Abstract
Mesenchymal stem cells (MSCs) are potent immunoregulators and have shown clinical utility in suppressing immunity. MSC function is modulated by cytokines, since inflammatory cytokines, such as IFNγ concomitant with TNFα, induce their immunoregulatory capability. Here, we show that IFNγ and TNFα act synergistically to induce high levels of expression of IL-6 and several other immune-related molecules in MSCs in vitro. We further found that, while either IFNγ or TNFα alone induced minor expression of C/EBPβ in MSCs, this transcription factor was dramatically upregulated when these cytokines were added together. A causal relationship between C/EBPβ upregulation and IL-6 expression was demonstrated by siRNA knockdown of C/EBPβ. C/EBPβ knockdown also inhibited the synergistic expression of CXCL1, iNOS, and CCL5 in response to concomitant IFNγ and TNFα . We conclude that C/EBPβ is a key transcription factor in synergistic gene upregulation by IFNγ and TNFα Importantly, C/EBPβ similarly mediated synergistic gene induction in response to IFNγ accompanied by IL-1β or LPS, suggesting that synergy between IFNγ and other stimuli share C/EBPβ as common mechanism. Furthermore, while STAT1 is critical in IFNγ signaling, we found that STAT1 knockdown in MSCs did not affect C/EBPβ expression or the synergistic induction of IL-6 and CXCL1 by IFNγ and TNFα. Thus, C/EBPβ is not regulated by STAT1. These results demonstrate the importance of cytokine interactions in MSC immunobiology, a better understanding of which will allow improved clinical application of these cells.
Keywords: C/EBP beta, Mesenchymal stem cells, IFN-γ, TNF
Introduction
Bone marrow-derived mesenchymal stem cells (MSCs) can differentiate into multiple lineages such as osteoblasts, chondrocytes, adipocytes, and hematopoiesis-supportive stroma (1, 2). These MSCs have recently been shown to have a strong immunoregulatory capability (3), a function that has already been harnessed medically to treat some conditions including severe graft-versus-host disease (4, 5). We have recently reported that this immunomodulatory effect is induced by specific combinations of inflammatory cytokines, such as IFNγ and TNFα, or IFNγ and IL-1 (6).
Under pathophysiological conditions, multiple cytokines are present in the local microenvironment of damaged sites. The combined effect of these cytokines on various cells is integral to the initiation and termination of immune processes. Therefore, it is important to understand the interactions between these cytokines. Among them, the pleiotropic cytokines IFNγ and TNFα are critical during many inflammatory processes. These cytokines are increased in many disease states and play a critical role in the etiology of several diseases (7, 8). While either IFNγ or TNFα alone can exert multiple biological activities independently, they have also been shown to act synergistically to induce gene expression in various cells (9, 10). In fact, IFNγ and TNFα have been shown to synergize in regulating function and gene expression in bone marrow-derived MSCs (6, 11). Little is known, however, about the mechanism underlying these effects.
In the present study, we examined the molecular mechanism that mediates the synergistic response to IFNγ and TNFα in gene expression by MSCs. Individually, IFNγ (12) and TNFα (13) are reported to regulate the activity of C/EBPβ, a well-known transcription factor for IL-6 (14). We found that IL-6 production is also highly upregulated by MSCs in response to IFNγ concomitant with TNFα. Therefore, we asked whether C/EBPβ is involved in the synergistic upregulation of IL-6 by these cytokines. We found that knockdown of C/EBPβ dramatically reduced IL-6 expression in MSCs treated with IFNγ and TNFα. Similarly, we found that C/EBPβ is involved in the expression of other genes induced by IFNγ and TNFα, such as CCL5, iNOS and CXCL1. Thus, C/EBPβ is a key transcription factor through which IFNγ and TNFα act synergistically on MSCs. We show this is also true for responses to IFNγ concomitant with IL-1β or LPS. Therefore, C/EBPβ is a common molecule in the synergy between IFNγ and several other inflammatory stimuli in the modulation of MSC function. These new findings about the biology of MSCs in the inflammatory environmentare expected to lead to better clinical application of MSCs.
