Abstract
Background
We hypothesize that a therapy that improves LV pump function early after infarction should decrease the need for compensation through sympathetic activation and dilation, thereby reducing the risk of developing heart failure. The mechanical properties of healing myocardial infarcts are an important determinant of left ventricular (LV) function, yet improving function by altering infarct properties has proven unexpectedly difficult. Using a computational model, we recently predicted that stiffening a large anterior infarct anisotropically (in only one direction) would improve LV function, while isotropic stiffening, the focus of previous studies and therapies, would not. The goal of this study was to test the novel strategy of anisotropic infarct reinforcement.
Methods and Results
We tested the effects of anisotropic infarct reinforcement in 10 open-chest dogs with large anteroapical infarcts that depressed LV pump function. We measured regional mechanics, LV volumes, and cardiac output at a range of preloads at Baseline, 45 minutes after coronary ligation (Ischemia), and 30 minutes later, following surgical reinforcement in the longitudinal direction (Anisotropic). Ischemia shifted the end-systolic pressure-volume relationship (ESPVR) and cardiac output curves rightward, decreasing cardiac output at matched end-diastolic pressure (EDP) by 44%. Anisotropic reinforcement significantly improved systolic function without impairing diastolic function, recovering half the deficit in overall LV function.
Conclusions
We conclude that anisotropic reinforcement is a promising new approach to improving LV function following a large myocardial infarction.
Keywords: myocardial infarction, mechanics, cardiac output, surgery
Introduction
Each year, over one million Americans experience a myocardial infarction (MI).1 Despite current therapies, the risk of developing heart failure following MI remains substantial, particularly for older patients and those with reduced LV function.1, 2 Following infarction, the mechanical properties of healing myocardial infarcts are a critical determinant of both depression of pump function and LV remodeling,3 suggesting that it might be possible to improve pump function and/or reduce adverse post-infarction remodeling by manipulating infarct mechanical properties.
In fact, several novel post-infarction therapies that work at least in part by modifying infarct mechanics are now in development. Injecting polymers into the infarct has been reported to improve heart function and/or reduce remodeling,4–7 as have altering metalloproteinase activity pharmacologically8–12 or through electrical stimulation,13 application of surgical devices and meshes originally designed to arrest dilation in patients with advanced heart failure,14–16 and peri-infarct pacing.17–19
Depressed pump function and left ventricular (LV) dilation both contribute to the development of heart failure following infarction. Therefore, improving pump function and limiting LV dilation are both important therapeutic goals. These two effects are easily confused in studies that rely on ejection fraction (EF) as the primary indicator of function, since reducing LV volumes increases EF even if stroke volume does not change. By contrast, studies that report cardiac output curves over a range of filling pressures can separate effects on dilation and pump function. Polymer injection, surgical reinforcement, and computational modeling studies that report cardiac output curves consistently show that these approaches reduce or limit left ventricular (LV) dilation and subsequent functional deterioration, but do not improve LV pump function acutely.7, 15, 20, 21 We therefore sought to develop a complementary therapeutic approach that could improve LV pump function early after a large myocardial infarction.
Normal myocardium deforms in a complex three-dimensional pattern with each heartbeat,22–24 and healing myocardial infarcts can be highly anisotropic (having different mechanical properties in different directions).25, 26 By contrast, current strategies for therapeutically modifying infarct mechanical properties provide isotropic reinforcement. We recently reported that selectively increasing infarct stiffness in the longitudinal direction improved predicted pump function in a computational model of a canine heart with a large anteroapical infarct, while isotropic or circumferential reinforcement did not.27 In the present study, we tested this prediction experimentally in 10 dogs with large, acute anteroapical infarcts and significant depression of pump function by surgically reinforcing the infarct region in the longitudinal direction.
Methods
Surgical Preparation and Instrumentation
This study was approved by the University of Virginia Animal Care and Use Committee. 22 adult mongrel dogs of both sexes (body weight 23.3±3.6kg) were anesthetized with sodium pentobarbital (30mg/kg iv induction, 5mg/kg-hr maintenance), intubated, and ventilated with room air. A heating pad was used to maintain constant body temperature. A left thoracotomy was performed at the fifth intercostal space, the pericardium was opened and the heart suspended in a pericardial cradle. A snare made of cloth umbilical tape was placed around the inferior vena cava (IVC) to facilitate temporary IVC occlusion. The left anterior descending artery (LAD) was isolated and ligation suture was placed distal to the first diagonal.
A Millar pressure catheter (Mikro-Tip SPC-454D, Millar Instruments, Houston, Texas) was inserted through the left carotid artery to measure left ventricular pressure (LVP). A catheter in the femoral artery was connected to a fluid-filled Gould manometer to monitor arterial pressure. An ultrasonic flow probe (A20, Transonic Systems, Ithaca, NY) was placed around the ascending aorta (AO) to measure left ventricular stroke volume (SV). Ten sonomicrometer crystals (2T-36S-40-RS, Sonometrics, London, Ontario, Canada) were inserted into the LV midwall using a metal introducer. Six crystals were used to measure changes in the three axes of the LV (Figure 1A): anterior-posterior (AP), septal-lateral (SL), and base-apex (BA). Four additional crystals were placed in the anterior LV wall, in the region supplied by the LAD, to measure regional strains.
