Abstract
Extinguishing abnormally strengthened learned responses to cues associated with drugs of abuse remains a key tactic for alleviating addiction. To assist in developing pharmacotherapies to augment exposure therapy for relapse prevention, investigation into neurobiological underpinnings of drug-cue extinction learning is needed. We used regional analyses of c-Fos and GluR2 protein expression to delineate neural activity and plasticity that may be associated with cocaine-cue extinction learning. Rats were trained to self-administer cocaine paired with a light cue, and later underwent a single 2hr extinction session for which cocaine was withheld but response-contingent cues were presented (cocaine-cue extinction). Control groups consisted of rats yoked to animals self-administering cocaine and receiving saline non-contingently followed by an extinction session, or rats trained to self-administer cocaine followed by a no-extinction session for which levers were retracted, and cocaine and cues were withheld. Among 11 brain sites examined, extinction training increased c-Fos expression in basolateral amygdala and prelimbic prefrontal cortex of cocaine-cue extinguished rats relative to both control conditions. In dorsal subiculum and infralimbic prefrontal cortex, extinction training increased c-Fos expression in both cocaine-cue and saline-cue extinguished rats relative to the no-extinction control condition. GluR2 protein expression was not altered in any site examined after extinction or control training. Findings suggest that basolateral amygdala and prelimbic prefrontal cortex neurons are activated during acquisition of cocaine-cue extinction learning, a process that is independent of changes in GluR2 abundance. Other sites are implicated in processing the significance of cues that are present early in extinction training.
Keywords: Basolateral amygdala, c-Fos protein, Cocaine, Extinction learning, GluR2 protein, Self-administration
1. Introduction
Through the process of associative learning, cues paired with drugs of abuse gain enhanced salience to exert long-lasting and powerful influences over the behavior of individuals abusing drugs [1]. Extinguishing abnormally strengthened learned responses to cues associated with drugs of abuse remains a key tactic for alleviating addiction. Exposure-based behavioral therapies make use of an extinction strategy whereby individuals are confronted repeatedly with cues in a controlled setting in an effort to reduce cue salience [2]. In animal models of exposure therapy for cocaine addiction, cocaine-paired stimuli are presented repeatedly during extinction training without the delivery of cocaine [3–6]. These studies suggest that the combination of extinction training with cognitive enhancing pharmacotherapy facilitates extinction learning and deters relapse behavior in rats and monkeys trained to self-administer cocaine. These encouraging preclinical findings prompt investigation into the neurobiological underpinnings of cocaine-cue extinction learning. This knowledge may assist in the development of improved pharmacotherapies to use in conjunction with exposure therapy for relapse prevention in addicts. While current knowledge of substrates and mechanisms of cocaine-cue extinction learning is limited [7], it is likely that this form of extinction learning involves neurosubstrates that are similar to those involved in extinction of conditioned fear [8]. Brain regions such as the ventromedial prefrontal cortex (vmPFC) and basolateral amygdala (BLA) may have a prominent role in this regard.
In the present investigation, expression of c-Fos protein, a member of the Fos family of proteins and a product of the immediate early gene c-fos, was measured to determine which brain sites may become acutely active during acquisition of cocaine-cue extinction learning. Previous studies in rats have shown increased c-Fos protein expression within striatum, hippocampus, prefrontal cortex and/or amygdala after cocaine-cue reinstatement tests that followed a period of abstinence [9] and [10] or response extinction training for which both cocaine and response-contingent cocaine-paired cues were withheld [11] and [12]. Our cue exposure paradigm, in which response-contingent cues are presented in the absence of cocaine injections, differs significantly from other extinction training paradigms in which both cocaine and cues are withheld. This is an important distinction because clinical treatment protocols utilize cue exposure as a means to extinguish conditioned responses [13].
While the design of the present investigation was optimized for measuring c-Fos protein acutely expressed in response to cocaine-cue extinction learning (see Section 2), the expression of GluR2-containing AMPA receptors also was evaluated. AMPA receptors containing the GluR2 subunit predominate in principal neurons throughout the adult brain [14] and co-express with c-Fos protein in larger numbers than other subunits [10]. GluR2-containing AMPA receptors within the amygdala may play a crucial role in the formation extinction memory that leads to reduction in previously established conditioned fear responses [15]. Previous studies in cocaine-trained rats have shown that intracellular, surface and/or total GluR2 subunit expression is not altered in nucleus accumbens (NAc) after response extinction training or cocaine-cue reinstatement tests following a period of abstinence [16] and [17]. In the present study, we determined if GluR2 subunit expression in several brain sites was altered after cocaine-cue extinction training.
2 Material and methods
2.1 Subjects
Male Wistar rats (Crl(WI)BR; 275–300 g) were housed individually in a temperature- (21–23 °C) and light- (08:00 h on, 20:00 h off) controlled facility. Rats were maintained in accordance with the 1996 NIH Guide for Care and Use of Laboratory Animals. The Boston University Institutional Animal Care and Use Committee approved research protocols. Animals were implanted with indwelling venous catheters using the surgical procedures described previously [3].
2.2 Drugs
Cocaine HCl (gift from NIDA, Bethesda, MD) was dissolved in sterile 0.9% saline containing 3 IU heparin/ml to a concentration of 1.6 mg/ml. For intravenous (i.v.) self-administration of cocaine, a 0.3 mg/kg unit dose was delivered at a rate of 0.03 ml/sec. For yoked-control rats, saline vehicle was infused at the same rate as cocaine.