Materials and Methods
Reagents, antibodies and mice
C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed under specific pathogen-free conditions in the animal facility at Robert Wood Johnson Medical School, accredited by American Association for Accreditation of Laboratory Animal Care. All animal use was approved by the Institutional Animal Care and Use Committee. Antibodies against C/EBPβ, STAT1 and β-actin were from Cell Signaling Technology (Danvers, MA).
Derivation of MSC from bone marrow
Bone marrow was flushed from the tibia and femur bones isolated from 6 to 10-week-old C57BL/6 mice. Cells were filtered through a 40 μm nylon mesh then plated in 25 cm2 tissue culture flasks in complete medium, consisting of RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 2 mM glutamine, and 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). All non-adherent cells were removed after 24 hours and the medium was replenished every three days. MSC clones were prepared as described previously (6, 15). These cells are capable of differentiating into adipocytes and osteocytes under corresponding differentiating conditions (6, 15). For all experiments, cloned MSCs from before the 15th passage were used.
RNA interference
siRNAs specific for mouse C/EBPβ or STAT1, and control siRNA with scrambled sequences, were purchased from Dharmacon (Lafayette, CO). In each transfection, 5ul siRNAs (100pM) were introduced into MSCs (1.5×106 cells) using the Amaxa Nucleofector device, with program U-023 and Nucleofector kit V (Amaxa, Cologne, Germany). The efficiency of transduction was determined for a control plasmid containing eGFP cDNA (Pmax, part of Nucleofector kit), for which the expression of eGFP was evaluated by flow cytometry and fluorescence microscopy, resulting in typically >90% efficiency.
Real time PCR
Total RNA was isolated from cell pellets using the RNeasy MiniKit (Qiagen, Valencia, CA). Genomic DNA was removed from total RNA prior to cDNA synthesis using the RNase-free DNase set for DNase digestion during RNA purification (Qiagen). First-strand cDNA synthesis was performed for each RNA sample using Sensiscript RT Kit (Qiagen). Random hexamers were usedto prime cDNA synthesis. Expression of mRNAwas determined by real-time PCR using SYBR Green Master Mix (Applied Biosystems, Foster City, CA). Nucleotide sequences of specific primers were as follows:
IL-6, forward 5′-GAGGATACCACTCCCAACAGACC-3′,
reverse 5′-AAGTGCATCATCGTTGTTCATACA-3′;
STAT1, forward-5′TGGTGAAATTGCAAGAGCTG-3′,
reverse-5′TGTGTGCGTACCCAAGATGT-3′;
C/EBPβ forward 5′-ACTTCAGCCCCTACCTGGAG-3′,
reverse 5′-GAGGTCGGAGAGGAAGTCGT-3′;
iNOS forward 5′-CAGCTGGGCTGTACAAACCTT-3′,
reverse 5′-CATTGGAAGTGAAGCGTTTCG-3′;
CCL5 forward 5′-ATATGGCTCGGACACCACTC-3′,
reverse 5′-GTGACAAACACGACTGCAAGA-3′;
CXCL1 forward 5′-CTGCACCCAAACCGAAGTC-3′,
reverse 5′-AGCTTCAGGGTCAAGGCAAG-3′, and
β-actin forward 5′-GCTCTGGCTCCTAGCACCAT-3′,
reverse5′-CCACCGATCCACACAGAGTAC-3′. Thermocycler conditions included an initial hold at 50°Cfor 2 minutes, then 95°C for 10 minutes; this was followed by a 2-step PCR program of 95°C for 15 seconds and 60°Cfor 50 seconds repeated for 40 cycles on an Mx4000 system (Stratagene), on which data were collected and quantitatively analyzed. Total sample RNA was normalized to endogenous β-actin mRNA. Expression level of mRNA is presented as fold change relative to an untreated control.
Western blot analysis
Protein samples were diluted in Laemmli buffer (62.5 mM Tris HCl, pH 6.9, 2% SDS, 1% β-mercaptoethanol, 10% glycerol, and 0.04% bromophenol blue) and separated on a 10% SDS–polyacrylamide gel. Proteins were then electroblotted onto a nitrocellulose membrane (Roche Molecular Biochemicals, Laval, QC) and revealed using rabbit or goat polyclonal antibodies against C/EBPβ, STAT1, orβ-actin (Cell signaling, MA) overnight at 4 °C, followed by chemiluminescent detection (Amersham ECL™, GE Healthcare, Piscataway, NJ) according to the manufacturer’s instructions. All results are representative of three independent experiments.