Figure 1.
A Diagram of sonomicrometer and patch placement; see text for details. B Photograph of modified Dacron patched sewed to the epicardial surface of the left ventricle. Black line on patch is aligned with the long axis of the LV; LAD occluder is visible near left edge of photo.
Experimental Protocol
After instrumentation, 2mg/kg propranolol and 0.2mg/kg atropine were administered to minimize reflex changes in heart rate and intrinsic myocardial contractility.28 Blockade was confirmed by test injection of 2.5μg/kg dobutamine. Baseline data were then recorded while preload was varied by temporary IVC occlusion (Baseline). The LAD was ligated between the first and second diagonal branches; arteries providing collateral flow to the lower 2/3 of the anterior wall were also ligated as needed to produce a large, dyskinetic ischemic region. Lidocaine (10 mg i.v.) was administered in 11 animals to control arrhythmias and defibrillation was required in 7. Hemodynamic and sonomicrometry data were recorded every 5 minutes following LAD occlusion. 45 minutes after LAD ligation, acute ischemia data were recorded during IVC occlusion (Ischemia).
Immediately following the Ischemia recording, the ischemic region was anisotropically reinforced by sewing a modified Dacron patch (Hemashield Knitted Double Velour Fabric, Maquet Cardiovascular LLC, Wayne, NJ) onto the epicardial surface of the heart over the ischemic area (Figure 1). The patches, typically used for LV aneurysm resection and repair,29 were modified by creating longitudinally oriented slits that allowed virtually unrestricted circumferential deformation in ex vivo mechanical tests, and were sewn to the epicardial surface under as much longitudinal tension as the surgeon felt could be applied without risking tearing of the sutures from the myocardium; this resulted in a remarkably consistent 11.4±2.9% reduction in longitudinal segment lengths in the center of the infarct. Following reinforcement, data were again acquired during IVC occlusion (Anisotropic, acquired 30.7±7.3 minutes after Ischemia). At the end of the experiment, animals were euthanized with an overdose of pentobarbital and hearts were excised for confirmation and imaging of sonomicrometer placement.
Data Analysis
Hemodynamic data were processed using the Sonometrics software, SonoSoft®, and custom Matlab software. To assess the state of LV function at each experimental condition, measurements were averaged over three to five cardiac cycles at constant preload. To analyze the response of the LV to preload changes, cycles covering the full range of pressure variation during IVC occlusion were analyzed and the resulting parameters fitted to obtain end-diastolic (EDPVR) and end-systolic (ESPVR) pressure-volume relationships as well as cardiac output curves.
For each cardiac cycle, end-diastole (ED) was defined as the point immediately before the sharp increase in LV pressure and end-systole (ES) was identified as the point of maximal elastance.30 LV volumes were computed from AP, SL, and BA axis lengths, assuming a truncated ellipsoidal geometry.30 Because sonomicrometers were located in the LV midwall, these volumes included both cavity volume and approximately half the LV wall volume, which is essentially constant over the cardiac cycle. Stroke volume was computed by integrating the AO flow signal as described and validated by Steingart, neglecting retrograde (coronary) flow.31 At baseline, stroke volumes computed by subtracting sonomicrometer-derived EDV and ESV values (SVsono) agreed with stroke volume computed by integrating the AO flow signal (SVflow). During ischemia, SVsono was less accurate due to systolic bulging of the ischemic region. Therefore, we report here only SVflow and compute ESV as EDV – SVflow.
ESPVR, EDPVR, and cardiac output curves for each condition were fitted and used to interpolate data for comparison at matched pressures. The linear ESPVR was obtained by fitting a straight line to ESP vs. ESV data (mean±SD R2 for fits to data from individual animals = 0.78±0.23), the exponential EDPVR and CO curves by fitting a straight line to ln(EDP) vs. EDV or CO data (R2 0.90±0.09 for EDPVR, 0.88±0.12 for CO curve). Interpolated volume and cardiac output values at matched pressures were also averaged to construct mean EDPVR, ESPVR, and CO curves. To assess changes in LV shape, we fitted ln(EDP) vs. ED segment length curves and compared interpolated BA, AP, and SL dimensions at matched pressures.
Regional strains were computed from three or more segment lengths measured by the 4 sonomicrometers in the center of the ischemic region. Post-mortem photographs were used to construct unit vectors describing the orientation of each crystal pair relative to the short axis of the LV. Real-time segment lengths were used to compute two-dimensional systolic regional strains reflecting circumferential shortening/stretching, longitudinal shortening/stretching, and shearing in the anterior midwall from end diastole to end systole.32 Segment lengths interpolated at matched pressures from linear fits of ln(EDP) vs. ED segment length data were used to compute remodeling strains reflecting changes in end-diastolic configuration across different states (Baseline, Ischemia, Anisotropic).