2.3 Behavioral Procedures
The experimental design is outlined in Table 1. During daily 1hr cocaine self-administration sessions, rats that later underwent cocaine-cue extinction training (Group 1) were initially trained to press a lever designated as active to obtain 0.3 mg/kg i.v. injections of cocaine paired with the simultaneous presentation of a distinctive visual stimulus (2-sec light) under a fixed-ratio (FR) 1 schedule of reinforcement. Responses on a second inactive lever had no scheduled consequences. Training continued until rats self-administered cocaine under a second-order reinforcement schedule. A second-order schedule was used because it exposes animals to a greater number of response-contingent drug-paired cues during sessions compared to a fixed ratio schedule. A high cue exposure baseline provides a stringent test of how well drug-seeking responses are reduced in later extinction tests. For this schedule, the 2-sec cue light was presented under an FR5 contingency during the entire session. The delivery of cocaine co-occurred with cue light presentation upon completion of the first FR5 after each 5-min fixed interval (FI) of time elapsed. After cocaine was delivered, the FI component was again in effect. This schedule is specified as an FI 5-min [FR5:S] second-order schedule, where the S refers to the 2-sec light cue. During 1 hr daily sessions, rats could self-administer a maximum 11 cocaine injections.
Table 1.
Experimental design and number of responses and infusions (mean ± SEM) during 1hr cocaine self-administration or yoked-saline sessions at baseline.
| Group | N | Experimental Design | Baseline Responses | Infusions | |||
|---|---|---|---|---|---|---|---|
| Training | Cues | Test | Active | Inactive | |||
| 1 | 8 | Cocaine | Paired | EXT | 378.1 ± 59.5 | 61.5 ± 15.5 | 9.6 ± 0.4 |
| 2 | 7 | Saline | Paired | EXT | 21.9 ± 4.8 | 9.8 ± 3.0 | N/A |
| 3 | 8 | Saline | Unpaired | EXT | 19.9 ± 3.6 | 8.2 ± 2.7 | N/A |
| 4 | 4 | Cocaine | Paired | No-EXT | 249.4 ± 56.9 | 59.2 ± 7.9 | 9.3 ± 0.3 |
| 5 | 4 | Cocaine | Unpaired | No-EXT | 153.3 ± 23.5 | 31.7 ± 10.8 | 9.4 ± 0.1 |
Training continued for a minimum of 30 sessions until rats self-administered cocaine reliably. After baseline cocaine self-administration was stable (i.e., the absence of increasing or decreasing trends and ≤ 10% individual variability day-to-day), rats underwent 2 weeks of abstinence prior to the extinction test session. During the abstinence period, rats received ten 1hr sessions in operant chambers for which levers were retracted, and without presentation of either the cocaine-paired stimulus or the delivery of cocaine. The multiple abstinence sessions were included to dampen the ability of the contextual cues in the test environment to acutely express c-Fos protein on test day [18–20]. In addition, multiple abstinence sessions were used to restore the ability of discrete cue presentations and lever pressing to acutely express c-Fos protein on test day [11] as well as to reveal neural activation patterns associated with the novel cocaine-cue extinction learning. Following the abstinence period, rats underwent a single 2hr cocaine-cue extinction test session. During the extinction test, an FI 5-min [FR5:S] second-order schedule also was used whereby the 2-sec cue light was presented under an FR5 contingency during the entire session. In addition, saline was substituted for cocaine and the delivery of saline co-occurred with cue light presentation upon completion of the first FR5 after each FI 5-min elapsed.
Several control groups were included in this experiment to evaluate the extent to which the molecular changes were selectively associated with cocaine-cue extinction learning (Table 1). Rats receiving saline (Groups 2 and 3) were yoked to rats self-administering cocaine (Group 1) and received saline infusions non-contingently. One group had infusions paired with the 2-sec light cue (Group 2), whereas the other group did not (Group 3). Following the 2-week abstinence period as described above, rats in Groups 2 and 3 were given a yoked-extinction test, which was identical to their yoked-training sessions. Additional control groups were trained to self-administer cocaine. One group was trained to self-administer cocaine in the usual manner (Group 4) and the other group was trained to self-administer cocaine with cues presented in a random non-contingent manner (Group 5). Following the 2-week abstinence period as described above, rats in Groups 4 and 5 were given an additional abstinence session rather than an extinction session as the test (No-EXT session). With this design, it was possible to determine if changes in c-Fos or GluR2 protein expression on test day were associated specifically with cocaine-cue extinction learning (Group 1), or if changes also were evident simply as a matter of lever pressing and receiving an i.v. injection of saline following paired or unpaired discrete cue presentation (Groups 2 and 3) or of chronic exposure to the cocaine context following a history of paired or unpaired discrete cue presentation during cocaine self-administration training (Groups 4 and 5). A home cage control group was not evaluated because our primary interest was in determining which brain sites exhibited enhanced neural activity while rats underwent extinction to the discrete cues paired with cocaine. The appropriate controls for this precise question were groups 2–5.
2.4 Neurochemical procedures
Expression of c-Fos and GluR2 proteins were measured immunohistochemically. As c-Fos protein has been shown to peak in neural expression 60–120min after exposure to an acute stimulus or novel event [21], rats were sacrificed by an overdose of sodium pentobarbital 60min following completion of the 2hr test session. This time-point was selected for sacrifice because it corresponds to the time c-Fos protein would hypothetically reach peak expression following extinction learning, an event that occurred during the last 30min of the 2hr extinction session (90min before sacrifice). Rats were perfused with 4% paraformaldehyde solution and the brains were extracted, flash frozen in isopentane and stored at −80°C. Different sets of 40μm coronal brain slices were collected and stored in ethylene glycol solution at −20°C for the assays. One set of these brain slices was used for c-Fos detection and another set for GluR2 detection. Sections taken at +3.72 to +2.52 mm contained the prelimbic and infralimbic prefrontal cortices (PL and IL), the anterior cingulate (ACC) and M1 motor cortices (M1); sections taken at +2.76 to −1.20 mm contained the nucleus accumbens core and shell (NAc core and NAc shell); sections taken at +2.50 to −0.48 mm contained the caudate putamen (CPu); sections taken at −1.5 to −3.48 mm contained the basolateral amygdala (BlA); sections taken at −3.00 to −5.50 mm contained dorsal hippocampal subiculum (dSUB); sections taken at −4.50 to −6.50mm contained the ventral hippocampal subiculum (vSUB) and paraventricular thalamic nucleus (PVN). These sites previously have been shown to be relevant for extinction learning and addiction [22], [23] and [24]. The M1 motor cortex (M1) served as a control site.