Proliferation assay
MSCs were plated in 96-well plates at 1 × 104 cells per well in 100 μl of complete medium. Splenocytes (3 × 105/well) isolated from C57BL/6 mice were then added in 100 μl of complete medium, resulting in a cell ratio of 1:30 (MSCs-to-splenocytes). Anti-CD3 antibody was added at 1μg/ml, and cell proliferation was determined by incorporation of 3H-thymidine (0.5 μCi/well) after 48 h.
Results
IFNγ and TNFα induce IL-6 expression synergistically in MSCs
Previous studies have shown that cytokines such as IFNγ and TNFα can markedly affect the physiological functions of bone marrow-derived MSCs (6, 16–19). Recently, IL-6 also has been shown to play an important role in MSC function (20, 21). To investigate whether IFNγ and TNFα affect the expression of IL-6, we added recombinant cytokines to cultures of cloned murine MSCs, cultured the cells for ?? days, and assayed for IL-6 mRNA by real-time PCR. We found that, when added concomitantly to MSCs in vitro, IFNγ and TNFα acted synergistically to dramatically increase IL-6 expression (Fig. 1A), while either cytokine was much less effective individually. This effect was concentration-dependent and occurred as early as three hours, and endured for at least three days (Fig. 1B).
Figure 1. IFNγ and TNFα induce IL-6 expression synergistically in MSCs.

MSCs were cultured with IFNγ (10 ng/ml) and graded concentrations of TNFα (0, 0.01, 0.1, 1, 10 ng/ml) for 24 h (A) or with IFNγ, TNFα, or their combination (each at 10 ng/ml) for the indicated times (B) and expression of IL-6 determined by real time PCR. Expression level of mRNA is presented as fold increase relative to an untreated control. Values are given as mean ± SEM of triplicates from a representative of two independent experiments.
C/EBPβ mediates the synergistic induction of IL-6 by IFNγ and TNFα
The transcription factor C/EBPβ is well-known to regulate IL-6 expression (14). To determine whether C/EBPβ is involved in the synergistic effect of IFNγ and TNFα on IL-6 expression in MSCs, we used siRNA technology to inhibit C/EBPβ production. This knockdown resulted in >95% decrease in C/EBPβ mRNA in MSCs in vitro (Fig. 2A), a result that was verified at the protein level by western blotting analysis (Fig. 2B). We found that knockdown of C/EBPβ resulted in markedly reduced IL-6 upregulation in MSCs in response to IFNγ and TNFα, added alone or in combination (Fig. 2C). These results suggest that C/EBPβ is critical in the synergistic induction of IL-6 by IFNγ and TNFα.
Figure 2. C/EBPβ is involved in the IL-6 expression induced by IFNγ and TNFα.