Statistical Analysis
The goal of this study was to test the model prediction that longitudinal reinforcement can mitigate acute depression of pump function following a large antero-apical infarction. Because this hypothesis can only be tested if pump function is depressed, we used depression of pump function as reflected by a rightward shift in the Ischemia cardiac output curve as an inclusion criterion for this study. Dogs with no shift in cardiac output curve during ischemia were excluded from further analysis. In the remaining animals, parameters with independent numerical values at each state (Baseline, Ischemia, Anisotropic) were analyzed by one-way repeated-measures ANOVA, with Newman-Keuls posthoc comparisons when the ANOVA showed significant variation among the groups. For parameters that were zero at baseline by definition, such as remodeling strains and changes in volumes, we tested the effect of ischemia using a one-sample t-test against a hypothetical mean of zero, and the effect of anisotropic reinforcement by paired two-tail t-test of Anisotropic vs. Ischemia data. A P value < 0.05 was used as the threshold for statistical significance for all tests.
Results
A total of 22 dogs underwent LAD ligation for this study. Of these, 10 animals showed a shift in the ventricular function curve (cardiac output vs. end-diastolic pressure) 45 minutes after LAD ligation and were included in the study. Of the remaining 12 animals, 3 died of ventricular fibrillation following LAD ligation, 8 showed no shift in the ventricular function curve following LAD ligation, and 1 was excluded due to failure of the data acquisition system during the study.
Hemodynamics
Heart rate and maximum dP/dt did not vary during the study, demonstrating effective blockade of sympathetic and parasympathetic reflex changes (Table 1). Since the animals were unable to compensate for reduced pump function by increasing heart rate or intrinsic contractility, they relied on the Frank-Starling mechanism, maintaining stroke volume (SV) and cardiac output (CO) by significantly increasing end-diastolic pressure (EDP) and volume (EDV).
Table 1.
Hemodynamic Parameters
| Parameter | Baseline | Ischemia | Anisotropic |
|---|---|---|---|
| EDP (mmHg) | 11.3±4.3 | 17.3±6.1† | 15.9±7.5 |
| EDV* (ml) | 78.2±15.5 | 89.0±18.0† | 82.2±16.2‡ |
| ESP (mmHg) | 96.1±14.8 | 94.7±14.3 | 92.2±9.7 |
| ESV* (ml) | 62.7±13.5 | 75.0±17.7† | 66.85±15.4‡ |
| SV (ml) | 15.5±3.8 | 14.0±3.9 | 15.4±3.5 |
| HR (bpm) | 108±10 | 109±15 | 111±15 |
| CO (L/min) | 1.69±0.51 | 1.54±0.54 | 1.70±0.44 |
| max dP/dt (mmHg/s) | 1227±337 | 1220±431 | 1342±249 |
Hemodynamic data from 10 dogs that showed a shift in cardiac output curve 45 minutes after coronary ligation. Dogs were pretreated with propranolol and atropine to prevent reflex changes in heart rate and intrinsic myocardial contractility; by design, end-systolic pressure (ESP), heart rate (HR), and maximum dP/dt did not vary significantly during the experiment. During Ischemia, animals compensated for reduced systolic function via the Frank-Starling mechanism, increasing end-diastolic pressure (EDP) and volume (EDV) to maintain stroke volume (SV) and cardiac output (CO) despite increased end-systolic volume (ESV). Anisotropic surgical reinforcement significantly reduced EDV and ESV.
Midwall volumes include cavity volume and approximately half the LV wall volume.
P<0.001 vs. baseline;
P<0.001 vs. ischemia.
Mechanics of Acute Ischemia and Anisotropic Reinforcement
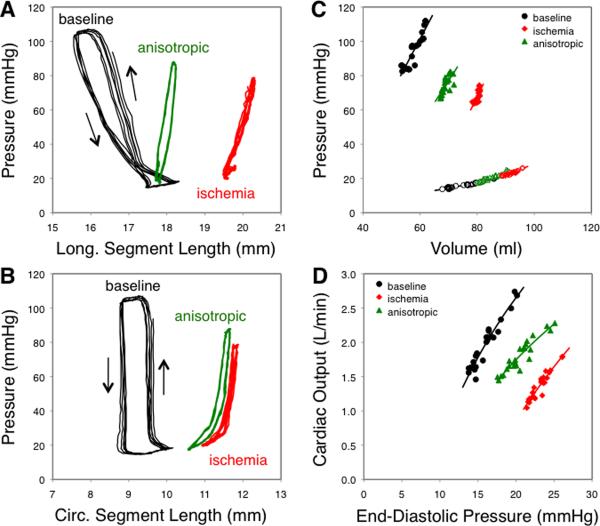
Figure 2 illustrates the impact of ischemia and anisotropic reinforcement on regional and global function in a single animal. At baseline, pressure-segment length loops indicated active contraction in the anterior wall (Figure 2A,B). Hemodynamic data acquired during IVC occlusion produced the expected exponential EDPVR, linear end-ESPVR, and relatively steep working portion of the cardiac output curve (Figure 2C,D). Forty-five minutes after LAD ligation, pressure-segment loops indicated passive lengthening rather than active contraction, the ESPVR was shifted rightward, and the ventricular function curve was shifted down and to the right, with a >50% reduction in cardiac output at matched EDP. Anisotropic surgical reinforcement selectively reduced longitudinal segment lengths (Figure 2A), shifted the ESPVR back to the left without affecting the EDPVR, and reversed about half the shift in the cardiac output curve.
