Abstract
Approximately one-third of persons infected with the hepatitis C virus (HCV) evidence mild cognitive impairment that is consistent with frontostriatal systems dysfunction, including cognitive dyscontrol, and impacts everyday functioning. The present study examined the effects of HCV on neurocognitive dispersion, or within-person variability in neurocognitive performance across domains, which may be a function of poor sustained cognitive control. High dispersion was also hypothesized to increase risk for unemployment. The study sample included 37 individuals with HCV infection (HCV+) and 45 demographically comparable uninfected comparison participants (HCV−). Dispersion was operationalized as an intraindividual standard deviation (ISD) calculated across the demographically-adjusted T-scores of 13 standard neuropsychological tests. Multiple linear regression and logistic regression approaches were used to evaluate associations between dispersion and HCV serostatus and employment status, respectively. HCV serostatus was significantly associated with higher dispersion, independent of mean level of neurocognitive ability, psychiatric factors, and liver disease severity. Within the HCV+ group, higher dispersion was associated with an increased risk of unemployment among individuals with higher overall mean neurocognitive ability. Increased neurocognitive dispersion among HCV+ individuals may indicate vulnerability to cognitive dyscontrol expressed as poor regulation of performance across tasks. Higher dispersion may manifest as functional difficulties in daily life, particularly among neurocognitively normal HCV-infected persons, which speaks to the potential clinical value of considering intra-individual variability when evaluating risk for everyday function problems in this population.
Keywords: Hepatitis C, neuropsychological assessment, variability, employment, everyday functioning
INTRODUCTION
Infection with the hepatitis C virus (HCV) is a considerable global public health problem, including the United States where the number of adults with chronic infection has reached approximately 1.6 million (Shepard, Finelli, & Alter, 2005). In addition to being a leading cause of advanced liver disease, including cirrhosis and liver cancer (Seeff & Hoofnagle, 2003), HCV is also potentially neurovirulent. Likely entering the central nervous system (CNS) via a “Trojan horse” mechanism by crossing the blood brain barrier (BBB) in infected peripheral circulating monocytes, there is increasing evidence of HCV replication in the cerebrospinal fluid (Laskus et al., 2002) and brain parenchyma (Forton, Karayiannis, Mahmud, Taylor-Robinson, & Thomas, 2004; Letendre et al., 2007; Radkowski et al., 2002). Clinical studies also show alterations in metabolic markers of neuroinflammation (e.g., elevated choline/creatine ratios; Taylor-Robinson, 2001) and neuronal loss (e.g., reduced N-acteyl aspartate; Taylor et al., 2004) in basal ganglia and frontal white matter among HCV-seropositive (HCV+) individuals.
These findings of CNS alterations in HCV infection suggest that the virus itself (either directly or indirectly) plays a role in the etiology of the commonly reported cognitive complaints related to the infection, which would be a separate, albeit parallel in some cases, pathway from cognitive changes resulting from advanced liver disease (e.g., Weissenborn et al., 2001). Indeed, approximately one-third of infected individuals demonstrate neurocognitive impairment on formal neuropsychological testing (see Forton, Taylor-Robinson, & Thomas, 2006, for a review). Interestingly, it appears as though individuals with low cognitive reserve might be particularly susceptible to the neurocognitive effects of the virus (Bieliauskas et al., 2007). Further complicating matters, studies vary in the degree to which the potentially confounding effects of liver disease, fatigue, and psychiatric comorbidity were addressed methodologically and statistically (Perry, Hilsabeck, & Hassanien, 2008). When present, neurocognitive deficits are generally mild and most reliably suggest impairments in domains reliant upon frontostriatal systems, including fine-motor speed (e.g., Cherner et al., 2005; Letendre et al., 2005), learning (e.g., deficient encoding strategies; McAndrews et al., 2005; Posada, et al., 2009), and information processing efficiency (e.g., Hilsabeck et al., 2002; 2003). HCV also affects a range of executive functions, including reasoning, abstraction, and mental flexibility (e.g., Bieliauskas et al., 2006; Cherner et al., 2005; Huckans et al., 2009; Weissenborn et al., 2004), as well as verbal response inhibition (Cordoba et al., 2003; Martin et al., 2004). Furthermore, there is evidence that HCV infection impacts decision-making as measured by delay discounting, or consistent selection of smaller immediate rewards rather than larger delayed awards, which is suggestive of increased impulsivity (Huckans et al., 2011). It is not clear whether the neurocognitive effects of HCV are reversible following treatment and viral clearance (e.g., Kraus et al., 2005), or whether there are lasting effects (e.g., Patullo, McAndrews, Damyanovich, & Heathcote, 2011).
These early data are compelling, but identification of a sensitive clinical measure of early cognitive change may be useful for understanding the neurocognitive effects of HCV infection. One potentially interesting possibility is that the expression of HCV-associated neurocognitive impairment may be somewhat variable across and within individuals, akin to what has been observed for many years in persons living with HIV infection (e.g., Butters et al., 1990; Morgan et al., 2011). Therefore, a novel approach to summarizing neurocognition whereby variability in performance is directly measured might detect an important neurocognitive signal in HCV infection. Specifically, intraindividual variability (IIV) is an index of within-person variation in cognitive performance that can be directly measured in a single testing session across trials of an individual test or across test scores that represent different domains. In contrast to focusing on average level of performance, IIV paradigms that explore variability in performance can be directly measured for each individual across a battery of tests (i.e., dispersion). IIV is believed to be a behavioral marker of reduced neural integrity (see MacDonald, Li, & Backman for a review) because it has been demonstrated across several populations (e.g., aging, dementia HIV infection) in which it has been linked to poor cognitive and daily functioning outcomes.
Elevated IIV has typically been observed in populations with frontal systems dysregulation and poor cognitive control of performance (Bellgrove et al., 2004; West et al., 2002). In support of the role of cognitive dyscontrol, robust evidence has strongly linked IIV with frontal grey and white matter lesions (e.g., Stuss et al., 2003), executively-demanding task conditions (Bellgrove et al., 2004; West et al., 2002), and elevated patterns of blood-oxygen level-dependent (BOLD) activation in frontal regions (e.g., Bellgrove et al., 2004). Given that the neurocognitive profile of HCV infection primarily involves executive dysfunction and attentional deficits (particularly the executive aspects of attention), it is reasonable to hypothesize that HCV seropositive individuals might evidence increased levels of cognitive variability that may be clinically important. For example, increased IIV has been demonstrated among HIV-seropositive individuals, in whom it is also associated with medication nonadherence (Ettenhofer et al., 2010; Levine et al., 2008).