2.4.1 Fos Immunohistochemistry
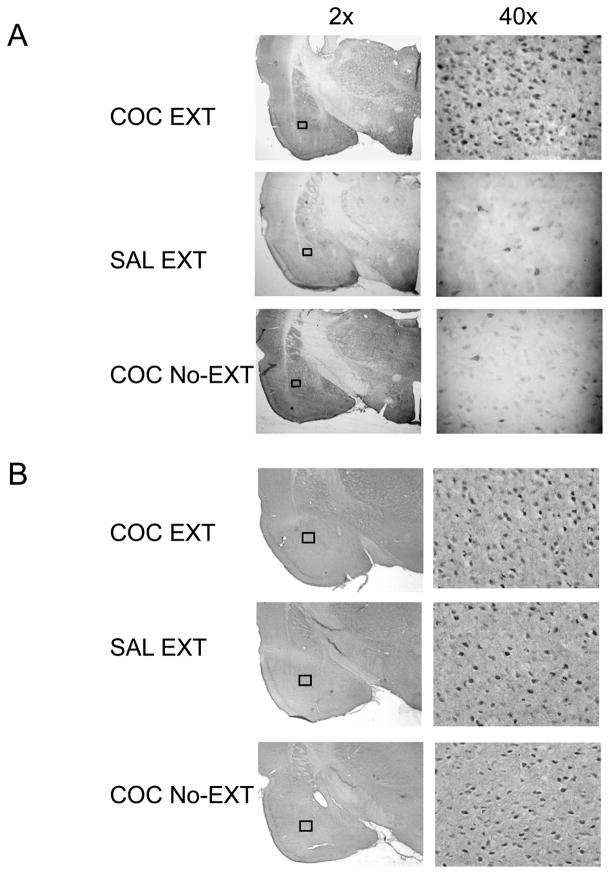
Methods for processing c-Fos protein expression were adapted from published reports [25] and [26]. To control for methodologically induced variability, sections from each behavioral group were processed at the same time, such that each immunocytochemical batch contained tissue from each experimental group. Free-floating sections (40μm) were washed (3 times for 10min each) in PBS, incubated for 1hr in 3% normal goat serum (NGS) in PBS with 0.20% Triton X-100 (PBS-Tx), and incubated overnight at 4°C with rabbit anti-c-Fos primary antibody (c-Fos sc-52, Santa Cruz Biotechnology, Santa Cruz, CA) and diluted 1:4000 in 1% NGS in PBS-Tx. Sections were then washed in PBS and incubated for 1hr with biotinylated anti-rabbit IgG secondary antibody (BA-1000, Vector Laboratories, Burlingame, CA) diluted 1:600 in 1% NGS in PBS-Tx. Sections were again washed in PBS and incubated in avidin-biotin-peroxidase complex (ABC Elite kit, PK-6100, Vector Laboratories) in PBS containing 0.5% Triton X-100 for 45min. Sections were then rinsed three times in PBS for 10min each and reacted with 0.025% 3,3′-diaminobenzidine with nickel intensification (DAB kit) for 5min and finally rinsed three times for 10min in PBS. Sections were then mounted on slides, dehydrated, and coverslipped with Permount (Fisher Scientific, Springfield, NJ). Images were captured using a 40X objective microscope and a Nikon DXM 1200 digital camera using Image Pro Plus 4.5 Software Media Cybernetics, Silver Spring, MD. Fos immunoreactivity was identified as a blue-black oval-shaped nucleus distinguishable from background (see Fig. 2a). The number of c-Fos-immunoreactive (Fos-IR) cells was counted manually by a reviewer blind to the status of the animal. The c-Fos-IR staining was quantified using the mean positive counts of left and right hemispheres within a sampling area of 0.1 mm2 and analyzing 2–3 coronal sections from each brain region of individual subjects. Brain regions were identified using the Paxinos and Watson atlas [27].
Figure 2.
Photomicrographs of representative BLA images for (A) c-Fos and (B) GluR2 staining at 2× and 40× magnification. Examples of staining after cocaine-cue extinction (COC EXT) and control training (SAL EXT or COC No-EXT) are included.
