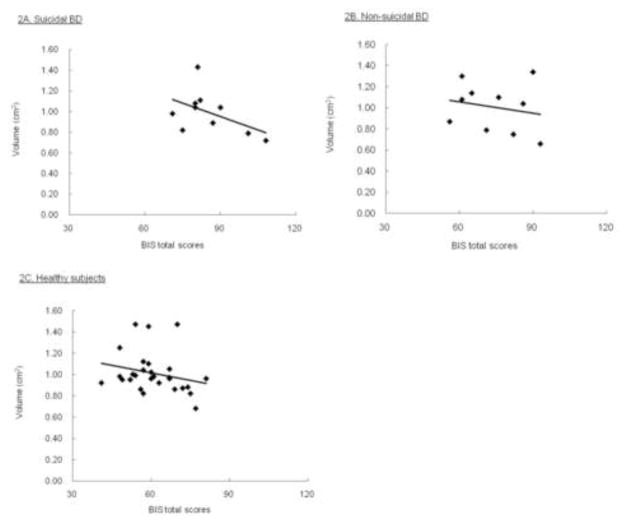
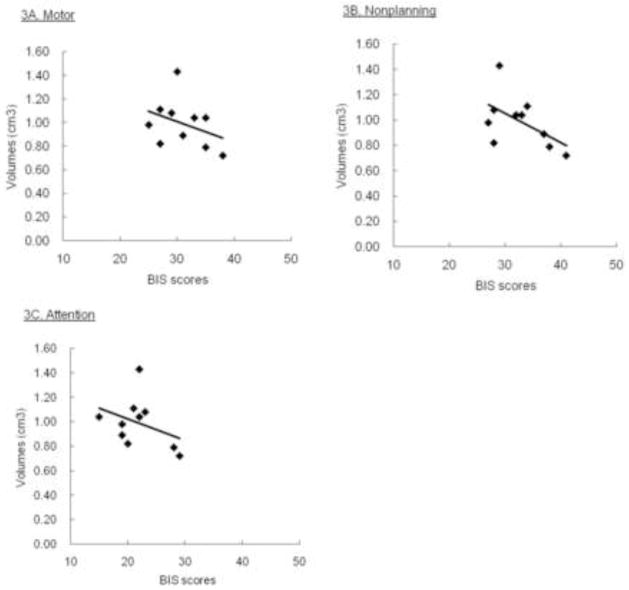
Contrary to our prediction, there were not significant differences in CC regional areas among the suicidal BD, non-suicidal BD and healthy subjects by ANCOVA (Table 1). However, as we predicted, the suicidal BD patients showed a significant inverse partial correlation between the BIS total score and the anterior genu area (r=−0.75, p=0.04) after adjusting for TBV and age. This association was not present for the whole sample (r=−0.18, p=0.24), the non-suicidal BD (r=0.11, p=0.79) or the healthy subjects (r=−0.19, p=0.36) (Fig. 1). Partial correlation analyses focusing on the three BIS subscales revealed that the suicidal BD patients also exhibited a significant and inverse correlation between the anterior genu area and the BIS motor (r=−0.79, p=0.02) and non-planning (r=−0.79, p=0.02) but not the attention score (r=−0.42, p=0.30) (Fig. 2). These correlation were not found in the non-suicidal BD; for the motor (r=−0.25, p=0.56), for the non-planning (r=0.30, p=0.46) or for the attention (r=0.05, p=0.91).
Figure 1.
Scatter plots showing the association between the BIS total scores and anterior genu CC areas.
Figure 2.
Scatter plots showing the association between the BIS subscales and anterior genu CC areas in the suicidal BD patients.
Turning to the clinical variables, the BD patients did not show a significant correlation between the regional CC areas and the four clinical variables: HAM-D, age of illness onset, length of illness or YMRS (Ps>0.05). There also was no significant association between CC areas and history of substance abuse (F1,16=0.30, P=0.59), history of mixed state (F1,16=0.60, P=0.45), medication (F1,16=0.51, P=0.48) or rapid cycling (F1,16=3.33, P=0.09).
DISCUSSION
This study shows the relationships among impulsivity, suicidality and brain structures in BD patients. The finding that suicidal patients with BD were more impulsive than the comparison groups adds to the evidence pointing to an association between suicidal behaviors and impulsivity in BD [33, 34]. The current study also supports our hypothesis that smaller anterior CC area predicts higher impulsivity among suicidal BD patients. These findings suggest that, in the suicidal BD patients, the medial anterior regions of the brain may participate in neural circuits relevant to impulsiveness and suicidal behaviors. To date, there is little evidence regarding CC pathology in patients with mood disorders. A structural study showed that BD patients have smaller total CC, anterior body, posterior body and isthmus areas compared to healthy subjects and that mania (measured by YMRS) is inversely correlated with total, posterior body and isthmus areas [2]. Another structural study showed that there are significant differences in total, genu, posterior body and isthmus areas between BD patients and healthy adults [8]. However, other studies failed to show any difference of CC areas between BD patients and healthy subjects [17, 39]. The current study did not support our hypothesis that suicidal BD patients have smaller anterior CC areas compared to non-suicidal BD and healthy subjects. Studies of MDD subjects also generated inconsistent findings [3, 21]. The reasons why the results of CC structural studies in mood disorders are so variable are unknown, but may be partly related to sample size and differences across studies in subject selection regarding mean age in participation, length of illness, onset age of illness, and imaging methods, e.g. manual and computerized. Despite study variability, a meta-analysis of five case control studies concluded that BD patients show smaller CC areas compared to healthy subjects [1].