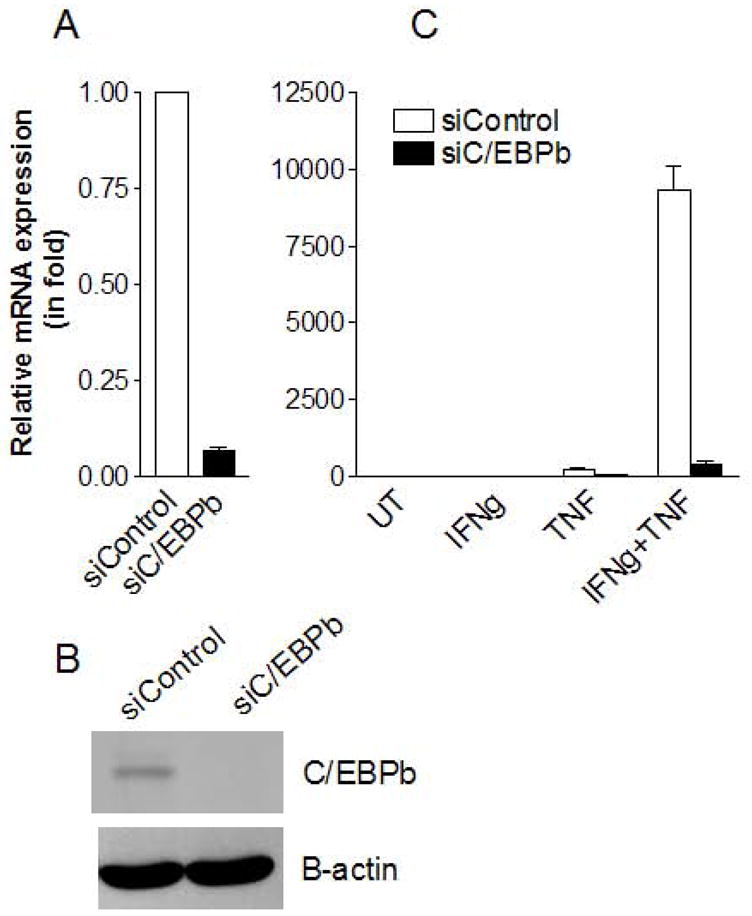
MSCs were transfected with siRNA specific for C/EBPβ (siC/EBPβ) or a scrambled siRNA control (siControl). After a 72 h incubation, expression levels of C/EBPβ were determined by real time PCR for mRNA (A) and by Western blotting analysis for protein (B). siRNA-transfected MSCs were stimulated with IFNγ, TNFα, or both 48 h after transfection, incubated for an additional 24 h, and IL-6 mRNA assayed by real time PCR (C). Values are given as mean ± SEM of triplicates from a representative of three independent experiments (also applies to Fig. 3–6).
To further verify the role of C/EBPβ in the production of IL-6 induced by TNFα and IFNγ, we examined the expression of C/EBPβ mRNA and protein in MSCs. IFNγ and TNFα each induced respectable levels of C/EBPβ expression, and much greater expression occurred when both cytokines were present (Fig. 3). Therefore, the synergistic induction of IL-6 in MSCs in response to IFNγ and TNFα is likely to occur through the upregulation of C/EBPβ expression.
Figure 3. IFNγ and TNFα induce C/EBPβ expression synergistically in MSCs.
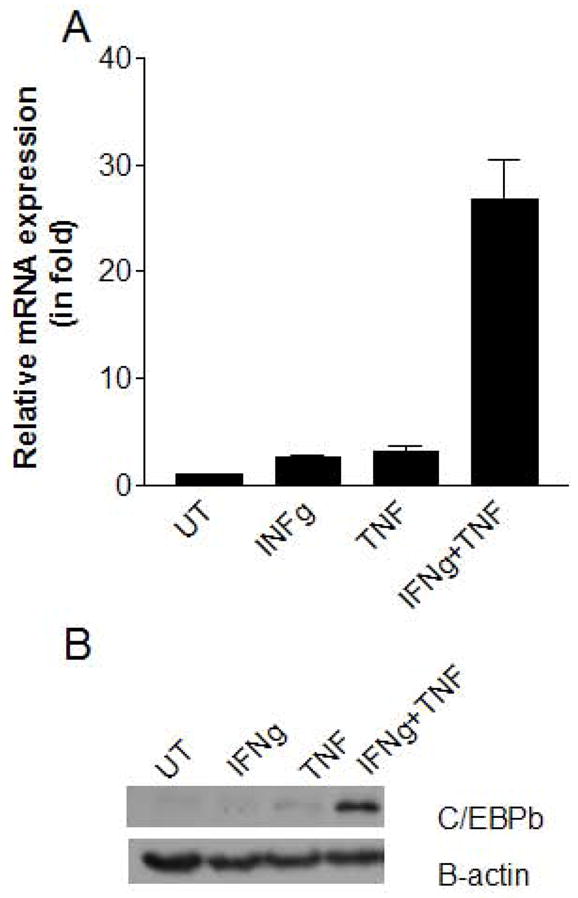
MSCs were treated with IFNγ, TNFα, or both (or untreated, “UT”) for 14 h. and C/EBPβ levels were determined by real time PCR for mRNA (A) and by Western blotting analysis for protein (B).