2.4.2 GluR2 Immunohistochemistry
Coronal brain sections (40μm) were processed using a free-floating method [28]. As above, to control for methodologically induced variability, sections from each behavioral group were processed at the same time, such that each immunocytochemical batch contained tissue from each experimental group. Brain sections were first washed 3 times in ice-cold phosphate buffer (PBS, pH 7.4) and then washed overnight at 4°C. Sections were transferred to 1.5% normal goat serum from the ABC kit (Santa Cruz Biotechnology) diluted in PBS for 1 hour at room temperature, then incubated overnight at 4°C in rabbit GluR2 affinity-purified polyclonal antibody 1:1000 (AB9209) in 1.5% goat serum (Millipore, Billerica, MA). Sections were washed 3 times in PBS and incubated in biotinylated secondary antibody, goat anti-rabbit IgG 1:2000 ABC kit (Santa Cruz Biotechnology). Immunoreactivity was visualized by the biotin-streptavidin ABC Staining System (Santa Cruz Biotechnology) using DAB (Thermo Scientific, Malvern, PA) as the chromagen. Brain sections were washed in PBS, mounted on chrome alum subbed slides, and coverslipped with Permount (Fisher Scientific). Images were obtained at 10X magnification and quantitative measurement was performed by a blind reviewer using a computer-assisted image analysis system, consisting of an Olympus BX51 bright field microscope interfaced with a color digital camera (MicroFire; Optronics, Goletta, CA), and a computer with Image-Pro Plus image processing and analysis software (Version 6.3; Media Cybernetics, Silver Spring, USA). GluR2 staining was quantified using the mean GluR2 positive counts of left and right hemispheres within a sampling area of 0.2 mm2 and analyzing 3–4 coronal sections from each brain region of individual subjects. GluR2 immunoreactivity was identified as signals showing grayscale contrast levels over 100 (see Fig. 2b). Brain regions were identified using the Paxinos and Watson atlas [29].
3 Results
3.1 Evidence of cocaine-cue extinction learning in animals with history of cocaine self-administration
The last five cocaine self-administration or yoked-saline sessions were used to establish baseline behavior, which is presented as the mean total number of responses per 1hr (Table 1). One-factor ANOVA across groups revealed significant differences in active lever responding at baseline [F(4,28)=16.6, p = 0.001]. Post-hoc tests (Holm-Sidak) indicated greater responding (p ≤ 0.01) in groups receiving cocaine paired with discrete cues (Groups 1 and 4) than groups receiving yoked-saline (Groups 2 and 3). In addition, active lever responding was higher (p ≤ 0.01) if a discrete cue was paired with cocaine delivery than if unpaired (Group 1 vs. Group 5), suggesting that paired cues are motivationally salient. Infusions were not significantly different among the cocaine groups, and their inactive lever responses were ≤ 24% of active lever responses.
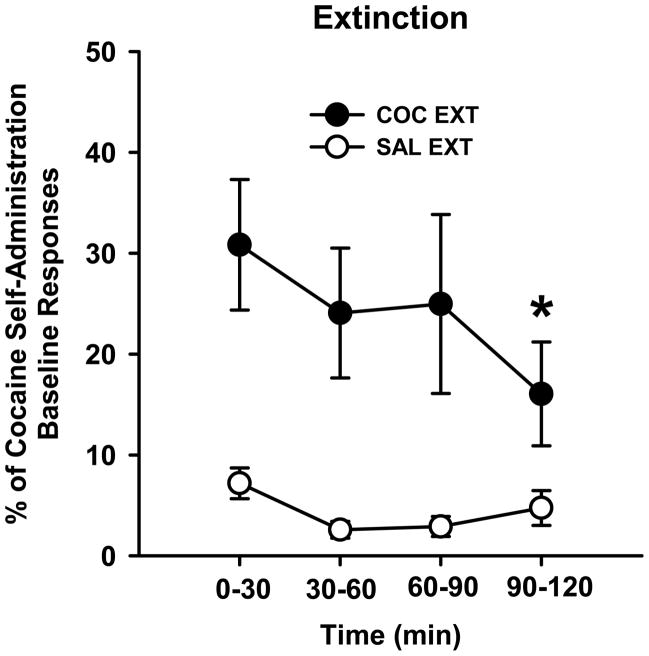
Prior to analysis of extinction responding in 30-min bins, data were expressed as the percent of the individual subject’s baseline responses due to the 5-fold difference in baseline responses of individual rats in Group 1 (COC EXT group). Responses per 30 min were first computed for baseline sessions prior to calculating the percent of baseline measure. In addition, values from each rat in the saline group were expressed as a percentage of the cocaine self-administration baseline, using the rat to which it was yoked to calculate this number. The two yoked-saline groups were combined for this analysis (SAL EXT group) because they exhibited similar levels of responding during the 2-hr extinction test session. The percent of baseline active lever responses was analyzed by ANOVA using the between-subjects factor of drug (cocaine, saline) and the repeated measures factor of time (four 30-min bins). The Holm-Sidak test was used for post-hoc analysis. Drug [F(1,23)=19.6, p ≤ 0.001] and time [F(3,69)=2.8, p ≤ 0.05] were significant factors. As expected, the COC EXT group exhibited significantly more active lever responding than the SAL EXT group throughout extinction training (p ≤ 0.001; Fig. 1). The SAL EXT group had low levels of responding during the entire 2hr session. Importantly, responding was significantly lower during the last 30-min bin compared to the first 30-min bin in the COC EXT group (p ≤ 0.05; Fig. 1). This suggests that cocaine-trained rats learned to attenuate lever pressing for the motivationally salient response-contingent cues over the course of the 2hr session and showed evidence of extinction learning by the last 30min of extinction training. Inactive lever responses were ≤ 11 % of active lever responses for cocaine-trained animals (not shown).
Figure 1.
Time course of cocaine-cue extinction learning in animals previously trained to self-administer cocaine (COC EXT) or receiving yoked-saline (SAL EXT) under a second-order schedule of reinforcement. Mean ± SEM values are expressed as the percentage of cocaine self-administration baseline responses (per 30min) for each group. Sequential 30min bins over the 2hr extinction training session were examined. * p<0.05 compared to the first 30min of extinction training.