On the contrary, signal intensity and diffusion tensor imaging (DTI) studies of CC genu have consistently supported differences between BD and healthy subjects [7, 10]. The ROI and voxel-based DTI studies revealed that BD patients had decreased fractional anisotropies (FA) of the genu, rostral, anterior midbody and middle of CC compared to healthy subjects [37]. Another DTI study demonstrated higher FA of the genu in BD patients compared to healthy subjects [42]. These DTI studies suggest that BD patients have abnormal integrity of CC since DTI can measure cerebral white matter and neural fiber tracts, and a large anisotropy shows organized structures [35, 36]. Future studies combining areas with these microstructure methods would further clarify the association between CC genu, suicidality and impulsivity in BD.
We also showed that the suicidal BD patients with higher impulsivity had smaller anterior genu area but the non-suicidal BD and healthy subjects did not. Further, the associations of the suicidal BD patients were observed for the motor impulsivity. However, the non-suicidal BD patients did not have these findings. Our prior study using a different sample found that smaller rostral ACC volumes of the BD patients related to higher the BIS total and motor impulsivity [24]. The rostral ACC connects with the OFC, amygdala, nucleus accumbens, hypothalamus, anterior insula and hippocampus that play a role of emotional and motivational processing [9]. These connections of the right and left hemispheres go through the genu and anterior CC area. The BIS motor impulsivity is reportedly a risk factor correlated with violence [25]. Together with them, these findings suggest that smaller anterior medial region in BD patients with high impulsivity may lead to impulsive risky behaviors including suicide attempts.
Some methodological limitations should be taken into account. The present study with a small number of participants supports the association of anterior CC regions with abnormal impulsivity and suicidal behaviors of BD. Prior gray matter volume studies suggest that orbitofrontal cortex, anterior cingulate and medial prefrontal cortex play an important role in abnormal emotion processing and impulsivity regulation [5]. It will be necessary to confirm the relationship between anterior CC area, impulsivity and suicidality in BD using gray and white matter volumes of the relevant cortical structures in larger sample size. Another study limitation is that we did not compare the CC areas of patients with and without comorbid cluster B disorders or with and without a family history of risk factors for suicidality, and our sample included only women. Sixty percent of the patients were taking psychiatric medication. Although, to our knowledge, there is no evidence that medications affect CC areas, and there were no significant differences between medicated and unmedicated patients on any CC areas in the current study, the medication may potentially impact the results. Nonetheless, the current study may add to some evidence that the anterior medial regions of the CC are associated with impulsivity and suicidality in BD.
Acknowledgments
This research was partly supported by grants MH 068662, MH 068766, RR 020571, AFSP, NARSAD, UTHSCSA GCRC (M01-RR-01346), the Krus Endowed Chair in Psychiatry (UTHSCSA) and the Veterans Administration (VA Merit Review).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnone D, McIntosh AM, Chandra P, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in bipolar disorder. Acta Psychiatr Scand. 2008;118:357–362. doi: 10.1111/j.1600-0447.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 2.Atmaca M, Ozdemir H, Yildirim H. Corpus callosum areas in first-episode patients with bipolar disorder. Psychol Med. 2007;37:699–704. doi: 10.1017/S0033291706009743. [DOI] [PubMed] [Google Scholar]
- 3.Ballmaier M, Kumar A, Elderkin-Thompson V, Narr KL, Luders E, Thompson PM, Hojatkashani C, Pham D, Heinz A, Toga AW. Mapping callosal morphology in early- and late-onset elderly depression: an index of distinct changes in cortical connectivity. Neuropsychopharmacol. 2008;33:1528–1536. doi: 10.1038/sj.npp.1301538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barratt ES. Impulsiveness and aggression. In: Monahan J, Steadman HJ, editors. Violence and Mental Disorder: Developments in Risk Assessments. University of Chicago Press; Chicago, IL: 1994. pp. 61–79. [Google Scholar]
- 5.Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- 7.Brambilla P, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Keshavan MS, Soares JC. Corpus callosum signal intensity in patients with bipolar and unipolar disorder. J Neurol Neurosurg Psychiatry. 2004;75:221–225. [PMC free article] [PubMed] [Google Scholar]
- 8.Brambilla P, Nicoletti MA, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Magnetic resonance imaging study of corpus callosum abnormalities in patients with bipolar disorder. Biol Psychiatry. 2003;54:1294–1297. doi: 10.1016/s0006-3223(03)00070-2. [DOI] [PubMed] [Google Scholar]