A particular subtype of IIV called dispersion reflects the performance of a single person across multiple tasks on a single occasion (Hultsch, MacDonald, & Dixon, 2002; Hilborn, Strauss, Hultsch, & Hunter, 2009). The nomenclature of IIV varies considerably across studies (e.g., MacDonald et al., 2009), but the for the purposes of the current study this form of IIV has been labeled dispersion. Dispersion has been shown to increase with advanced age (Christensen et al., 1999; Hultsch et al., 2002; Hilborn et al., 2009), and higher levels of dispersion were harbingers of cognitive decline in longitudinal studies of aging (e.g., Christensen et al., 1999; Rapp, Schnaider-Beeri, Sano, Silverman, & Haroutunian, 2005). Although even healthy individuals evidence dispersion as evidenced by relative strengths and weaknesses in their neurocognitive profile (Schretlen, Munro, Anthony, & Pearlson, 2003), such neurocognitive variability is exacerbated in clinical populations (e.g., advanced age, neuromedical illness, or dementia) in which it is suggestive of altered cognitive integrity secondary to underlying neuropathology (e.g., MacDonald, Li, & Backman, 2009; Wetter et al., 2006). Notably, dispersion has been significantly correlated with other forms of IIV (Hultsch et al., 2002), supporting the notion that a similar mechanism of cognitive dyscontrol may underlie the emergence of dispersion. Emerging evidence suggests that dispersion may represent an important neurocognitive signal in neurotropic infectious disease populations. For example, a recent study from our group observed significantly greater neurocognitive dispersion in older HIV-infected adults (Morgan et al., 2011). Importantly, increased within-session IIV has been associated with poorer medication adherence in HIV (Ettenhofer et al., 2010; Levine et al., 2008). These findings could suggest that deficient cognitive control via frontal systems dysregulation in HCV infection may be expressed as increased dispersion.
It may also be useful to examine the potential association between HCV-related dispersion and everyday functioning outcomes. Evidence indicates that HCV infection negatively impacts employment, as evidenced by higher rates of unemployment (e.g., Jacobs et al., 2003), increased absenteeism (e.g., Su et al., 2010), and lower work productivity (DiBonaventura et al., 2011). Yet little is known about neurocognitive predictors of unemployment among HCV+ individuals. Several studies have reported alterations across a range of everyday behavior and functioning activities in HCV infection, including a nearly three-fold increase in clinically elevated neurobehavioral symptoms in daily life (per self-report) relative to seronegative individuals (Posada et al., 2010) and greater risk for problematic changes in functional status HCV-related neurocognitive impairment, including dependence in performing activities of daily living (e.g., Vigil et al., 2008). Relatedly, among individuals infected with HIV, poorer neurocognitive performance is a predictor of difficulties with employment (e.g., Heaton, Marcotte et al., 2004). Taken together, this evidence indicates that neuropsychiatric factors, including aspects of neurocognitive performance (such as dispersion), may be important predictors of employment difficulties in HCV infection.
Accordingly, the present study aimed to examine dispersion as a function of HCV serostatus, with the hypothesis that HCV+ individuals would demonstrate higher levels of dispersion relative to HCV-seronegative (HCV−) counterparts. Relatedly, it was expected that the HCV effect on dispersion would not be dependent on factors such as advanced liver disease, depression, or fatigue. Furthermore, in the HCV+ group it was hypothesized that greater dispersion would be associated with poorer daily functioning, as evidenced by unemployment.
METHOD
Participants
The current sample of 82 participants was drawn from projects funded by the National Institute on Drug Abuse to study the effects of methamphetamine, HIV, and HCV on the central nervous system. Participants in the parent study were recruited from the San Diego community and local clinics (e.g., flyers). HCV serostatus was determined by enzyme-linked immunosorbent assay, results of which were used to define the HCV− and HCV+ groups. In order to facilitate investigation of the effect of HCV on cognition, individuals with prior or current treatment for HCV were excluded from the parent study at baseline. Additionally, participants who reported having a history of neurological disorders (e.g., seizure disorders, closed head injuries with loss of consciousness greater than 15 minutes, stroke), severe psychiatric illnesses (e.g., psychotic disorders), or medical comorbidities known to affect cognitive functioning (e.g., HIV co-infection) were also excluded. Notably, the parent study focused on methamphetamine dependence, and to eliminate that potential confounding factor (as well as that posed by any current substance dependence) only individuals with no current substance dependence diagnosis were identified for the present study. However, given the high comorbidity of substance dependence and HCV infection, individuals with lifetime histories of substance dependence were included to achieve a representative sample, and substances for which the rates of lifetime prevalence differed between the groups were included in the analyses. Additional exclusion criteria applied for the present study only include having met criteria for current mood disorders, current or lifetime Attention-Deficit/Hyperactivity Disorder (ADHD), or Antisocial Personality Disorder (ASPD). Once these criteria specific to the present study were applied, the resulting sample sizes were n = 45 for the HCV− group and n = 37 for the HCV+ group.
As shown in Table 1, the groups of HCV− and HCV+ individuals were generally comparable with regard to demographic characteristics. As expected, the groups differed in the rates of lifetime substance dependence diagnoses for alcohol and several other substances (e.g., cocaine). The HCV+ group also endorsed significantly higher levels of current affective distress (p = .04; likely driven by significantly higher scores on the Fatigue/Inertia subscale among the HCV+ group, p = .008), but there were no group differences in the rates of lifetime Major Depressive disorder or Bipolar disorder. The groups did not significantly differ with regard to the rate of self-reported learning disability (HCV− = 22.2% vs. HCV+ = 35.1%, p = .20). Table 1 also displays standard laboratory markers of liver function, which demonstrate that none of the HCV+ individuals evidenced advanced liver disease. Specifically, the aminotransferase (AST)-to-platelet ratio index (APRI) is an indirect measure of liver fibrosis based on a non-invasive procedure derived from serum laboratory values. No participants in the HCV+ group evidenced APRI values suggestive of severe liver fibrosis (i.e., Stage F3 or greater; Loaexa-del-Castillo et al., 2008). Table 1 also displays HCV RNA levels, which provide a measure of HCV replication.
Table 1.