3.2 Extinction-related neuronal activity as measured by c-Fos protein expression
For each of the 11 brain sites examined, similar levels of c-Fos protein expression were observed in the two yoked-saline groups that had received extinction training (t(14) = 0.04 to 1.87; p>0.05) and in the two cocaine-trained groups that had received no extinction training (t(6) = 0.02 to 0.90; p>0.05). Therefore, data were combined from each of the two groups to represent the saline (SAL EXT) and cocaine (COC No-EXT) control groups, respectively. For each brain site, these groups were compared by one-factor ANOVA to the cocaine-trained group that had received extinction training (COC EXT). The Holm-Sidak test was used for post-hoc analysis. Representative images of c-Fos protein staining in the BLA are shown in Fig. 2A.
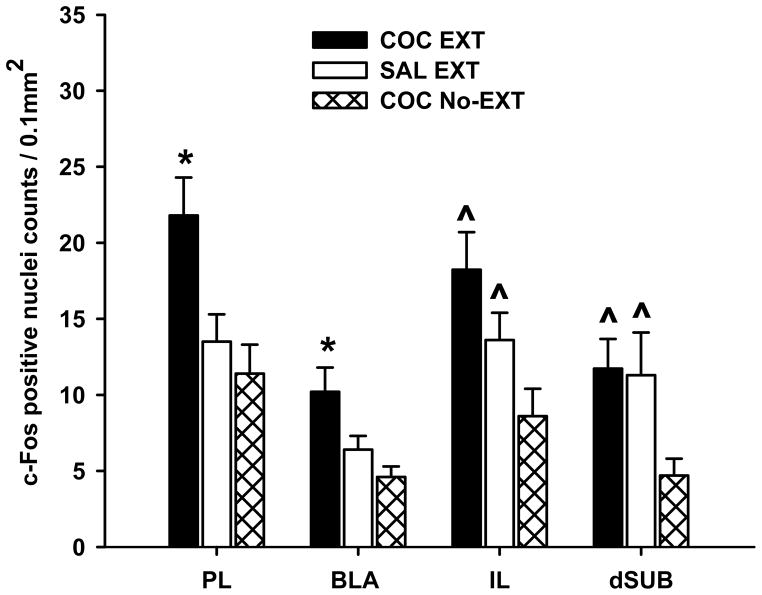
Two different patterns of change in c-Fos expression were observed. For the first change, c-Fos protein expression was significantly greater in the COC EXT group compared to the SAL EXT and COC No-EXT groups (p ≤ 0.05; Fig. 3). This type of change was found in the PL [F(2, 31) = 5.6; p ≤ 0.05] and BLA [F(2, 31) = 3.8; p ≤ 0.05]. For the second change, c-Fos protein expression was significantly greater in the COC EXT and SAL EXT groups compared to the COC No-EXT group (p ≤ 0.05; Fig. 3). This type of change was found in the IL [F(2, 31) = 5.2; p ≤ 0.05] and dSUB [F(2, 31) = 3.3; p ≤ 0.05]. For the remaining brain sites, c-Fos protein expression did not differ significantly among the three groups (Table 2).
Figure 3.
Expression of c-Fos protein after cocaine-cue extinction (COC EXT) and control training (SAL EXT or COC No-EXT). Values are the mean ± SEM number of c-Fos-positive cell counts per 0.1 mm2 in brain regions where significant differences were found. * p<0.05 compared to SAL EXT and COC No-EXT control groups; ^ p<0.05 compared to the COC No-EXT control group.
Table 2.
Expression of c-Fos protein (mean ± SEM) in brain sites where no significant group differences were detected after cocaine-cue extinction (COC EXT) and control training (SAL EXT or COC No-EXT). Values are the mean ± SEM number of c-Fos-positive cell counts per 0.1 mm2.
| Brain Site | Group | ||
|---|---|---|---|
| COC EXT | SAL EXT | COC No-EXT | |
| M1 | 12.6 ± 2.7 | 15.6 ± 2.5 | 10.4 ± 1.7 |
| NAc Core | 15.0 ± 3.8 | 14.8 ± 1.5 | 13.8 ± 2.4 |
| NAc Shell | 11.1 ± 2.2 | 10.0 ± 1.0 | 7.5 ± 1.1 |
| CPu | 10.1 ± 3.6 | 6.6 ± 1.1 | 8.8 ± 2.6 |
| vSUB | 10.1 ± 3.3 | 8.0 ± 1.0 | 8.5 ± 2.0 |
| PVN | 24.3 ± 7.1 | 21.6 ± 2.6 | 16.6 ± 2.6 |
To determine if the magnitude of c-Fos protein expression were simply a reflection of the number of active lever responses emitted during the last 30 min of the extinction session, correlation analyses were conducted in the different brain sites. Using data from COC EXT and SAL EXT groups, we observed significant positive correlations only for the NAc core (Pearson’s r = 0.431; p ≤ 0.05) and CPu (Pearson’s r = 0.556; p ≤ 0.05). These analyses showed no significant correlations for brain sites in which c-Fos protein expression was enhanced after extinction training (BLA, PL, IL and dSUB).
3.3 Extinction-related changes in GluR2 protein expression
For each brain site examined for GluR2, the control groups were combined as in the c-Fos analysis above (SAL EXT and COC No-EXT). Data were analyzed by one-factor ANOVA to measure changes in these groups relative to the COC EXT group. Representative images of GluR2 staining in the BLA are shown in Fig. 2B. ANOVA analyses revealed that GluR2 protein expression did not significantly differ among the COC EXT, SAL EXT and COC No-EXT groups for any of the 7 brain sites examined, including the BLA, PL, IL and dSUB (Table 3).
Table 3.