Figure 2.
Effect of ischemia and anisotropic reinforcement in a single animal. A,B Infarct region pressure-segment length data. At baseline (black), loops indicated active contraction in both the longitudinal (A) and circumferential (B) directions. During ischemia (red), both segments demonstrated passive stretch and recoil along a single curve. Anisotropic reinforcement (green) reduced longitudinal segment length throughout the cardiac cycle. C Ischemia shifted the end-systolic pressure-volume relationship (ESPVR, closed symbols) rightward and anisotropic reinforcement returned ESPVR towards baseline, with little effect on the end-diastolic relationship (EDPVR, open symbols). Note that volumes are midwall volumes, including both cavity volume and approximately half the LV wall volume. D Cardiac output curves shifted down and to the right with ischemia and returned towards baseline with anisotropic reinforcement.
Effect of Anisotropic Reinforcement on Regional Mechanics and LV Shape
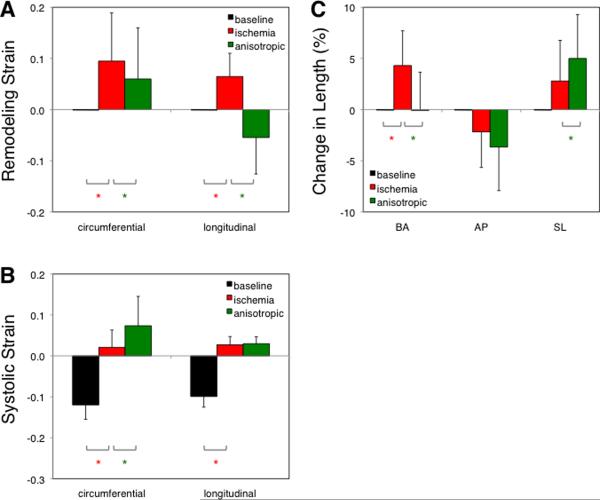
Remodeling strains in the center of the ischemic region showed increased circumferential and longitudinal dimensions at a matched EDP of 12 mmHg during Ischemia compared to Baseline (Figure 3A). Anisotropic reinforcement reversed longitudinal remodeling (P < 0.001 vs. Ischemia), while modestly reducing circumferential remodeling (P < 0.01). Systolic strains were negative at Baseline, indicating circumferential and longitudinal shortening during contraction (Figure 3B). Acute ischemia abolished shortening in both directions (P < 0.001 vs. Baseline), and anisotropic reinforcement slightly increased systolic circumferential stretching without affecting longitudinal strain. Globally, ischemia increased the base-apex (BA) dimension significantly (Figure 3C) but did not induce significant changes in either short-axis dimension, while anisotropic reinforcement restored BA length to baseline and increased the septal-lateral (SL) dimension.
Figure 3.
Effect of ischemia and anisotropic reinforcement on regional strains and LV dimensions. A Remodeling strains indicating changes in end-diastolic dimensions at a matched EDP of 12 mmHg are zero at baseline by definition. Ischemia increased both circumferential and longitudinal dimensions relative to baseline, while anisotropic reinforcement dramatically reduced longitudinal remodeling. B Systolic strains indicating deformation from end diastole to end systole are negative at baseline, indicating systolic shortening, and positive during ischemia, indicating systolic stretching. C Ischemia increased average base-apex (BA) dimensions at a matched end-diastolic pressure of 12 mmHg without significantly affecting anterior-posterior (AP) or septal-lateral (SL) dimensions. Anisotropic reinforcement restored BA to baseline and significantly increase SL. *P<0.05 for comparison indicated.
Effect of Anisotropic Reinforcement on Systolic and Diastolic Function
Mean EDPVR and ESPVR curves showed clearly that ischemia shifted the ESPVR rightward and anisotropic reinforcement partially reversed this shift with little effect on the EDPVR (Figure 4A). We quantified shifts in pressure-volume behavior using volumes interpolated from individual ESPVR and EDPVR curves at identical pressures for all animals. As expected from the average curves, end-systolic volume at a pressure of 85 mmHg (ESV85) increased significantly with ischemia (Figure 4B), while end-diastolic volume at a pressure of 12 mmHg (EDV12) did not change. Anisotropic reinforcement recovered more than half of the change in ESV85 (P < 0.01 vs. ischemia) without affecting EDV12.
Figure 4.