Synergistic regulation of other genes by IFNγ and TNFα is also C/EBPβ-dependent
IFNγ and TNFα also synergistically induced the expression of additional genes, such as iNOS, CCL5, and CXCL1, as shown in Fig 4. We therefore asked if upregulation of these genes is also C/EBPβ-dependent. We knocked down C/EBPβ in MSCs, and measured the expression of iNOS, CCL5, and CXCL1 in response to IFNγ and TNFα. C/EBPβ knockdown crippled the normal upregulation of iNOS, CCL5 and CXCL1 (Fig. 4), suggesting that C/EBPβ is a key molecule in the synergistic effects of IFNγ and TNFα on gene expression in MSCs.
Figure 4. C/EBPβ is involved in the synergistic upregulation of several genes byIFNγ and TNFα.
MSCs transfected with siRNA specific for C/EBPβ or control siRNA were stimulated with IFNγ, TNF or both (as in Fig. 2) and resultant expression of iNOS, CCL5, and CXCL1 was assayed by real time PCR.
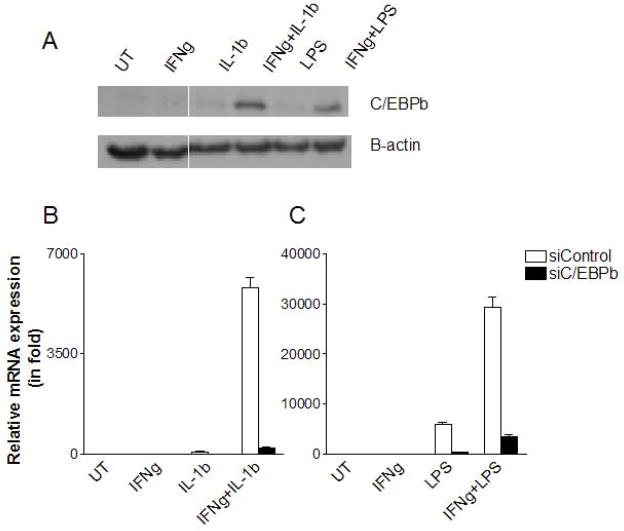
C/EBPβ also mediates the synergistic induction of IL-6 by IFNγ combined with IL-1β or LPS
Previous experiments have shown that IFNγ can synergize with IL-1β or LPS, as well as TNFα, to induce gene expression in various cell types (6, 22–24). To examine the possible role of C/EBPβ in the response of MSCs to IFNγ and IL-1β or LPS, we measured C/EBPβ expression in cytokine-stimulated MSCs and found that both IL-1β and LPS act synergistically with IFNγ to induce C/EBPβ (Fig. 5A). To determine whether the increased levels of IL-6 observed after concomitant stimulation with IFNγ and IL-1β or IFNγ and LPS is mediated by C/EBPβ, we measured IL-6 mRNA by real time PCR in MSCs with or without C/EBPβ knockdown after culture with these cytokine pairs. Both IL-1β and LPS displayed dramatic synergy with IFNγ in the induction high levels of IL-6 expression. Furthermore, C/EBPβ knockdown severely curtailed this effect (Fig. 5B–C). Therefore, C/EBPβ is a critical mediator of the synergy between IFNγ and several other proinflammatory stimuli in upregulating gene expression in MSCs.
Figure 5. C/EBPβ is involved in the synergistic upregulation of IL-6 by IFNγ in combination with IL-1β or LPS.
MSCs were treated with IFNγ accompanied by IL-1β or LPS for 14 h. C/EBPβ expression was determined by Western blotting analysis (A). MSCs were transfected with C/EBPβ siRNA or control siRNA. 48 h later, these MSCs were stimulated with the indicated combinations of IFNγ and L-1β (B), or IFNγ and LPS (C), incubated for 24 h., and then IL-6 mRNA was assayed by real time PCR.