Expression of GluR2 protein (mean ± SEM) in brain sites where no significant group differences were detected after cocaine-cue extinction (COC EXT) and control training (SAL EXT or COC No-EXT). Values are the mean ± SEM number of GluR2-positive counts per 0.2mm2.
| Brain Site | Group | ||
|---|---|---|---|
| COC EXT | SAL EXT | COC No-EXT | |
| PL | 17.0 ± 2.7 | 16.1 ± 1.8 | 21.0 ± 2.7 |
| IL | 16.8 ± 1.3 | 17.4 ± 1.7 | 18.8 ± 1.6 |
| NAc Core | 17.8 ± 3.6 | 20.9 ± 2.7 | 21.5 ± 2.8 |
| NAc Shell | 14.9 ± 2.2 | 20.6 ± 2.5 | 21.4 ± 4.0 |
| CPu | 27.3 ± 3.7 | 28.4 ± 2.4 | 21.3 ± 1.7 |
| BLA | 14.2 ± 2.6 | 16.2 ± 2.7 | 15.7 ± 2.9 |
| dSUB | 27.3 ± 3.5 | 25.4 ± 4.0 | 24.5 ± 3.4 |
4. Discussion
The results of this investigation suggest that sites within amygdala, prefrontal cortex, hippocampus and striatum are actively engaged during cocaine-cue extinction training. Based on the patterns and correlations of c-Fos protein expression, however, the different sites may mediate distinct processes during extinction training. Expression of c-Fos protein was selectively increased in BLA and PL by cocaine-cue extinction training. These findings suggest that these brain areas are associated with cocaine-cue extinction learning. Importantly, c-Fos protein expression within the BLA and PL did not correlate with the number of lever presses emitted during the last 30min of extinction training, suggesting that c-Fos was activated by cocaine-cue extinction learning and not by motor performance.
Association of the BLA with cocaine-cue extinction learning is not entirely surprising given the wealth of evidence implicating learning mechanisms in BLA for acquisition and reinstatement of cocaine-seeking responses [30–35]. Studies showing a disruption of the acquisition and consolidation of cocaine-cue extinction learning after pre-session neuronal inactivation [36] and post-session protein synthesis inhibition [37] of BLA, respectively, strengthen this conclusion. These findings provide functional evidence that the BLA is critically involved in mediating cocaine-cue extinction learning and are consistent with the present findings establishing enhanced c-Fos expression in BLA as a neural correlate of cocaine-cue extinction learning. In PL and IL, an increase in c-Fos protein expression was observed in rats undergoing cocaine-cue extinction training relative to abstinence control training, However, in IL, c-Fos protein expression also was increased in yoked-saline controls undergoing yoked-extinction training relative to cocaine controls receiving abstinence training. These patterns of change suggest that IL may be involved in processing the significance of cues present early in the acquisition phase of extinction training, whereas PL may be involved in mediating cocaine-cue extinction learning. These conclusions are difficult to reconcile in light of previous reports that used site-selective inactivation procedures and found that IL, as opposed to PL, regulated consolidation of extinction [38] and [24]. As extinction in these reports took place in a fear context or a cocaine self-administration context, the different effects in our study regarding prefrontal cortex control of extinction may relate to use of discrete vs. context cues during extinction training, or to examination of acquisition vs. consolidation of extinction. Consistent with our findings, others reported an increase in c-Fos protein expression in PL after presentation of a cocaine-paired, but not saline-paired, discrete stimulus in rats [12], whereas there was no difference in c-Fos protein expression in IL after presentation of cocaine-paired vs. saline-paired discrete stimuli [39].
Past studies show that neuronal inactivation of the dSUB disrupts acquisition of cocaine-cue extinction learning [36]. Similar disruptive effects on acquisition of fear extinction were observed after neuronal inactivation of DH [40]. However, the pattern of change in c-Fos protein expression in dSUB (enhanced expression after both cocaine-cue and yoked-saline extinction training) suggest a role for the hippocampus in processing the significance of cues that are present early in extinction training rather than mediating cocaine-cue extinction learning per se. Notably, c-Fos protein expression in dSUB was independent of the rate of responding during the last 30min of the test session, suggesting that changes in c-Fos protein were not simply a reflection of motor behavior. One function of the hippocampal system is to process the significance of a cue in relation to other cues present in the environment and store the relations among them to support declarative memory [41]. This aspect of hippocampal system function may account for the elevated c-Fos protein expression in dSUB following both cocaine-cue and yoked-saline extinction training as well as for a deficit in cocaine-cue extinction learning following neuronal inactivation of the dSUB [36].
Expression of c-Fos protein after cocaine-cue extinction training did not differ from abstinence control training or yoked-extinction training in yoked-saline controls in NAc core, NAc shell or CPu. These findings differ to some degree from Zavala et al. [10] who showed increased c-Fos expression in these sites in rats given a cue test after undergoing abstinence control training relative to rats given a cue test after undergoing cocaine-cue or yoked-saline extinction training. Differences in time of sacrifice after the test session coupled with the degree of cue exposure prior to sacrifice may account for these different effects. In the current study, cues were restored on test day under the cocaine-cue and yoked-saline extinction training conditions after a 2-week drug-free period consisting of abstinence (no-extinction) training in the operant chambers. This design was used to optimize measurement of c-Fos protein acutely expressed in response to cocaine-cue extinction learning, the most salient event experienced 90–120 min prior to sacrifice in the COC EXT group. We used this time course for sacrifice because any expression of c-Fos protein associated with cue exposure, lever pressing or i.v. saline injection experienced at the start of the test session would have dissipated by the time of sacrifice 3hr later. In Zavala et al. [10], the cues were novel only in the abstinence-training group on test day, as rats from the cocaine-cue and yoked-saline extinction conditions had experienced cues for 22 consecutive days just prior to test day. Rats from these training conditions would have been tolerant to any c-Fos protein induction by cues on test day. Since their rats were sacrificed immediately after the 90min test session, the enhanced expression of c-Fos protein only in the abstinence-training group is likely associated with cocaine-seeking responses on test day rather than cocaine-cue extinction learning. The fact that c-Fos protein expression in NAc core, NAc shell and CPu did not differ between training conditions in the present study suggests these sites are not associated with cocaine-cue extinction learning. However, positive correlations between c-Fos expression in NAc core and CPu and the rate of responding during the last 30min of the test session were found. These findings suggest that NAc core and CPu may mediate motor behavior during extinction training. As the NAc core is the gateway from the ventral to the dorsal striatum, both sites do have the capacity to influence motor outcome [42].