- 9.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 10.Caetano SC, Silveira CM, Kaur S, Nicoletti M, Hatch JP, Brambilla P, Sassi R, Axelson D, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Abnormal corpus callosum myelination in pediatric bipolar patients. J Affect Disord. 2008;108:297–301. doi: 10.1016/j.jad.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YW, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other Axis I disorders. Biol Psychiatry. 1996;39:896–899. doi: 10.1016/0006-3223(95)00295-2. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich S, Breeze JL, Hesdorffer DC, Noam GG, Hong X, Alban RL, Davis SE, Renshaw PF. White matter hyperintensities and their association with suicidality in depressed young adults. J Affect Disord. 2005;86:281–287. doi: 10.1016/j.jad.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 13.First MB, Spitzer RL, Gibbon M, Janet W. Structured Clinical Interview for DSM-IV Axis I Disorders- Non- Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- 15.Guze SB, Robins E. Suicide and primary affective disorders. Br J Psychiatry. 1970;117:437–438. doi: 10.1192/bjp.117.539.437. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser P, Dauphinais ID, Berrettini W, DeLisi LE, Gelernter J, Post RM. Corpus callosum dimensions measured by magnetic resonance imaging in bipolar affective disorder and schizophrenia. Biol Psychiatry. 1989;26:659–668. doi: 10.1016/0006-3223(89)90100-5. [DOI] [PubMed] [Google Scholar]
- 18.Jackson GD, Duncan JS. MRI Anatomy: A New Angle on the Brain. Churchill Livingstone; New York: 1996. [Google Scholar]
- 19.Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, Courtet P, Phillips ML. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry. 2008;165:740–748. doi: 10.1176/appi.ajp.2008.07081239. [DOI] [PubMed] [Google Scholar]
- 20.Keshavan MS, Diwadkar VA, Harenski K, Rosenberg DR, Sweeney JA, Pettegrew JW. Abnormalities of the corpus callosum in first episode, treatment naive schizophrenia. J Neurol Neurosurg Psychiatry. 2002;72:757–760. doi: 10.1136/jnnp.72.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacerda AL, Brambilla P, Sassi RB, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of corpus callosum in unipolar depression. J Psychiatr Res. 2005;39:347–354. doi: 10.1016/j.jpsychires.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Lacerda AL, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry. 2004;55:353–358. doi: 10.1016/j.biopsych.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monahan J, Steadman HJ, Appelbaum PS, Robbins PC, Mulvey EP, Silver E, Roth LH, Grisso T. Developing a clinically useful actuarial tool for assessing violence risk. Br J Psychiatry. 2000;176:312–319. doi: 10.1192/bjp.176.4.312. [DOI] [PubMed] [Google Scholar]
- 26.Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, Sassi RB, Mallinger AG, Keshavan MS, Soares JC. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. 2007;12:360–366. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- 27.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 28.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. JClin Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Pompili M, Ehrlich S, De Pisa E, Mann JJ, Innamorati M, Cittadini A, Montagna B, Iliceto P, Romano A, Amore M, Tatarelli R, Girardi P. White matter hyperintensities and their associations with suicidality in patients with major affective disorders. Eur Arch Psychiatry Clin Neurosci. 2007;257:494–499. doi: 10.1007/s00406-007-0755-x. [DOI] [PubMed] [Google Scholar]
- 30.Pompili M, Innamorati M, Mann JJ, Oquendo MA, Lester D, Del Casale A, Serafini G, Rigucci S, Romano A, Tamburello A, Manfredi G, De Pisa E, Ehrlich S, Giupponi G, Amore M, Tatarelli R, Girardi P. Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1501–1507. doi: 10.1016/j.pnpbp.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 32.Standring S. Gray’s Anatomy The Anatomical Basis of Clinical Practice. Churchill Livingstone; 2005. pp. 411–414. [Google Scholar]
- 33.Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry. 2005;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- 34.Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disord. 2009;11:280–288. doi: 10.1111/j.1399-5618.2009.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taber KH, Pierpaoli C, Rose SE, Rugg-Gunn FJ, Chalk JB, Jones DK, Hurley RA. The future for diffusion tensor imaging in neuropsychiatry. J Neuropsychiatry Clin Neurosci. 2002;14:1–5. doi: 10.1176/jnp.14.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 37.Wang F, Kalmar JH, Edmiston E, Chepenik LG, Bhagwagar Z, Spencer L, Pittman B, Jackowski M, Papademetris X, Constable RT, Blumberg HP. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry. 2008;64:730–733. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 39.Yasar AS, Monkul ES, Sassi RB, Axelson D, Brambilla P, Nicoletti MA, Hatch JP, Keshavan M, Ryan N, Birmaher B, Soares JC. MRI study of corpus callosum in children and adolescents with bipolar disorder. Psychiatr Res. 2006;146:83–85. doi: 10.1016/j.pscychresns.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 41.Yuh WTC, Tali ET, Afini AK, Sahinoglu K, Gai F, Bergman RA. MRI of Head & Neck Anatomy. Churchill Livingstone; New York: 1994. [Google Scholar]
- 42.Yurgelun-Todd DA, Silveri MM, Gruber SA, Rohan ML, Pimentel PJ. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007;9:504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]