Effect of ischemia and anisotropic reinforcement on average pressure-volume curves. A Ischemia shifted the end-systolic pressure-volume relationship (ESPVR, closed symbols) rightward and anisotropic reinforcement returned ESPVR towards baseline, with little effect on the end-diastolic relationship (EDPVR, open symbols). B Comparing volumes interpolated at matched pressures (dashed lines in Panel A) showed no change in end-diastolic volume at 12 mmHg (EDV12), but a significant increase in end-systolic volume at 85 mmHg (EDV85) with ischemia and significant reduction of EDV85 with anisotropic reinforcement. Note that volumes are midwall volumes, including both cavity volume and approximately half the LV wall volume. *P<0.05 for comparison indicated.
Effect of Anisotropic Reinforcement on Pump Function
A shift in cardiac output curve during ischemia was an inclusion criterion for this study. Comparing the mean Baseline and Ischemia curves, average CO at a matched EDP of 12 mmHg dropped 44%, and a 50% increase in EDP to 18 mmHg would have been required to return to baseline CO (Figure 5A). Using the baseline EDP in each individual animal and interpolating to find CO at matched pressures in the other states confirmed a significant reduction in CO with ischemia (P < 0.01, Figure 5B). Anisotropic reinforcement recovered more than half the deficit in cardiac output (P < 0.05).
Figure 5.
Effect of ischemia and anisotropic reinforcement on average cardiac output curves. A Cardiac output curves shifted down and to the right with ischemia and returned towards baseline with anisotropic reinforcement. B Comparing cardiac output interpolated at matched end-diastolic pressures (baseline EDP) in each animal showed a significant drop in cardiac output with ischemia and recovery of 50% of the deficit with anisotropic reinforcement. *P<0.05 for comparison indicated.
Discussion
Acute myocardial infarcts stretch and bulge during systole, wasting contractile energy expended by remaining non-infarcted myocardium and reducing overall pump function. The changes are reflected in a rightward shift in both the end-systolic pressure-volume relationship (ESPVR) and cardiac output curve (Figures 4, 5) – for any level of preload, the heart is able to generate less pressure and eject less stroke volume. Intuition suggests that stiffening these acute infarcts should reduce systolic stretching of the infarct and improve function. Yet computational modeling and experimental studies have consistently shown that while a stiffer infarct improves systolic function, it also impairs diastolic function, shifting not only the ESPVR but also the end-diastolic pressure-volume relationship (EDPVR) to the left. The systolic and diastolic effects offset, producing no net improvement in LV function.15, 20, 21, 33
Until recently, such studies considered only isotropic stiffening (increasing the stiffness equally in all directions). However, the complex three-dimensional nature of deformation in the heart wall22–24 and the fact that healing myocardial infarcts can be highly anisotropic in some animal models25, 26 led us to consider whether anisotropic stiffening or reinforcement might be a better strategy. We used a computational model to ask what infarct mechanical properties would provide the best pump function following a large anterior infarction, and found that the combination of high stiffness in the longitudinal direction and low stiffness in the circumferential direction provided the best predicted function.27 Infarct anisotropy appeared to circumvent the trade-off between systolic and diastolic function, improving systolic function with little impact on diastolic function. In the present study, we tested this model prediction by surgically reinforcing acute anteroapical infarcts in dogs with post-infarction depression of LV pump function. We found that anisotropic reinforcement in these animals did in fact improve systolic function with little effect on filling, recovering half of the deficit in the cardiac output curve associated with acute ischemia. We conclude that anisotropic reinforcement is a novel and promising approach to improving LV function following a large myocardial infarction.
Effects of Anisotropic Reinforcement
Globally, anisotropic reinforcement improved systolic function without impairing diastolic filling. Circumferential systolic stretching in the infarct actually increased following reinforcement, suggesting that simply reducing systolic dyskinesis is not the primary mechanism for improved systolic function. Rather, our prior modeling study and current data suggest that anisotropic reinforcement re-shapes the heart at end diastole (ED). Comparing remodeling strains and global dimensions at matched EDP (Figures 3A,C), we found that ischemia not only induced acute circumferential and longitudinal expansion of the infarct, but also increased the base-apex dimension (BA) of the LV significantly. Anisotropic reinforcement reversed longitudinal expansion in the infarct and returned BA to baseline. Since reinforcement restricted longitudinal expansion during diastole but did not reduce diastolic volumes, we would expect increased stretching along at least one other axis, and we in fact saw a significant increase in the ED septal-lateral (SL) dimension after patch application. Therefore, anisotropic reinforcement may increase preload in circumferentially oriented remote myocardium. Additional studies are clearly required to better understand the impact of anisotropic reinforcement on border and remote myocardium, as well as transmural variations in regional strains following reinforcement (this study measured midwall strains).