STAT1 is not involved in the induction of IL-6 expression in MSCs by IFNγ and TNFα
It is well known that IFNγ activates the JAK1/2-STAT1 signaling pathway in a variety of cell types, and this pathway accounts for the majority of IFNγ-mediated biological effects. Consistent with previous reports, we also found that IFNγ induces STAT1 expression in MSCs (Fig. 6a). To better understand the molecular mechanism by which IFNγ synergizes with TNFα to induce gene expression, we knocked down STAT1. Transfection of MSCs with STAT1-specific siRNA resulted in > 95% elimination of STAT1 mRNA (Fig. 6b) and similar prevention of STAT1 protein production (Fig. 6c). Interestingly, however, STAT1 knockdown did not inhibit the upregulation of IL-6 or CXCL1 induced by IFNγ + TNFα, while iNOS and CCL5 were greatly reduced (Fig. 6d). This strongly suggests that while STAT1 is induced by IFNγ in MSCs, it regulates only some genes (iNOS and CCL5, but not IL-6 or CXCL1), unlike C/EBPβ, which upregulates all of these genes.
Figure 6. The role of STAT1 in the synergistic induction of gene expression by IFNγ and TNFα.
MSCs were treated with IFNγ, TNFα, or both for 24 h., and STAT1 expression was determined by real time PCR (A). MSCs transfected with siRNA specific for STAT1 or a scrambled siRNA control were tested after 72 h. for STAT1 expression by real time PCR (B) or by Western blotting analysis (C), or siRNA-transfected MSCs were stimulated with IFNγ, TNFα, or both 48 h after transfection, incubated for an additional 24 h, and the expression of IL-6, iNOS, CCL5 and CXCL1 was determined by real time PCR (D).
STAT1 does not affect C/EBPβ expression in MSCs
As described above, knockdown of C/EBPβ, but not STAT1, inhibited the expression of IL-6 and CXCL1. This suggests that STAT1 does not affect C/EBPβ expression. To further verify this, we measured C/EBPβ levels in MSCs and found indeed that knockdown of STAT1 had no effect on C/EBPβ expression (Fig. 7). Therefore, even for STAT1-dependent genes such as iNOS and CCL5, transcription factors STAT1 and C/EBPβ are likely to act in parallel rather than by regulating each other.
Figure 7. Knockdown of STAT1 does not affect C/EBPβ expression.
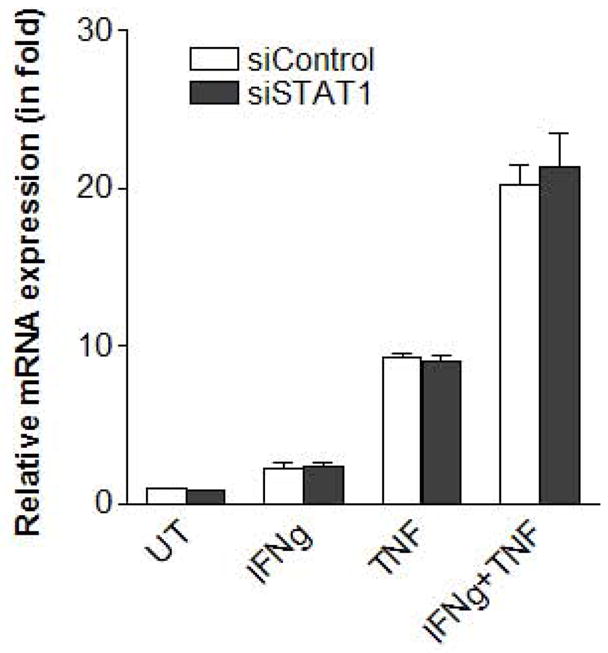
siRNA-transfected MSCs were stimulated with IFNγ, TNFα, or both 48 h after transfection, incubated for an additional 14 h, and C/EBPβ expression was determined by real time PCR. Values are given as mean ± SEM of triplicates from a representative of two independent experiments.
Knockdown of C/EBPβ partly inhibited the immunosuppression by MSCs
We have shown that the same cytokine combinations that induce the immunomodulatory function of MSCs also synergistically upregulate C/EBPβ. We have also reported that chemokines and iNOS play important roles in effecting immunosuppression by MSCs (6). Since C/EBPβ mediates the synergistic expression of iNOS and chemokines, we predicted that knockdown of C/EBPβ would reverse the immunosuppressive function of MSCs. To test this, siC/EBPβ-transfected MSCs were cultured with splenocytes and the effect on proliferation in response to anti-CD3 was determined. As shown in the supplemental figure, knockdown of C/EBPβ in MSCs inhibited their ability to suppress splenocyte proliferation, indicating that the immunosuppressive function is at least in part dependent on C/EBPβ.