Demographic, Psychiatric, and Medical Characteristics of the Study Sample
| Variable | HCV− (n = 45) | HCV+ (n = 37) | p |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 42.2 (9.2) | 45.3 (6.2) | .09 |
| Education (years) | 12.9 (2.2) | 12.4 (2.3) | .34 |
| Sex (% male) | 62.2 | 51.4 | .32 |
| Ethnicity | |||
| Caucasian (%) | 66.7 | 70.3 | .73 |
| African American (%) | 17.8 | 13.5 | -- |
| Hispanic (%) | 13.3 | 8.1 | -- |
| Other (%) | 2.2 | 8.1 | -- |
| Mean NP Performance T-score | 50.7 | 49.0 | .21 |
| Global Deficit Score (GDS)a | 0.18 [0.05, 0.27] | 0.22 [0.11, 0.52] | .10 |
| GDS Impaired (%) | 15.6 | 24.3 | .32 |
| Psychiatric Characteristics b | |||
| Total Mood Disturbance (POMS) | 39.3 (28.1) | 54.6 (38.0) | .04 |
| Tension/Anxiety | 6.6 (5.8) | 8.9 (6.5) | .09 |
| Depression/Dejection | 5.8 (8.7) | 8.6 (1.6) | .19 |
| Anger/Hostility | 5.6 (8.3) | 7.2 (7.9) | .38 |
| Vigor/Activation | 19.8 (6.6) | 16.7 (7.5) | .05 |
| Fatigue/Inertia | 4.8 (4.4) | 8.5 (7.7) | .008 |
| Confusion/Bewilderment | 4.3 (3.6) | 6.2 (5.3) | .06 |
| Lifetime Major Depressive Disorder (%) | 26.7 | 35.1 | .41 |
| Lifetime Bipolar Disorder (%) | 0.0 | 5.4 | .11 |
| Lifetime Substance Use Disorders (%) | |||
| Alcohol dependence | 6.7 | 35.1 | .001 |
| Stimulant dependence | 0.0 | 37.8 | <.0001 |
| Cannabis dependence | 6.7 | 24.3 | .02 |
| Opioid dependence | 0.0 | 32.4 | <.0001 |
| Cocaine dependence | 2.2 | 43.2 | <.0001 |
| Other substance dependence | 0.0 | 5.4 | .13 |
| Employment Characteristics | |||
| Unemployed (%) | 40.9 | 50.0 | .42 |
| Employed (%) | 59.1 | 50.0 | -- |
| Full-time (%) | 73.1 | 72.2 | .95 |
| Part-time (%) | 26.9 | 27.8 | -- |
| Highest position achieved (HH z-score) | 0.20 (0.22) | −0.12 (0.17) | .26 |
| HCV Disease Characteristics a | |||
| HCV RNA serum (log copies/mL) | -- | 5.8 [3.0, 6.1] | -- |
| Total Bilirubin (mg/dL) | -- | 0.7 [0.5, 0.9] | -- |
| Albumin (mg/dL) | -- | 4 [3.8, 4.2] | -- |
| Platelets (×103 cells/mm3) | -- | 224 [187, 284] | -- |
| AST Level (mg/dL) | -- | 49 [29.5, 90.5] | -- |
| ALT Level (mg/dL) | -- | 63 [45.5, 91] | -- |
| APRI | -- | 0.4 [0.3, 0.9] | -- |
Note. NP = Neuropsychological; HH = Hollingshead;
data presented are medians [interquartile ranges];
No current psychiatric diagnoses, no lifetime ADHD in either group;
POMS = Profile of Mood States; AST = aspartate aminotransferase; ALT = alanine aminotransferase; APRI = AST-to-platelet ratio index
Materials and Procedure
After providing written informed consent, participants completed standardized, comprehensive neuropsychological, psychiatric, and medical research evaluations.
Psychiatric Diagnoses and Affective distress
The Composite International Diagnostic Interview 2.1 (CIDI 2.1; World Health Organization, 1998) was administered for determination of relevant psychiatric diagnoses. This computer-assisted assessment tool is a structured interview administered by trained evaluators. The CIDI yields psychiatric diagnoses that are consistent with Diagnostic and Statistical Manual – Fourth Edition (DSM-IV; American Psychiatric Association, 1994) criteria, which were used for determining eligibility for the study (i.e., exclusion criteria) and for characterization of the resulting sample (e.g., lifetime substance dependence diagnoses, MDD). To measure current affective distress, participants also completed the Profile of Mood States (POMS) questionnaire (McNair, Lorr, & Droppleman, 1981), which is a self-report questionnaire in which participants rate the recent (i.e., past two weeks) frequency of several types of psychiatric symptoms, including depression, anxiety, confusion, and fatigue. The raw Total Mood Disturbance score is a summary score that is collapsed across the various symptoms to yield a measure of current affective distress (range = 0 to 200). Raw scores from the subscales of the POMS (e.g., Fatigue/Inertia; range = 0 to 28) are also presented in Table 1.
Neurocognitive Evaluation and Measurement of Dispersion
All participants performed above the cutoff for suboptimal effort (i.e., > 90% accuracy) on the Hiscock Digit Memory Test (Hiscock & Hiscock, 1989). Study participants were administered an approximately 3-hour neuropsychological battery, which comprised standardized clinical tests across several cognitive domains. An index of dispersion, which was the specific subtype of IIV investigated in the present study, was derived for the primary criterion by selecting standard summary measures from tests within each cognitive domain sampled. Following a procedure previously used to operationalize dispersion (e.g., Christensen et al., 1999), the standard deviation of the T-scores from all selected summary scores was calculated for each participant, yielding an intraindividual standard deviation (ISD) across those T-scores. Higher scores reflected a greater degree of within-person variability across the battery.
In order to minimize the effect of demographic characteristics, such as age, education, sex, and ethnicity, on neuropsychological test performance, raw scores from the measures listed above were converted to demographically-corrected T-scores using the best available normative data (Heaton, Miller et al., 2004; Norman et al., 2011). The selected T-scores included the following, grouped by domain to demonstrate that tasks were selected to represent multiple cognitive domains (but domain groupings were not used in the calculation of the dispersion score); Speed of Information Processing: Wechsler Adult Intelligence Scale – Third Edition Symbol Search and Digit Symbol subtests (WAIS-III; Psychological Corporation, 1997), Trail Making Test Part A (TMT-A; Reitan & Wolfson, 1993); Verbal Fluency: Controlled Oral Word Association Test Total Correct (COWAT-FAS; Benton, Hamsher, & Sivan, 1994), Animal Fluency Total Correct (Benton, Hamsher, & Sivan 1994); Learning: Hopkins Verbal Learning Test-Revised Trials 1–3 Total (HVLT-R; Benedict, Schretlen, Groninger, & Brandt, 1998), Brief Visuospatial Learning Test Trials 1–3 Total (BVMT-R; Benedict, 1997); Executive Functions: Wisconsin Card Sorting Test-64 Card Version Perseverative Responses (WCST-64; Kongs, Thompson, Iverson, & Heaton, 2000), Trail Making Test Part B (TMT-B; Reitan & Wolfson, 1993), Halstead Category Test Total Errors (Reitan & Wolfson, 1993); Working Memory: Paced Auditory Serial Addition Test Total Correct (PASAT, Gronwall, 1977), Letter-Number Sequencing subtest from the Wechsler Memory Scales – Third Edition (WMS-III; Psychological Corporation, 1997); Motor Skills: Grooved Pegboard Dominant and Nondominant Total Time (GP: Kløve, 1963). A higher ISD, or dispersion score, reflected greater variability in performance across the battery (range = 6.3 to 14.8). A mean neuropsychological performance T-score variable was also calculated from the measures that comprise the dispersion variable. In addition, a Global Deficit Score (GDS) was calculated for all participants, which is an empirically-supported measure that summarizes neurocognitive performance across a comprehensive battery by weighting impaired performance to enhance sensitivity (see Carey et al., 2004 for more details). Individual T-scores are converted to deficit scores that range from 0 (T > 40) to 5 (T < 20), which are then averaged to generate the GDS. As shown in Table 1, this traditional measure of neurocognitive performance did not differ between the groups (p = .10), nor did the rate of global cognitive impairment (p = .32), which was based on a validated clinical cutpoint (i.e., scores ≥ 0.05 indicate impairment; Carey et al., 2004).