Therapeutic Options Following Myocardial Infarction
Following a large myocardial infarction, sympathetic activation increases myocardial contractility and heart rate, and elevated filling pressures increase diastolic volume and sarcomeric pre-stretch, enhancing contraction via the Frank-Starling mechanism. While sympathetic activation and LV dilation help preserve cardiac output acutely, they are also the initial steps in the pathogenesis of heart failure. Standard post-infarction therapy therefore includes beta blockers to counteract detrimental effects of chronic sympathetic activation and angiotension converting enzyme (ACE) inhibitors to slow LV remodeling.34 However, standard therapy tends to delay rather than prevent the development of failure, particularly in patients with large infarcts, and the prognosis for patients with heart failure is bleak.35 Several groups have therefore explored more aggressive approaches to preventing LV dilation, applying therapies originally developed for heart failure immediately after infarction to prevent LV dilation and functional deterioration rather than waiting to address them until they occur. Gorman and colleagues showed that early application of cardiac support devices limits dilation and functional deterioration.36 Several groups reported similar results with early post-infarction injection of polymers.4–7 Shuros et al. adapted biventricular pacing, originally developed for heart failure, to a strategy termed `peri-infarct pacing' for use early after myocardial infarction. By pre-exciting the border zone to reduce regional stroke work, they were able to attenuate post-infarction LV remodeling.19 Smalling and colleagues have even explored the potential of LV unloading with LVADs and aortic balloon pumps during initial reperfusion to decrease infarct size.37, 38
These efforts all seek to limit LV dilation and remodeling earlier in the course of post-infarction healing but do not provide immediate improvements in LV pump function. We sought to develop a complementary strategy for improving LV function early after infarction. We hypothesize that a therapy that improves LV pump function early after infarction should decrease the need for compensation through sympathetic activation and dilation, thereby reducing the risk of developing heart failure. The present study is exciting as a proof-of-concept that it is possible to improve LV pump function immediately after infarction by appropriately manipulating infarct mechanical properties. Chronic studies are required to determine whether this acute improvement in pump function will in fact reduce long-term dilation and development of heart failure. Our approach shares the goal of improving LV pump function early after infarction with regenerative approaches such as stem cell injection that aim to restore functioning muscle to the infarct region. However, much of the remodeling that predisposes to heart failure occurs in the first few days, while most regenerative approaches will require weeks to fully implement. We therefore propose that mechanical reinforcement early after infarction has significant potential not only as a stand-alone therapy but also as an adjunct to regenerative approaches.
Limitations
The goal of this study was to test the hypothesis that longitudinal reinforcement can improve depressed pump function acutely in dogs with large anteroapical infarcts. Because sympathetic activation can temporarily compensate for depressed pump function early after myocardial infarction, we used pharmacologic blockade to prevent changes in heart rate and intrinsic myocardial contractility.28 This approach allowed us to recognize and quantify changes in pump function through shifts in the cardiac output curve; it also facilitated comparisons with computational models, which typically hold intrinsic myocardial contractility and heart rate constant. However, additional studies are clearly required to test our hypothesis that anisotropic reinforcement will alter post-infarction sympathetic activation and remodeling when neurohumoral reflexes are intact.
Computational and experimental studies have consistently found that isotropic infarct reinforcement or stiffening does not improve LV function acutely,7, 15, 20, 21 while our data show that anisotropic reinforcement does improve LV function acutely in this animal model. We did not compare isotropic against anisotropic reinforcement in this study; additional studies would be needed to test definitively whether anisotropic reinforcement improves acute LV function more than isotropic reinforcement. In addition, for this proof-of-concept study we reinforced the infarct by sewing a modified Dacron patch to the epicardial surface. Clinically, sewing a modified or specially manufactured patch to the epicardial surface could be feasible in the subset of patients who undergo surgical revascularization early after infarction, but developing a minimally invasive approach to anisotropic reinforcement would extend its potential use to a much larger patient population.
Approximately one third of the animals entering this study were excluded because there was no evidence of altered pump function following infarction. Some of this variability among animals was likely due to variations in infarct size. Ligating the same artery in the same place can produce a wide range of infarct sizes in different dogs due to an extensive and variable network of coronary collaterals. Although we ligated collaterals where apparent, it is likely that infarct size varied substantially among animals, and that animals with small infarcts showed less depression of pump function. In addition, although changes in other hemodynamic parameters with ischemia were similar between the included and excluded groups, dP/dt increased significantly only in the excluded group, suggesting that pharmacologic blockade might have been incomplete in some of these animals.
Conclusions
In summary, we tested the model prediction that longitudinal reinforcement of acute canine anteroapical infarcts can acutely improve depressed LV pump function. Anisotropic reinforcement improved systolic function without impairing filling, restoring half the deficit in the cardiac output curve associated with acute ischemia. We conclude that anisotropic reinforcement is a novel and promising approach to improving LV function following a large myocardial infarction. The next step in the development of this potential therapy is to conduct chronic animal studies to evaluate the impact of anisotropic reinforcement on LV function, remodeling, and the development of heart failure.
Clinical Perspective.