Discussion
IFNγ and TNFα have been reported previously to synergize in the induction of gene expression (10, 25, 26) in various cells, but little is known about this phenomenon in MSCs. Furthermore, the mechanism of synergy between these two cytokines is not fully understood. In the present study, we show that IFNγ and TNFα act synergistically to induce the expression of several immune-related genes, such as IL-6, CCL5, CXCL1, and iNOS, and that the transcription factor C/EBPβ plays a key role in their upregulation. IFNγ similarly synergized with IL-1β, LPS, as well as TNFα, to induce gene expression in MSCs, and these effects were also C/EBPβ-dependent. Therefore, C/EBPβ is a major transcription factor in the synergistic responses to IFNγ and other inflammatory factors.
C/EBPβ is known to regulate many genes, and is reported to be induced by IFNγ (27), LPS, and IL-1β (14). However, there is little information about the role of C/EBPβ in the cooperation between IFNγ and TNFα or other cytokines in the induction of gene expression. In this study, we showed that IFNγ and TNFα act synergistically to induce C/EBPβ expression in MSCs, and that knockdown of C/EBPβ dramatically inhibits the expression of genes induced by IFNγ and TNFα. This suggests that synergistic gene upregulation by IFNγ and TNFα is due at least in part to cooperative induction of C/EBPβ expression. The combinations of IFNγ + IL-1β or IFNγ + LPS also acted synergistically to induce C/EBPβ expression, and knockdown of C/EBPβ dramatically inhibited the normal induction of gene expression by these pairs of stimuli, indicating that the synergy between them is also at least partially due to cooperative induction of C/EBPβ expression. Taken together, these results show that synergistic induction of C/EBPβ expression is one of the key mechanisms by which IFNγ and many other inflammatory factors act to synergistically induce high levels of gene expression in MSCs. Elucidation of the mechanism by which IFNγ interacts with other inflammatory factors such as TNFα to induce C/EBPβ awaits further study.
STAT1 is essential in IFNγ-induced signaling process (28, 29). When we examined its role in the synergistic effects of IFNγ and TNFα in MSCs, we found that upregulation of IL-6 and CXCL1 is independent of STAT1, implying that the activity of C/EBPβ is unrelated to STAT1. However, the synergistic upregulation of other genes such as CCL5 and iNOS is regulated by both STAT1 and C/EBPβ, suggesting that these two transcription factors cooperate to regulate gene expression. These results demonstrate the existence of both STAT1-dependent and STAT1-independent regulation of gene expression by IFNγ cooperating with other cytokines.
We expect that our findings will aid in the understanding of the synergism between IFNγ and TNFα in upregulating gene expression in MSCs. This new information illuminates not only the interaction between cytokines, but also the immunobiology of MSCs. Further studies will reveal details of how cytokines synergistically regulate C/EBPβ in MSCs. Also warranted is additional investigation into how cytokine modulation of C/EBPβ might be harnessed to control inflammatory responses in medical conditions perpetuated by pro-inflammatory factors such as IFNγ and TNFα.
Supplementary Material
Acknowledgments
This work was supported in part by New Jersey Commission on Science and Technology (NJCST-2042-014-84), USPHS grants (AI057596), and the National Space Biomedical Research Institute (IIH00405), which is supported by the National Aeronautics and Space Administration through Cooperative Agreement NCC 9-58.