Evaluation of Employment
Regarding employment, several types of information were collected. Relevant items from the Patient Assessment of Own Functioning (PAOFI; Chelune et al., 1986), a self-report measure in which an individual rates daily functioning difficulties, were used to classify employment status (i.e., employed, unemployed). The PAOFI was also used to classify employed individuals as employed on a part-time or full-time basis in order to characterize the sample, but these groups were collapsed into an “employed” group due to the limited sample size. One HCV+ participant and three HCV− comparison participants who were rated as “unemployed” by this measure were disabled, per chart review (these cases were included in the unemployed group). Using the dichotomous employment status variable, 26 HCV− participants were employed (59%) and 18 were unemployed (41%), and 18 HCV+ participants were employed (50%) whereas 18 were unemployed (50%). Information regarding the highest level of employment ever achieved by the participants is reflected in the Hollingshead (1975) system, which assigns scores to self-reported occupations to rank those positions in terms of sophistication and skill. Scores were assigned by a trained research assistant for all cases in which information on highest position held was available (group sizes appear below). Higher scores reflect lower level positions, and the scores range from 1 (highest) to 7 (lowest). Z-scores have been provided in Table 1 for ease of interpretation (scores reversed so that lower z-scores indicate lower employment level). Within the HCV− group, the employed individuals (n = 10, M = 0.33, SD = 0.79) did not differ from the unemployed individuals (n = 10, M = −0.15, SD = 1.0) on Hollingshead scores (p = 0.24). Similarly, the HCV+ employed individuals (n = 16, M = 0.12, SD = 0.97) did not differ from the HCV+ unemployed (n = 18, M = −0.34, SD = 0.91) on Hollingshead scores (p = .17).
Statistical Analyses
To evaluate the effect of HCV serostatus on dispersion, a simultaneous multiple linear regression approach was used, with the continuous dispersion variable as the criterion. The variables on which the HCV+ and HCV− groups differed (shown in Table 1) were included in the model as a priori covariates to account for their influence, including prevalence of lifetime substance dependence for several substances, including alcohol, cannabis, and cocaine, as well as self-reported current affective distress. Given that differences between the HCV serostatus groups on POMS Total Mood Disturbance appear to be driven by the Fatigue/Inertia subscale, it should be noted that the pattern of results did not differ when the subscale score was substituted for the summary score.
To evaluate the effect of dispersion on employment status within the HCV+ group, a logistic regression was conducted with employment status (dichotomous: employed versus unemployed) as the criterion. Notably, there were no significant demographic differences between the employed and unemployed individuals (ps > .05 for age, education, sex, ethnicity). The logistic regression analysis therefore included dispersion, mean neuropsychological performance T-score, and their interaction, which were regressed on a dichotomous employment status variable reflecting current employment (full-time or part-time) versus unemployment. The association between dispersion and the mean neuropsychological performance T-score was weak (r = 0.15, p = .36), supporting inclusion of both variables in the model to account for the influence of mean level of performance on dispersion.
RESULTS
Effect of HCV Serostatus on Dispersion
For descriptive purposes, Table 2 displays average levels of performance on the selected neuropsychological measures by HCV serostatus. As shown in Table 3, the primary analysis evaluating the effect of HCV serostatus on dispersion revealed that the overall model was significant (p = .03), and the only significant predictor in the model was HCV serostatus (p = .003). All other predictors were not significantly associated with dispersion (ps > .05) and inclusion of age in the model did not change the pattern of results. Given our small sample size and the lack of a significant association between dispersion and the other predictors, we also evaluated a reduced model with only HCV serostatus and mean neuropsychological T-score in the model, and the pattern of results was unchanged (i.e., HCV serostatus was significantly associated with dispersion despite controlling for mean level of performance). Planned comparisons showed that the HCV+ group demonstrated higher dispersion scores (M = 9.8, SD = 2.0) relative to the HCV− group (M = 8.5, SD = 2.0; p = .003; Cohen's d = 0.65), but there was no significant between-group difference in the mean level of performance (HCV−: M = 50.7, SD = 5.6; HCV+: M = 50.0, SD = 6.9; p = .21; Cohen's d = 0.11). Given that no participants in the HCV− group met criteria for lifetime substance dependence for methamphetamine or opioids, these variables could not be included in the regression; as such, planned post hoc group comparisons were conducted within the HCV+ group to determine whether dispersion differed as a function of these diagnoses. Results showed no significant relationships between these variables and dispersion (ps > .10), suggesting that these factors were not driving the observed HCV effect. Dispersion was not correlated with standard laboratory measures of liver function or with self-reported fatigue (as measured by the Fatigue/Inertia subscale of the POMS) within the HCV+ group, all ps > .10.
Table 2.
Mean Level of Performance on Neuropsychological Summary Scores Comprising Dispersion Index by HCV Serostatus
| Variable | HCV− (n = 45) | HCV+ (n = 37) | p |
|---|---|---|---|
| Speed of Information Processing | |||
| WAIS-III Symbol Search subtest | 52.2 (10.2) | 49.7 (11.2) | .29 |
| WAIS-III Digit Symbol subtest | 51.2 (9.5) | 50.5 (12.6) | .78 |
| Trail Making Test Part A | 52.3 (8.2) | 51.3 (14.1) | .69 |
| Verbal Fluency | |||
| COWAT-FAS Total Correct | 50.4 (9.9) | 51.2 (10.6) | .72 |
| Animal Fluency Total Correct | 51.9 (8.1) | 50.2 (10.5) | .44 |
| Learning | |||
| HVLT-R Trials 1–3 Total* | 48.2 (9.2) | 44.0 (10.9) | .07 |
| BVMT-R Trials 1–3 Total* | 50.4 (12.0) | 45.7 (11.6) | .08 |
| Executive Functions | |||
| WCST-64 Perseverative Responses | 48.5 (11.4) | 51.3 (13.1) | .32 |
| Trail Making Test Part B | 53.8 (10.7) | 56.0 (11.0) | .37 |
| Halstead Category Test Total | 48.1 (9.8) | 45.3 (9.4) | .19 |
| Working Memory | |||
| PASAT 200 Total Correct | 49.5 (10.4) | 45.3 (9.6) | .06 |
| WMS-III Letter-Number Sequencing subtest | 53.2 (9.2) | 50.3 (8.8) | .16 |
| Motor Functions | |||
| Grooved Pegboard Dominant Total Time | 51.6 (10.1) | 47.5 (13.0) | .11 |
| Grooved Pegboard Nondominant Total Time | 50.2 (10.2) | 47.7 (13.9) | .35 |
Note. WAIS-III = Wechsler Adult Intelligence Test – Third Edition; COWAT = Controlled Oral Word Association Test; HVLT-R = Hopkins Verbal Learning Test-Revised; BVMT-R = Brief Visuospatial Memory Test-Revised; WCST = Wisconsin Card Sorting Test; PASAT = Paced Auditory Serial Addition Test; WMS-III = Wechsler Memory Scales – Third Edition;
indicates that significant differences were observed using a 1-tailed significance test (HCV+ < HCV−), ps < .05
Table 3.
Multiple regression showing a significant association between HCV serostatus and dispersion (N=82)
| Variable | Model | β | p-value |
|---|---|---|---|
| Adjusted R2 | 0.1 | ||
| F (6, 75) | 2.5 | .03 | |
| HCV serostatus [negative] | −0.38 | .003 | |
| Mean NP Performance T-score | 0.20 | .08 | |
| Current Affective Distress | −0.05 | .70 | |
| Alcohol Dependencea | 0.14 | .26 | |
| Cannabis Dependencea | −0.07 | .54 | |
| Cocaine Dependencea | −0.02 | .91 |
Note: NP = neuropsychological; Mean NP Performance T-score = average of T-scores included in dispersion variable; Current Affective Distress = Profile of Mood States Total Mood Disturbance;
lifetime diagnosis of substance dependence.