Regarding AMPA receptor subunit expression, GluR2 protein expression after cocaine-cue extinction training did not differ from abstinence control training or extinction training in yoked-saline controls in any of the brain sites examined in the present study. These findings replicate previous observations of no changes in GluR2 protein expression in the PFC, NAc, CPu and DH of rats undergoing cocaine-cue extinction training [10]. In another study that used a model of extinction training for which both cocaine delivery and cue presentation were withheld (response extinction training in a cocaine self-administration context), a significant increase in GluR2 protein expression was observed in CPu after response extinction training compared to extinction training in yoked-saline controls [43]. Others report that the level of GluR2 protein expression was greater in the NAc shell of rats receiving response extinction vs. abstinence training [16]. While many differences exist between our study and these previous studies, including the duration of the withdrawal period, single versus multiple extinction tests and GluR2 detection methods, these results highlight further the marked neurobiological changes in the brain following response vs. cue extinction training [44], [16] and [45].
Regulation of c-Fos protein expression in the amygdala following exposure to cocaine-paired cues has been shown to involve stimulation of D1 receptors [12] and activation of members of the ERK pathway [9] and [48]. The co-expression of c-Fos and GluR2 proteins in cortical brain areas following cocaine cue extinction training [10] in addition to the reciprocal relationship between c-Fos and GluR2 expression in the hippocampus following contextual fear extinction [49] support the candidacy of GluR2 as a potential partner regulating c-Fos protein expression. In the present study, enhanced c-Fos protein expression in the BLA and PL following cocaine-cue extinction training and the lack of a concomitant effect on GluR2 protein expression, would suggest that changes in GluR2 abundance does not drive the increased c-Fos expression under these conditions, although co-expression of GluR2 and c-Fos was not determined in this study. An additional limitation relates to the fact that immunocytochemistry was performed under permeabilized conditions; the results represent an overall level of GluR2 subunits of both the cell surface and intracellular compartments. The abundance of AMPA receptors at the cell surface is dynamically regulated by vesicle-mediated trafficking processes and is not always proportional to total AMPA receptor amount [48]. It is possible that during cocaine-cue extinction learning, changes occur only in AMPA receptor distribution between cell surface vs. cytosol, or synaptic vs. extrasynaptic domains. This change in receptor distribution may not be detected by monitoring total AMPA receptor amount. Unraveling the amount, activity, and subcellular targeting contribution of these trafficking pathways in cocaine-cue extinction learning may require detection based on other methods such as western blotting of subfractionated (synaptic versus non-synaptic vs. intracellular) tissue or biotinylation processes. Alternatively, it may be that other AMPA receptors, potentially the GluR1 subunit, is implicated in cue extinction learning following cocaine self-administration, as past research has shown that rapid loss of calcium permeable AMPA receptors (GluR1-containing) in BLA is associated with fear extinction learning [49] and [50].
There is a general assumption that cocaine-cue extinction may involve circuits and use mechanisms of synaptic plasticity similar to those of fear extinction. Even though the areas and, to an extent, the molecular mechanisms involved in fear extinction learning vary with the task employed, research in rats using pharmacological manipulations, correlative molecular studies and electrophysiology emphasize interactions among several brain regions, most notably the amygdala and IL in fear extinction learning [51]. The fact that cocaine-cue extinction training did not selectively enhance c-Fos protein expression in the IL in the present study suggests that the circuitry for fear extinction and cocaine-cue extinction may differ to some degree. This may be related to the use of aversive stimulation in the case of fear conditioning, which recruits the central amygdala that is densely connected to the IL [52], and the use of positive reinforcement in the case of cocaine conditioning, which recruits the PL [53]. Moreover, it is highly likely that the substrates identified as important for cocaine-cue extinction would extend to other drugs of abuse, particularly other psychostimulants, but future work is needed to confirm this point. In conclusion, further understanding of BLA and PL mechanisms underlying cocaine-cue extinction learning may assist in establishing viable strategies to increase the efficacy of exposure therapy for cocaine addiction, which contrary to expectation, is minimally effective as a stand-alone treatment modality [54].
Research Highlights.
Cocaine-cue extinction increased c-Fos expression in BLA and PL
Cocaine-cue and saline-cue extinction increased c-Fos expression in dSUB and IL
Number of lever responses correlated with c-Fos expression in NAc core and CPu
GluR2 expression was not altered after cocaine-cue extinction or control training
Understanding extinction mechanisms may improve exposure therapy in cocaine addicts
Acknowledgments
This study was supported by DA024315, DA024315-S1 and seed funding from the Boston University Center for Neuroscience. We thank Carolyn Kirkman, Angela J. Young and Marsha D. Guy for expert technical assistance.