Following myocardial infarction, both depressed pump function and gradual dilation and remodeling of the left ventricle contribute to the development of heart failure. Although it seems logical that mechanically reinforcing the healing infarct should improve pump function, multiple studies have found that mechanical therapies such as surgical reinforcement and polymer injection can prevent dilation, but do not directly improve pump function. Typically, stiffening the infarct improves systolic function but impairs filling, producing no net benefit on cardiac output. Importantly, these prior studies used methods that stiffen or reinforce the infarct similarly in all directions (isotropically). In the current study, we tested a new approach – reinforcing infarcts in only one direction (anisotropically) – in dogs with large anteroapical infarcts, and found that anisotropic reinforcement can improve systolic function without impairing filling, significantly enhancing cardiac output curves. Long-term studies are now needed to determine whether anisotropic reinforcement early after infarction improves pump function enough to reduce the subsequent risk of heart failure.
Acknowledgments
The authors wish to acknowledge the surgical assistance of N. Craig Goodman.
Funding Sources University of Virginia Coulter Translational Research Program (GA, JWH); NIH/NHLBI R01 HL-075639 (JWH).
Footnotes
Conflict of Interest Disclosures None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol. 2003;42:1446–1453. doi: 10.1016/s0735-1097(03)01057-x. [DOI] [PubMed] [Google Scholar]
- 3.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 4.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 5.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee R, Zavadzkas JA, Saunders SM, McLean JE, Jeffords LB, Beck C, Stroud RE, Leone AM, Koval CN, Rivers WT, Basu S, Sheehy A, Michal G, Spinale FG. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann Thorac Surg. 2008;86:1268–1276. doi: 10.1016/j.athoracsur.2008.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan LP, Matsuzaki K, Noma M, Jackson BM, Eperjesi TJ, Plappert TJ, St John-Sutton MG, Gorman JH, 3rd, Gorman RC. Dermal filler injection: a novel approach for limiting infarct expansion. Ann Thorac Surg. 2009;87:148–155. doi: 10.1016/j.athoracsur.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006;48:15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 9.Lindsey ML, Gannon J, Aikawa M, Schoen FJ, Rabkin E, Lopresti-Morrow L, Crawford J, Black S, Libby P, Mitchell PG, Lee RT. Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation. 2002;105:753–758. doi: 10.1161/hc0602.103674. [DOI] [PubMed] [Google Scholar]
- 10.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 11.Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation. 2003;108:1487–1492. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 12.Yarbrough WM, Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Escobar GP, Joffs C, Lucas DG, Crawford FA, Jr., Spinale FG. Matrix metalloproteinase inhibition modifies left ventricular remodeling after myocardial infarction in pigs. J Thorac Cardiovasc Surg. 2003;125:602–610. doi: 10.1067/mtc.2003.197. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee R, Rivers WT, Ruddy JM, Matthews RG, Koval CN, Plyler RA, Chang EI, Patel RK, Kern CB, Stroud RE, Spinale FG. Long-term localized high-frequency electric stimulation within the myocardial infarct: effects on matrix metalloproteinases and regional remodeling. Circulation. 2010;122:20–32. doi: 10.1161/CIRCULATIONAHA.110.936872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enomoto Y, Gorman JH, 3rd, Moainie SL, Jackson BM, Parish LM, Plappert T, Zeeshan A, St John-Sutton MG, Gorman RC. Early ventricular restraint after myocardial infarction: extent of the wrap determines the outcome of remodeling. Ann Thorac Surg. 2005;79:881–887. doi: 10.1016/j.athoracsur.2004.05.072. discussion 881–887. [DOI] [PubMed] [Google Scholar]
- 15.Kelley ST, Malekan R, Gorman JH, 3rd, Jackson BM, Gorman RC, Suzuki Y, Plappert T, Bogen DK, Sutton MG, Edmunds LH., Jr. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation. 1999;99:135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- 16.Liao SY, Siu CW, Liu Y, Zhang Y, Chan WS, Wu EX, Wu Y, Nicholls JM, Li RA, Benser ME, Rosenberg SP, Park E, Lau CP, Tse HF. Attenuation of left ventricular adverse remodeling with epicardial patching after myocardial infarction. J Card Fail. 2010;16:590–598. doi: 10.1016/j.cardfail.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Chung ES, Dan D, Solomon SD, Bank AJ, Pastore J, Iyer A, Berger RD, Franklin JO, Jones G, Machado C, Stolen CM. Effect of peri-infarct pacing early after myocardial infarction: results of the prevention of myocardial enlargement and dilatation post myocardial infarction study. Circ Heart Fail. 2010;3:650–658. doi: 10.1161/CIRCHEARTFAILURE.110.945881. [DOI] [PubMed] [Google Scholar]