Effect of Dispersion on Employment Status in HCV+ Individuals
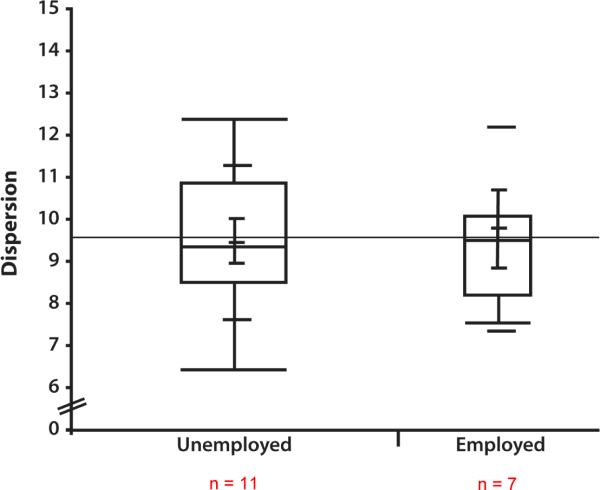
Next, a logistic regression was conducted within the HCV+ group to evaluate whether dispersion was associated with employment status. Notably, there were no significant demographic differences between the employed and unemployed individuals (i.e., age, education, sex, ethnicity). Furthermore, the groups were compared on a vascular risk variable that indicated presence of physical health conditions including diabetes mellitus, hypertension, and coronary artery disease; the rate of vascular risk was very low in both the employed (15.4%) and unemployed (11.8%) groups, which did not differ significantly. The logistic regression analysis therefore included dispersion, mean T-score, and their interaction, which were regressed on a dichotomous employment status variable reflecting current employment (full-time or part-time) versus unemployment. The overall model was significant, X2 (df=3) = 17.2, p = .0007, and all predictors were significant (ps < .05), including the interaction term (p = .0016). This pattern of results was not different when the psychiatric variables on which the employment status groups differed were included in the model, including demographically-corrected POMS Total z-score and lifetime cannabis dependence, although these were also significant predictors in their respective models (ps < .05). Inclusion of APRI as an index of liver disease severity in the model did not change the pattern of results and it was not a significant predictor of employment status. To probe the interaction, the HCV+ group was stratified on mean level of performance by creating a dichotomous variable defined by a median cutpoint. Individuals whose mean T-scores were above the median (> 50.6) fell in the “high mean performance” group, and those with mean T-scores below the median were in the “low mean performance” group. Group comparisons on dispersion between the employed and unemployed individuals in the stratified HCV+ sample were examined, which revealed a significant difference in dispersion as a function of employment status in the high mean performance group: That is, unemployed individuals evidenced significantly higher mean dispersion scores (n = 7, M = 11.4, SD = 0.87) relative to those who were employed (n = 11, M = 9.2, SD = 2.2; p = .03, Cohen's d = 1.4), as displayed in Figure 1a. A simple logistic regression analysis conducted in the high mean performance group revealed that for each unit increase in dispersion the likelihood of being unemployed increased by 2.4 [X2 (df=1) = 6.3, p = .01, 95% CI = 1.2, 8.3]. In contrast, there was no difference in level of dispersion between the unemployed (n = 11, M = 9.4, SD = 0.6) and employed (n = 7, M = 9.7, SD = 0.8, p = .77, Cohen's d = 0.4) groups in the low mean performance group, shown in Figure 1b, and each unit increase in dispersion was associated with a 0.9 increased likelihood of unemployment [X2 (df=1) = 0.1, p = .75, 95% CI = 0.5, 1.5].
Figure 1a.
The box and whisker plots display Dispersion values for the unemployed versus employed groups within the HCV+ sub-sample with higher mean neuropsychological performance, revealing that all unemployed individuals evidenced higher levels of dispersion relative to a range of dispersion values in the employed group. (Note. The employment status for 1 participant was not available, and therefore that individual was excluded for this analysis.)
Figure 1b.
Dispersion values for the unemployed versus employed groups within the HCV+ sub-sample with lower mean neuropsychological performance were not significantly different, as shown in the box and whisker plots.
An additional post hoc analysis was conducted in the entire sample to investigate whether the interactive effect of dispersion and mean level of performance was stronger in the HCV+ group relative to the HCV− group. A logistic regression was run with the employment status variable as the criterion and HCV serostatus, dispersion, mean neuropsychological performance T-score, and all interactions, including a 3-way interaction. The full model was significant [X2 (df=7) = 19.8, p = .006) and the 3-way interaction was significant [X2 (df=1), = 10.3, p = .001). To probe this interaction, we stratified the groups by HCV serostatus, and then investigated potential group differences on dispersion as a function of employment status. The results for the HCV+ group are presented in the post hoc findings above, and no differences were observed between employed and unemployed HCV− individuals at high or low levels of mean neuropsychological performance (ps > .10).
DISCUSSION
Accumulating biomarker, neuroimaging, and neuropathological evidence suggests that chronic HCV infection negatively impacts the CNS independent of advanced liver disease (e.g., Forton et al., 2006), and up to one-third of infected individuals demonstrate mild neurocognitive impairment (see Forton et al., 2006 for a review) that may lead to difficulties with everyday functioning (e.g., Vigil et al., 2008) and behavioral dysregulation, (Huckans et al., 2011; Posada et al., 2010). These prior studies have used traditional neuropsychological summary scores based on mean level of performance, which have consistently revealed impairments in domains such as executive functions (e.g., Bieliauskas et al., 2006; Huckans et al., 2009; Martin et al., 2004). Our study extended that literature by showing that HCV serostatus is a risk factor for within-person fluctuations in neurocognitive performance across a battery of tests (i.e., a specific type of IIV labeled dispersion). Furthermore, our findings suggest that elevated dispersion appears to impact vocational functioning. Although employment status has not previously been investigated in relation to HCV infection or dispersion, this finding contributes to the understanding of the daily functioning impact of HCV (Posada et al., 2010), and provides preliminary evidence regarding the clinical utility of dispersion.
Consistent with our a priori hypothesis, the present study demonstrated an HCV effect on IIV, which was marked by significantly higher levels of dispersion among HCV+ individuals as compared to their demographically-comparable seronegative counterparts. That is, across a battery of neuropsychological tests, individuals with HCV were more variable in their cognitive performance across measures that assess different domains. The HCV+ and HCV− groups were demographically-comparable, and demographically-adjusted normative T-scores were used in the calculation of the dispersion score. As shown in Table 2, the HCV+ group did not differ from the HCV− comparison group at the mean level of performance for any of the administered measures (with the exception of one-tailed significant findings indicating poorer performance by the HCV+ group relative to the HCV− comparison group on learning measures, which is consistent with prior literature; e.g., McAndrews et al., 2005; Posada, et al., 2009). The demonstration of a significant HCV effect on dispersion is therefore consistent with the hypothesis that measures of IIV, such as dispersion, may be early behavioral markers of poor neural integrity (e.g., MacDonald et al., 2009). Furthermore, the observed differences in dispersion as a function of HCV serostatus could not be better explained by liver disease severity (as indexed by APRI), fatigue, or psychiatric factors, such as lifetime histories of MDD diagnosis or substance dependence. Although not directly tested in the current study, evidence from prior research suggests that increased dispersion in HCV+ individuals may reflect deficient control in allocating cognitive resources as required for the task at hand (e.g., Bellgrove et al., 2004; West et al., 2002). As a unique and separable component of executive functions, cognitive dyscontrol within HCV infection would be consistent with prior evidence of frontal systems dysregulation and executive dysfunction demonstrated in this population (Forton et al., 2006). The link between the preferential impact of HCV on frontal cortex and white matter (e.g., Letendre et al., 2007; Taylor et al., 2004) and the evidence indicating that IIV, including dispersion, is related to frontal systems dysfunction (e.g., MacDonald et al., 2009), suggests that cognitive dyscontrol may be a primary mechanism underlying the observed dispersion findings. That is, poor adjustment and allocation of cognitive resources as needed across the tasks in the battery could result in higher dispersion scores among HCV+ individuals. This interpretation is necessarily tempered by the fact that all of our available executive function tasks were intentionally incorporated into our dispersion variable, which left us without an external criterion of executive functions to evaluate convergent validity free from criterion contamination. As such, we acknowledge the possibility that the increased dispersion observed among HCV+ individuals may have been related to impairment in basic attention or speed of information processing, both of which have been reported in the context of HCV infection (e.g., Hilsabeck et al., 2002; 2003).