Footnotes
Authors contribution
KK, BND, HM and GK were responsible for the study concept and design. BND, BL and NS contributed to the acquisition of animal data and c-Fos analysis. KL-M performed the GluR2 analysis. BND and KK performed data analysis and interpretation, and drafted the manuscript. GK, AL, HM, and KL-M provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47 (Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621–32. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nic Dhonnchadha BÁ, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, et al. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35(2):357–67. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nic Dhonnchadha BÁ, Pinard E, Alberati D, Wettstein JG, Spealman RD, Kantak KM. Inhibiting glycine transporter-1 facilitates cocaine-cue extinction and attenuates reacquisition of cocaine-seeking behavior. Drug Alcohol Depend. 2012;122(1–2):119–26. doi: 10.1016/j.drugalcdep.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torregrossa MM, Sanchez H, Taylor JR. D-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 2010;30(31):10526–33. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-cycloserine facilitates extinction of cocaine self-administration in rats. Synapse. 2011;65(9):938–44. doi: 10.1002/syn.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nic Dhonnchadha BÁ, Kantak KM. Cognitive enhancers for facilitating drug cue extinction: Insights from animal models. Pharmacol Biochem Behav. 2011;99(2):229–44. doi: 10.1016/j.pbb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan GB, Moore KA. The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav. 2011;99(2):217–28. doi: 10.1016/j.pbb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20(2):798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145(2):438–52. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, et al. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63(10):823–35. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98(4):1976–81. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann SG. Enhancing exposure-based therapy from a translational research perspective. Behav Res Ther. 2007;45(9):1987–01. doi: 10.1016/j.brat.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30(3):126–34. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Lin HC, Mao SC, Su CL, Gean PW. Alterations of excitatory transmission in the lateral amygdala during expression and extinction of fear memory. Int J Neuropsychopharmacol. 2010;13(3):335–45. doi: 10.1017/S1461145709990678. [DOI] [PubMed] [Google Scholar]
- 16.Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421(6918):70–5. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 17.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;518(24):10579–93. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77(1–2):23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 20.Bardo MT, Neisewander JL, Pierce RC. Novelty-induced place preference behavior in rats: effects of opiate and dopaminergic drugs. Pharmacol Biochem Behav. 1989;32(3):683–9. doi: 10.1016/0091-3057(89)90018-x. [DOI] [PubMed] [Google Scholar]
- 21.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–51. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 22.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29(4):802–12. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- 24.LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17(4):168–75. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halem HA, Cherry JA, Baum MJ. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J Neurobiol. 1999;39(2):249–63. [PubMed] [Google Scholar]
- 26.Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci. 2006;26(18):4852–9. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Sydney: Academic Press; 2008. [Google Scholar]
- 28.Leite-Morris KA, Fukudome EY, Shoeb MH, Kaplan GB. GABA(B) receptor activation in the ventral tegmental area inhibits the acquisition and expression of opiate-induced motor sensitization. J Pharmacol Exp Ther. 2004;308(2):667–78. doi: 10.1124/jpet.103.058412. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- 30.Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology. 1996;127(3):213–24. [PubMed] [Google Scholar]
- 31.Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87(2):139–48. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- 32.Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22(3):1126–36. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23(10):2809–13. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- 34.Mashhoon Y, Tsikitas LA, Kantak KM. Dissociable effects of cocaine-seeking behavior following D1 receptor activation and blockade within the caudal and rostral basolateral amygdala in rats. Eur J Neurosci. 2009;29(8):1641–53. doi: 10.1111/j.1460-9568.2009.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabriele A, See RE. Reversible inactivation of the basolateral amygdala, but not the dorsolateral caudate putamen, attenuates consolidation of cocaine-cue associative learning in a reinstatement model of drug-seeking. Eur J Neurosci. 2010;32(6):1024–9. doi: 10.1111/j.1460-9568.2010.07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szalay JJ, Morin ND, Kantak KM. Involvement of the dorsal subiculum and rostral basolateral amygdala in cocaine cue extinction learning in rats. Eur J Neurosci. 2011;33(7):1299–307. doi: 10.1111/j.1460-9568.2010.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szalay JJ, Hodges AB, Kantak KM. Neural regulation of the time course for cocaine cue extinction consolidation. College on Problems of Drug Dependence Annual Meeting; 2011. [Abstract # 704] [Google Scholar]
- 38.Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem. 2009;16(9):520–9. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- 39.Zavala AR, Browning JR, Dickey ED, Biswas S, Neisewander JL. Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. European Neuropsychopharmacology. 2008;18(8):600–11. doi: 10.1016/j.euroneuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25(39):8978–87. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eichenbaum H, Fagan A, Mathews P, Cohen NJ. Hippocampal system dysfunction and odor discrimination learning in rats: impairment or facilitation depending on representational demands. Behav Neurosci. 1988;102(3):331–9. doi: 10.1037//0735-7044.102.3.331. [DOI] [PubMed] [Google Scholar]
- 42.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14(6):784–95. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21(7):RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11(5):648–57. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8(2):212–9. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 47.Guedea AL, Schrick C, Guzman YF, Leaderbrand K, Jovasevic V, Corcoran KA, Tronson NC, Radulovic J. ERK-associated changes of AP-1 proteins during fear extinction. Mol Cell Neurosci. 2011;47(2):137–44. doi: 10.1016/j.mcn.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang G, Gilbert J, Man HY. AMPA receptor trafficking in homeostatic synaptic plasticity: functional molecules and signaling cascades. Neural Plast. 2012;2012:825364. doi: 10.1155/2012/825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao SC, Lin HC, Gean PW. Augmentation of fear extinction by infusion of glycine transporter blockers into the amygdala. Mol Pharmacol. 2009;76(2):369–78. doi: 10.1124/mol.108.053728. [DOI] [PubMed] [Google Scholar]
- 50.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330(6007):1108–12. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20(2):231–5. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinard CR, Mascagni F, McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience. 2012;205:112–24. doi: 10.1016/j.neuroscience.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamaterelease into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97(2):155–67. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]