- 18.Chung ES, Menon SG, Weiss R, Schloss EJ, Chow T, Kereiakes DJ, Mazur W, Salo RW, Galle E, Pastore JM. Feasibility of biventricular pacing in patients with recent myocardial infarction: impact on ventricular remodeling. Congest Heart Fail. 2007;13:9–15. doi: 10.1111/j.1527-5299.2007.05868.x. [DOI] [PubMed] [Google Scholar]
- 19.Shuros AC, Salo RW, Florea VG, Pastore J, Kuskowski MA, Chandrashekhar Y, Anand IS. Ventricular preexcitation modulates strain and attenuates cardiac remodeling in a swine model of myocardial infarction. Circulation. 2007;116:1162–1169. doi: 10.1161/CIRCULATIONAHA.107.696294. [DOI] [PubMed] [Google Scholar]
- 20.Bogen DK, Rabinowitz SA, Needleman A, McMahon TA, Abelmann WH. An analysis of the mechanical disadvantage of myocardial infarction in the canine left ventricle. Circulation Research. 1980;47:728–741. doi: 10.1161/01.res.47.5.728. [DOI] [PubMed] [Google Scholar]
- 21.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 22.Arts T, Costa KD, Covell JW, McCulloch AD. Relating myocardial laminar architecture to shear strain and muscle fiber orientation. Am J Physiol Heart Circ Physiol. 2001;280:H2222–2229. doi: 10.1152/ajpheart.2001.280.5.H2222. [DOI] [PubMed] [Google Scholar]
- 23.Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am J Physiol. 1999;276:H595–607. doi: 10.1152/ajpheart.1999.276.2.H595. [DOI] [PubMed] [Google Scholar]
- 24.Takayama Y, Costa KD, Covell JW. Contribution of laminar myofiber architecture to load-dependent changes in mechanics of LV myocardium. Am J Physiol Heart Circ Physiol. 2002;282:H1510–1520. doi: 10.1152/ajpheart.00261.2001. [DOI] [PubMed] [Google Scholar]
- 25.Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LH, Jr., Bogen DK. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 1994;89:2315–2326. doi: 10.1161/01.cir.89.5.2315. [DOI] [PubMed] [Google Scholar]
- 26.Holmes JW, Nunez JA, Covell JW. Functional implications of myocardial scar structure. Am J Physiol Heart Circ Physiol. 1997;272:H2123–2130. doi: 10.1152/ajpheart.1997.272.5.H2123. [DOI] [PubMed] [Google Scholar]
- 27.Fomovsky GM, Macadangdang JR, Ailawadi G, Holmes JW. Model-based design of mechanical therapies for myocardial infarction. J Cardiovasc Transl Res. 2011;4:82–91. doi: 10.1007/s12265-010-9241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little WC, O'Rourke RA. Effect of regional ischemia on the left ventricular endsystolic pressure-volume relation in chronically instrumented dogs. J Am Coll Cardiol. 1985;5:297–302. doi: 10.1016/s0735-1097(85)80050-4. [DOI] [PubMed] [Google Scholar]
- 29.Dor V, Sabatier M, Di Donato M, Maioli M, Toso A, Montiglio F. Late hemodynamic results after left ventricular patch repair associated with coronary grafting in patients with postinfarction akinetic or dyskinetic aneurysm of the left ventricle. J Thorac Cardiovasc Surg. 1995;110:1291–1299. doi: 10.1016/S0022-5223(95)70052-8. discussion 1300-1291. [DOI] [PubMed] [Google Scholar]
- 30.Holmes JW. Candidate mechanical stimuli for hypertrophy during volume overload. Journal of Applied Physiology. 2004;97:1453–1460. doi: 10.1152/japplphysiol.00834.2003. [DOI] [PubMed] [Google Scholar]
- 31.Steingart RM, Meller J, Barovick J, Patterson R, Herman MV, Teichholz LE. Pulsed doppler echocardiographic measurement of beat-to-beat changes in stroke volume in dogs. Circulation. 1980;62:542–548. doi: 10.1161/01.cir.62.3.542. [DOI] [PubMed] [Google Scholar]
- 32.Villarreal FJ, Waldman LK, Lew WYW. Technique for measuring regional two-dimensional finite strains in canine left ventricle. Circulation Research. 1988;62:711–721. doi: 10.1161/01.res.62.4.711. [DOI] [PubMed] [Google Scholar]
- 33.Janz RF, Waldron RJ. Predicted effect of chronic apical aneurysms on the passive stiffness of the human left ventricle. Circulation Research. 1978;42:255–263. doi: 10.1161/01.res.42.2.255. [DOI] [PubMed] [Google Scholar]
- 34.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Jr., Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210–247. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorman RC, Jackson BM, Burdick JA, Gorman JH. Infarct restraint to limit adverse ventricular remodeling. J Cardiovasc Transl Res. 2011;4:73–81. doi: 10.1007/s12265-010-9244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achour H, Boccalandro F, Felli P, Amirian J, Uthman M, Buja M, Smalling RW. Mechanical left ventricular unloading prior to reperfusion reduces infarct size in a canine infarction model. Catheter Cardiovasc Interv. 2005;64:182–192. doi: 10.1002/ccd.20271. [DOI] [PubMed] [Google Scholar]
- 38.LeDoux JF, Tamareille S, Felli PR, Amirian J, Smalling RW. Left ventricular unloading with intra-aortic counter pulsation prior to reperfusion reduces myocardial release of endothelin-1 and decreases infarction size in a porcine ischemia-reperfusion model. Catheter Cardiovasc Interv. 2008;72:513–521. doi: 10.1002/ccd.21698. [DOI] [PubMed] [Google Scholar]