Within the HCV-seropositive group, significantly higher levels of this type of IIV were observed among a group of unemployed individuals relative to those who were employed in a group whose mean level of neuropsychological performance was within normal limits. These data suggest that dispersion may have impact on real-world functioning even among individuals whose performance on traditional neurosychological measures has not declined. Notably, to our knowledge the present study is the first to examine neurocognitive predictors of unemployment among HCV+ individuals. Prior studies have reported evidence of dependence in instrumental activities of daily living in HCV infection related to neurocognitive impairment (i.e., speed of information processing and motor domains; Vigil et al., 2008) or clinically-elevated behavioral symptoms of inhibition (Posada et al., 2010). In extension of those findings, our results revealed that high levels of dispersion was demonstrated among the unemployed HCV+ individuals, and this novel finding regarding an important functional outcome in HCV infection may have clinical utility in terms of identifying individuals at risk for problems with daily functioning. Detection of this effect within a small sample suggests that it may represent an important signal in HCV, but this finding should be replicated with a larger group of HCV+ individuals. This finding also extends prior studies showing that higher levels of IIV are associated with poorer medication adherence in HIV infection (Ettenhofer et al., 2010; Levine et al., 2008). As such, there is gathering evidence regarding the functional impact of elevated IIV, which could be further substantiated by exploring the relationship of IIV to other instrumental functional outcomes in HCV infection, including medication adherence and automobile driving.
Although our study was cross-sectional in nature, thereby rendering inferences regarding causality speculative, our findings could nevertheless suggest that high levels of neurocognitive dispersion may have interfered with HCV-infected individuals' capacity to maintain (or obtain) gainful employment. Even more striking was the fact that this relationship was observed only among individuals with higher average neuropsychological performance (i.e., mean T-scores above the median). In contrast, among those with lower mean neuropsychological summary scores there was no difference between the employed and unemployed groups on dispersion. Although unexpected, this pattern of findings could provide additional evidence that dispersion has incremental validity in relation to average neuropsychological performance. While impaired global neuropsychological performance (i.e., based on average T-scores across a battery or a global deficit score) has already been demonstrated to be a risk factor for functional impairment (e.g., Heaton, Marcotte et al., 2004), dispersion may represent a risk factor for difficulty in daily functioning among individuals who are not impaired based on this traditional measure. IIV has been conceptualized as a potentially early marker of loss of neural integrity (MacDonald, et al., 2009), and therefore it may interfere with daily functioning before declines in average level in performance (i.e., mean) are detected. As such, this finding could also have potential clinical utility because consideration of dispersion might help identify individuals who would otherwise be overlooked as at risk for problems with everyday functioning because their overall neuropsychological performance falls within normal limits. Notably, our post hoc analysis conducted within the entire sample suggested that the relationship between high levels of dispersion and unemployment among individuals with higher levels of mean neuropsychological performance was unique to the HCV+ group. No differences were observed in dispersion as a function of employment status at either high or low levels of mean neuropsychological performance in the HCV− group. Although this does lend inferential support to the notion that dispersion may be an early signal of poor neuronal and cognitive integrity that is possibly related to presence of HCV infection, we would expect that high levels of dispersion could potentially interfere with successful performance even among HCV− individuals.
The current study has limitations worth noting. The small sample sizes were not ideal, but despite the resulting type II error risk, the effect sizes were substantial (as evidenced by Cohen's d and odds ratios) and therefore the effects were readily detected even in our small sample. As such, this is an intriguing finding and it is suggested that these findings be replicated with a larger sample that could address some of the additional limitations listed below. It is unlikely that suboptimal effort accounted for our findings because all participants performed with better than 90% accuracy on the HDMT, which is suggestive of adequate effort (Woods et al., 2003). The cross-sectional nature of the study is another limitation, and future studies should examine whether dispersion predicts cognitive decline in HCV infection. Such an investigation could be particularly beneficial in terms of following up our findings regarding the link between increased dispersion and employment status because individuals whose high levels of IIV cause functional problems even in the absence of global cognitive impairment may be at greater risk for future cognitive decline and additional functional decline. As with any complex clinical investigation, unmeasured variables may have influenced our findings. For example, we did not include details regarding the participants' extended medical histories or current medication regimens (e.g., psychotropic medications), which may have influenced the findings with regard to dispersion levels and rate of unemployment. In our study mood disorders were an exclusion criterion in order to isolate the effects of HCV infection, but future studies may also examine whether concurrent mood disorders, which are prevalent in disease populations such as HCV infection, exacerbate expression of dispersion. Furthermore, since current affective distress (i.e., POMS Total Mood Disturbance) was significantly associated with unemployment in HCV+ individuals, mood-related predictors of important functional outcomes such as vocational functioning should be further explored. Our sample size was not large enough to allow for investigation of the role of type of employment (e.g., using Hollingshead scores) in the relationship between dispersion and unemployment status in our HCV+ group, and therefore this is a suggestion for future work given the demonstrated effect of cognitive reserve in the expression of neurocognitive impairment in HCV infection (Bieliauskas et al., 2007). All individuals in our study were treatment naïve, and therefore future work may compare levels of IIV pre- and post-treatment to investigate whether increased dispersion may resolve (or be exacerbated by) with effective antiviral therapy (Kraus et al., 2005; Patullo et al., 2011). Also, although our study included liver laboratory values that allowed us to conclude that our results were not likely attributable to advanced liver disease, we did not examine biomarkers of immune activation and neuroinflammation (e.g., monocyte chemoattractant protein-1, MCP-1; tumor necrosis factor-alpha, TNF-alpha), or neuronal loss (e.g., N-acetyl aspartate on magnetic resonance spectroscopy), which may show a relationship to dispersion and would therefore demonstrate the neuropathological mechanisms underlying the HCV effect on IIV. An additional future direction toward that end includes various neuroimaging methods to examine the neural correlates of HCV-related dispersion. As mentioned above, our study was limited by the lack of external neuropsychological measures, which did not allow us to examine the cognitive mechanisms of dispersion in HCV, which we hypothesize to be cognitive dyscontrol relating to preferential impact on frontal-subcortical systems in HCV.