Footnotes
Guangwu Xu: Conception and design, Collection and assembly of data, Data analysis and interpretation, Manuscript writing
Yingyu Zhang: Conception and design, Collection and assembly of data, Data analysis and interpretation, Manuscript writing
Liying Zhang: Provision of study material, Administrative support
Arthur I. Roberts: Manuscript writing, Data analysis and interpretation
Yufang Shi: Conception and design, Manuscript writing, Final approval of manuscript, Financial support
References
- 1.Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 2.Kuznetsov SA, Krebsbach PH, Satomura K, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 3.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 4.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Billiau A. Interferon-gamma: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 8.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 9.Sanceau J, Wijdenes J, Revel M, et al. IL-6 and IL-6 receptor modulation by IFN-gamma and tumor necrosis factor-alpha in human monocytic cell line (THP-1). Priming effect of IFN-gamma. J Immunol. 1991;147:2630–2637. [PubMed] [Google Scholar]
- 10.Hattori A, Iwasaki S, Murase K, et al. Tumor necrosis factor is markedly synergistic with interleukin 1 and interferon-gamma in stimulating the production of nerve growth factor in fibroblasts. FEBS Lett. 1994;340:177–180. doi: 10.1016/0014-5793(94)80132-0. [DOI] [PubMed] [Google Scholar]
- 11.Oh I, Ozaki K, Sato K, et al. Interferon-gamma and NF-kappaB mediate nitric oxide production by mesenchymal stromal cells. Biochem Biophys Res Commun. 2007;355:956–962. doi: 10.1016/j.bbrc.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Gade P, Xiao W, et al. The interferon signaling network and transcription factor C/EBP-beta. Cell Mol Immunol. 2007;4:407–418. [PMC free article] [PubMed] [Google Scholar]
- 13.Ranjan P, Boss JM. C/EBPbeta regulates TNF induced MnSOD expression and protection against apoptosis. Apoptosis. 2006;11:1837–1849. doi: 10.1007/s10495-006-9530-0. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Isshiki H, Sugita T, et al. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. Embo J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu G, Zhang L, Ren G, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240–248. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 16.English K, Barry FP, Field-Corbett CP, et al. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007;110:91–100. doi: 10.1016/j.imlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Ryan JM, Barry F, Murphy JM, et al. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan JL, Tang KC, Patel AP, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 20.Xu G, Zhang Y, Zhang L, et al. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;361:745–750. doi: 10.1016/j.bbrc.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 22.Takami Y, Motoki T, Yamamoto I, et al. Synergistic induction of hepatocyte growth factor in human skin fibroblasts by the inflammatory cytokines interleukin-1 and interferon-gamma. Biochem Biophys Res Commun. 2005;327:212–217. doi: 10.1016/j.bbrc.2004.11.144. [DOI] [PubMed] [Google Scholar]
- 23.Proost P, Verpoest S, Van de Borne K, et al. Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-gamma in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids. J Leukoc Biol. 2004;75:777–784. doi: 10.1189/jlb.1003524. [DOI] [PubMed] [Google Scholar]
- 24.Jorens PG, Van Overveld FJ, Vermeire PA, et al. Synergism between interleukin-1 beta and interferon-gamma, an inducer of nitric oxide synthase, in rat lung fibroblasts. Eur J Pharmacol. 1992;224:7–12. doi: 10.1016/0014-2999(92)94811-9. [DOI] [PubMed] [Google Scholar]
- 25.Sekine N, Ishikawa T, Okazaki T, et al. Synergistic activation of NF-kappab and inducible isoform of nitric oxide synthase induction by interferon-gamma and tumor necrosis factor-alpha in INS-1 cells. J Cell Physiol. 2000;184:46–57. doi: 10.1002/(SICI)1097-4652(200007)184:1<46::AID-JCP5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Lee AH, Hong JH, Seo YS. Tumour necrosis factor-alpha and interferon-gamma synergistically activate the RANTES promoter through nuclear factor kappaB and interferon regulatory factor 1 (IRF-1) transcription factors. Biochem J. 2000;350(Pt 1):131–138. [PMC free article] [PubMed] [Google Scholar]
- 27.Roy SK, Wachira SJ, Weihua X, et al. CCAAT/enhancer-binding protein-beta regulates interferon-induced transcription through a novel element. J Biol Chem. 2000;275:12626–12632. doi: 10.1074/jbc.275.17.12626. [DOI] [PubMed] [Google Scholar]
- 28.Durbin JE, Hackenmiller R, Simon MC, et al. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 29.Meraz MA, White JM, Sheehan KC, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.