In summary, the findings of the current study demonstrated that neurocognitive dispersion is significantly increased in individuals with HCV infection in the absence of advanced liver disease, and this relationship does not appear to be related to fatigue or psychiatric factors. Furthermore, a novel finding linking dispersion to employment status among HCV+ individuals with higher mean neuropsychological performance suggests that dispersion may be an early marker of cognitive dyscontrol that can contribute to problems with daily functioning in the absence of global impairment. This finding is clinically relevant and potentially important because neurocognitively normal people are not typically considered to be at risk for problems with everyday functioning, and therefore would not be identified and provided services or targeted for tracking and follow-up.
Table 4.
Logistic regressions showing a significant association between dispersion and employment status among HCV+ individuals (n = 36a)
| Variable | Model | X2 | p-value | Odds Ratio |
|---|---|---|---|---|
| X2 (df=3) | 17.2 | .0007 | ||
| Dispersion | 5.88 | .001 | 2.0 | |
| Mean NP Performance T-score | 13.18 | .0003 | 0.70 | |
| Dispersion* Mean NP Performance T-score | 9.93 | .002 | -- |
Note: NP = neuropsychological;
employment status information was not available for 1 participant who was therefore excluded from the analyses
ACKNOWLEDGEMENTS
*The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H., Shannon LeBlanc; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Steven Paul Woods, Psy.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D. (Consultant); Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S., Tanya Wolfson, M.A.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
FUNDING This work was supported by National Institute on Drug Abuse (NIDA) grant P01 DA12065 to Dr. Igor Grant, and National Institute on Alcohol Abuse and Alcoholism Grant #5 T32 AA013525 to Dr. Edward Riley. The HIV Neurobehavioral Research Center (HNRC) is supported by Center award MH 62512 from the National Institute of Mental Health (NIMH).
REFERENCES
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed Author; Washington, D.C.: 1994. [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–6. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test - Revised. Psychological Assessment Resources, Inc.; Odessa, Florida: 1997. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Benton LA, Hamsher K, Sivan AB. Multi-lingual Aphasia Examination. 3rd ed. AJA; Iowa City, IA: 1994. Controlled Oral Word Association Test. [Google Scholar]
- Bieliauskas LA, Back-Madruga C, Lindsay KL, Snow KK, Kronfol Z, Lok AS, Padmanabhan L, Fontana RJ. Clinical relevance of cognitive scores in hepatitis C patients with advanced fibrosis. Journal of Clinical and Experimental Neuropsychology. 2006;28:1346–61. doi: 10.1080/13803390500473720. [DOI] [PubMed] [Google Scholar]
- Bieliauskas LA, Back-Madruga C, Lindsay KL, Wright EC, Kronfol Z, Lok AS, Halt-C Trial Group Cognitive reserve and neuropsychological functioning in patients infected with hepatitis C. Journal of the International Neuropsychological Society. 2007;13:687–692. doi: 10.1017/S1355617707070877. [DOI] [PubMed] [Google Scholar]
- Butters N, Grant I, Haxby J, Judd LL, Martin A, McClelland J, Stover E. Assessment of AIDS-related cognitive changes: recommendations of the NIMH Workshop on Neuropsychological Assessment Approaches. Journal of Clinical and Experimental Neuropsychology. 1990;12:963–78. doi: 10.1080/01688639008401035. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, et al. Initial validation of a screening battery for the detection of HIV-associated neurocognitive impairment. The Clinical Neuropsychologist. 2004;18:234–48. doi: 10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, HIV Neurobehavioral Research Center Group Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64:1342–47. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- Chelune GJ, Heaton RK, Lehman RAW. Neuropsychological and personality correlates of patients' complaints of disability. In: Gerald Goldstein RET, editor. Advances in clinical neuropsychology. Vol. 3. Plenum Press; New York: 1986. pp. 95–126. [Google Scholar]
- Christensen H, MacKinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P. Dispersion in cognitive ability as a function of age: A longitudinal study of an elderly community sample. Aging, Neuropsychology, & Cognition. 1999;6:214–28. [Google Scholar]
- Cordoba J, Flavia M, Jacas C, Sauleda S, Esteba JI, Vargas V, Esteban R, Guardia J. Quality of life and cognitive function in hepatitis C at different stages of liver disease. Journal of Hepatology. 2003;39:231–8. doi: 10.1016/s0168-8278(03)00189-2. [DOI] [PubMed] [Google Scholar]
- DiBoneventura M, Wagner JS, Yuan Y, L'Italien G, Langletm P, Ray Kim W. The impact of hepatitis C on labor force participation, absenteeism, presenteeism, and non-work activities. Journal of Medical Economics. 2011;14:253–61. doi: 10.3111/13696998.2011.566294. [DOI] [PubMed] [Google Scholar]
- Ettenhofer ML, Foley J, Behdin N, Levine AJ, Castellon SA, Hinkin CH. Reaction time variability in HIV-positive individuals. Archives of Clinical Neuropsychology. 2009;25:791–98. doi: 10.1093/arclin/acq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton DM, Karayiannis P, Mahmud N, Taylor-Robinson SD, Thomas HC. Identification of unique hepatitis C virus quasispecies in the central nervous system and comparative analysis of internal translational efficiency of brain, liver, and serum variants. Journal of Virology. 2004;78:5170–83. doi: 10.1128/JVI.78.10.5170-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton DM, Taylor-Robinson SD, Thomas HC. Central nervous system changes in hepatitis C virus infection. European Journal of Gastroenterological Hepatology. 2006;18:333–38. doi: 10.1097/00042737-200604000-00005. [DOI] [PubMed] [Google Scholar]
- Gronwall DMA. Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–375. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Rivera Mindt M, Sadek J, Moore DJ, Bentley H, the HNRC Group The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004;10:317–31. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources, Inc.; Lutz, FL: 2004. [Google Scholar]
- Hilborn JV, Strauss E, Hultsch DF, Hunter MA. Intraindividual variability across cognitive domains: investigation of dispersion levels and performance profiles in older adults. Journal of Clinical and Experimental Neuropsychology. 2009;31:412–24. doi: 10.1080/13803390802232659. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440–46. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. Journal of the International Neuropsychological Society. 2003;9:847–54. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- Hiscock M, Hiscock CK. Refining the forced-choice method for the detection of malingering. Journal of Clinical and Experimental Neuropsychology. 1989;11:967–74. doi: 10.1080/01688638908400949. [DOI] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. Unpublished manuscript, Yale University; New Haven, CT: 1975. [Google Scholar]
- Huckans M, Seelye A, Parcel T, Mull L, Woodhouse J, Bjornson D, Hauser P. The cognitive effects of hepatitis C in the presence and absence of a history of substance use disorder. Journal of International Neuropsychological Society. 2009;15:69–82. doi: 10.1017/S1355617708090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckans M, Seelye A, Woodhouse J, Parcel T, Mull L, Schwartz D, Hoffman W. Discounting of delayed rewards and executive dysfunction in individuals infected with hepatitis C. Journal of Clinical and Experimental Neuropsychology. 2011;33:176–86. doi: 10.1080/13803395.2010.499355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. Journal of Gerontology Series B: Social Sciences. 2002;57:P101–15. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Jacobs P, Ng YC, Stafinski T, Dodd R, Larke R, Wong W. Labor force participation among individuals with hepatitis C in the US. Pharmacoeconomics. 2003;21:565–72. doi: 10.2165/00019053-200321080-00003. [DOI] [PubMed] [Google Scholar]
- Kløve H. Grooved pegboard. Lafayette Instruments; Indiana: 1963. [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test - 64 Card Computerized Version. Psychological Assessment Resources; Odessa, FL: 2000. [Google Scholar]
- Kraus MR, Schafer A, Wissman S, Reimer P, Scheurien M. Neurocognitive changes in patients with hepatitis C receiving interferon alfa-2b and ribavirin. Clinical Pharmacology Therapy. 2005;77:90–100. doi: 10.1016/j.clpt.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Laskus T, Radkowski M, Bednardska A, Wilkinson DA, Nowicki M, Nikolopoulou GB, Rakela J. Detection and analysis of hepatitis C virus sequences in cerebrospinal fluid. Journal of Virology. 2002;76:10064–68. doi: 10.1128/JVI.76.19.10064-10068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, Grant I. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: Biological correlates of disease. AIDS. 2005;19:S72–8. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- Letendre S, Paulino AD, Rockenstein E, Adame A, Crews L, Cherner M, HIV Neurobehavioral Research Center Group Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. Journal of Infectious Diseases. 2007;196:361–70. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Hardy DJ, Barclay TR, Reinhard MJ, Cole MM, Hinkin CH. Elements of attention in HIV-infected adults: evaluation of an existing model. Journal of Clinical and Experimental Neuropsychology. 2008;30:53–62. doi: 10.1080/13803390601186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S, Heathcote EJ. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41:801–8. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Li SC, Backman L. Neural underpinnings of within-person variability in cognitive functioning. Psychological Aging. 2009;24:792–808. doi: 10.1037/a0017798. [DOI] [PubMed] [Google Scholar]
- Martin EM, Novak RM, Fendrick M, Vassileva J, Gonzalez R, Grbesic S, Sworoski L. Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. Journal of the International Neuropsychological Society. 2004;10:298–300. doi: 10.1017/S135561770410218X. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1981. [Google Scholar]
- Morgan EE, Woods SP, Delano-Wood L, Bondi MW, Grant I, HIV Neurobehavioral Research Program (HNRP) Group Intraindividual variability in HIV infection: Evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology. 2011;25:645–54. doi: 10.1037/a0023792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D, Cysique L, Ake C, Lazarretto D, Vaida F, Heaton RK. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. Journal of Clinical and Experimental Neuropsychology. doi: 10.1080/13803395.2011.559157. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattullo V, McAndrews MP, Damyanovich A, Heathcote EJ. Influence of hepatitis C virus on neurocognitive function in patients free from other risk factors: Validation from therapeutic outcomes. Liver International. 2011;31:1028–1038. doi: 10.1111/j.1478-3231.2011.02549.x. [DOI] [PubMed] [Google Scholar]
- Perry W, Hilsabeck RC, Hassanien TI. Cognitive dysfunction in chronic hepatitis C: A review. Digestive Diseases and Sciences. 2008;53:307–21. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- Posada C, Moore DJ, Woods SP, Vigil O, Ake C, Perry W, HIV Neurobehavioral Research Center Group Implications of hepatitis C virus infection for behavioral symptoms and activities of daily living. Journal of Clinical and Experimental Neuropsychology. 2010;32:637–44. doi: 10.1080/13803390903418900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada C, Morgan EE, Moore DJ, Woods SP, Letendre SL, Grant I, HIV Neurobehavioral Research Center Group Neurocognitive effects of the hepatitis C virus. Current Hepatitis Reports. 2009;8:158–66. [Google Scholar]
- Psychological Corporation . WAIS-III and WMS-III technical manual. Author; San Antonio, TX: 1997. [Google Scholar]
- Radkowski M, Wilkinson J, Nowicki M, Adair D, Vargas H, Ingui C, Rakela J, Laskus T. Search for hepatitis C virus negative-strand RNA sequences and analysis of viral sequences in the central nervous system: Evidence of replication. Journal of Virology. 2002;76:600–8. doi: 10.1128/JVI.76.2.600-608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Sano M, Silverman JM, Haroutunian V. Cross-domain variability of cognitive performance in very old nursing home residents and community dwellers: relationship to functional status. Gerontology. 2005;51:206–12. doi: 10.1159/000083995. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Neuropsychology Press; Tucson, AZ: 1993. [Google Scholar]
- Schretlen DJ, Munro CA, Anthony JC, Pearlson GD. Examining the range of normal intraindividual variability in neuropsychological test performance. Journal of the International Neuropsychological Society. 2003;9:864–70. doi: 10.1017/S1355617703960061. [DOI] [PubMed] [Google Scholar]
- Seeff LB, Hoofnagle JH. Appendix: The National Institutes of Health Consensus Development Conference management of hepatitis C 2002. Clinics in Liver Disease. 2003;7:261–87. doi: 10.1016/s1089-3261(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C infection. Lancet Infectious Diseases. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Su J, Brook RA, Kleinman NL, Corey-Lisle P. The impact of hepatitis C virus infection on work absence, productivity, and healthcare benefit costs. Hepatology. 2010;52:436–42. doi: 10.1002/hep.23726. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126:2362–80. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Letendre SL, Schweinsburg BC, Alhassoon OM, Brown GG, Gongvatana A, The HNRC Group Hepatitis C virus infection is associated with reduced white matter N-acetylaspartate in abstinent methamphetamine users. Journal of the International Neuropsychological Society. 2004;10:110–13. doi: 10.1017/S1355617704101161. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson SD. Applications of magnetic resonance spectroscopy to chronic liver disease. Clinical Medicine. 2001;1:54–60. doi: 10.7861/clinmedicine.1-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil O, Posada C, Woods SP, Atkinson JH, Heaton RK, Perry W, HIV Neurobehavioral Research Group Impairments in fine-motor coordination and speed of information processing predict declines in everyday functioning in hepatitis C infection. Journal of Clinical and Experimental Neuropsychology. 2008;30:805–15. doi: 10.1080/13803390701802354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schuler A, Ennen JC, Boker KW. Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. Journal of Hepatology. 2004;41:845–51. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- West R, Murphy KJ, Armillo ML, Craik FM, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cognition. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- Wetter SR, Delis DC, Houston WS, Jacobson MW, Lansing A, Cobell K, Salmon DP, Bondi MW. Heterogeneity in verbal memory: A marker of preclinical Alzheimer's disease? Aging, Neuropsychology, and Cognition. 2006;13:503–515. doi: 10.1080/138255890969492. [DOI] [PubMed] [Google Scholar]
- Woods SP, Conover E, Weinborn M, Rippeth JD, Brill RM, Heaton RK, HIV Neurobehavioral Research Center Group Base rate of Hiscock Digit Memory Test failure in HIV-associate neurocognitive disorders. The Clinical Neuropsychologist. 2003;17:383–9. doi: 10.1076/clin.17.3.383.18079. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Composite International Diagnostic Interview (CIDI, version 2.1) Author; Geneva, Switzerland: 1998. [Google Scholar]