Abstract
Mesenchymal stem cells (MSCs), also called multipotent mesenchymal stromal cells, exist in almost all tissues and are a key cell source for tissue repair and regeneration. Under pathological conditions, such as tissue injury, these cells are mobilized towards the site of damage. Tissue damage is usually accompanied by proinflammatory factors, produced by both innate and adaptive immune responses, to which MSCs are known to respond. Indeed, recent studies have shown that there are bidirectional interactions between MSCs and inflammatory cells, which determine the outcome of MSC-mediated tissue repair processes. Although many details of these interactions remain to be elucidated, we provide here a synthesis of the current status of this newly emerging and rapidly advancing field.
Mesenchymal stem cells (MSCs)
Stem cells have several distinct characteristics that distinguish them from other cell types. First, they are mostly unspecialized and are self-renewing. Second, they can be induced to differentiate into various specialized cell types, and thus hold promise for regenerative medicine [1]. Recent studies have suggested that resident in almost all tissues are a small number of dormant stem cells that can become activated and specifically migrate to sites of tissue damage, where they then perform repair functions. When derived from differentiated tissues, these cells are often referred to as ‘adult stem cells’, although they are also present in various tissues in embryos and infants. Thus, it is more appropriate to refer to them as tissue stem cells or MSCs [2]. MSCs have been isolated from many different tissues, including bone marrow, adipose tissue, nervous tissue, hair follicles, intestinal epithelium, cardiac tissue, amniotic fluid, placenta, and Wharton's jelly of the umbilical cord. In culture, most MSCs have a spindle morphology like fibroblasts, and can be maintained for several passages without significant alterations in their major properties [3]. MSCs are multi-potent and can differentiate into distinct cell types, such as chondrocytes, osteoblasts, and adipocytes [4]. MSCs derived from adult bone marrow can be cloned and expanded in vitro more than a million-fold without loss of differentiation potential; these bone marrow-derived MSCs are the most routinely used in studies [5]. However, many properties of these rare tissue-resident cells remain unknown [6].
Recent studies have suggested that MSCs can influence various physiological and pathophysiological processes, such as immune and inflammatory responses [2]. In 2002, it was reported that MSCs can modulate immune responses, with the finding that baboon MSCs could inhibit the mixed lymphocyte reaction in vitro, and prevent the rejection of allogeneic skin graft in vivo [7]. Subsequently, a large body of work has demonstrated that MSCs are immunosuppressive both in vitro and in vivo in other animal models and human studies [2]. These findings are important because, although the immunomodulatory capacity of MSCs could potentially be harnessed therapeutically, there may also be unwanted effects associated with immunosupression. Here, we review the evidence linking MSCs with immunosuppression and the mechanistic data explaining how immunomodulation occurs. We also examine how the immune status of the host might influence the immunomodulatory activity of MSCs. Finally, we consider the implications of these data for clinical studies of MSCs in disease.
MSCs in the damaged tissue microenvironment
Pathogenic tissue injury usually involves the activation of immune and inflammatory cells. Under normal conditions, apoptotic cells are silently cleared by resident phagocytes without causing inflammation. By contrast, acute tissue damage is usually followed by inflammation, even in cases of nonimmune or noninfectious injury [8,9]. Cellular components released from necrotic cells and microvasculature damage lead to enhanced vasopermeability and infiltration of macrophages and neutrophils. In addition to these innate immune cells, adaptive immune cells including B cells, CD4+ T cells and CD8+ T cells are also closely associated with tissue damage and repair [10,11]. Importantly, phagocytosis of necrotic cells results in the release of proinflammatory factors, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, various chemokines and leukotrienes, and free radicals [12]. Together with fibroblasts and endothelial cells, the most common cell types involved in the process of injury repair, these inflammatory cells and factors are finely regulated to achieve a balance in tissue homeostasis.
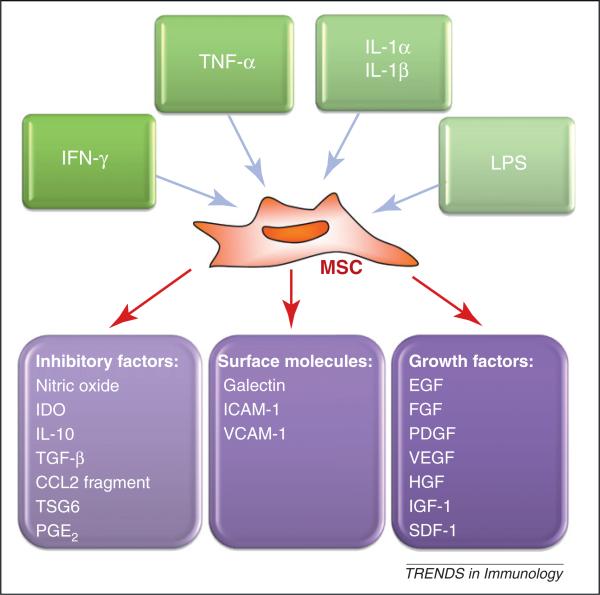
In recent years, MSCs have come to be recognized as one type of adult stem cells actively participating in tissue repair [6]. When tissue damage occurs, MSCs either in the immediate vicinity or those derived from bone marrow are believed to migrate into the damaged tissue. Details of their migration, differentiation and survival mechanisms at the damage sites remain elusive, however, mainly because of a lack of reliable tracing markers. Nevertheless, MSCs must interact closely with various stromal cells and inflammatory cells once they reach the site of damage to participate in tissue repair [13–15]. The mechanisms of MSC-mediated tissue repair are complex, but MSC-derived trophic factors play an important role. In animal models, MSC-conditioned medium could, to some extent, mimic the therapeutic effects of MSCs in enhancing wound healing, and improving cardiac function following myocardial infarction [16–18]. Genetic ablation of specific factors, such as TNF-inducible gene 6 protein (TSG6), largely abrogates the beneficial effect of MSCs in promoting the repair of myocardial infarction and corneal damage [19,20]. Several reports have demonstrated that MSCs can release an array of growth factors (Figure 1), such as epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor (TGF-β), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF)-1, angiopoietin-1 and stromal cell-derived factor (SDF)-1, many of which are generated by proinflammatory stimuli such as TNF-α, lipopolysaccha-ride, or hypoxia, in a nuclear factor (NF)-κB-dependent manner [21,22]. Further studies to investigate how the inflammatory milieu modulates growth factor production at sites of tissue damage will provide insights into the therapeutic mechanisms of MSC effects.
Figure 1.
Activation of MSCs by inflammatory cytokines, an inflammatory cytokine-licensing process of MSCs. MSCs and the immune system interact closely. During an immune response the inflammatory cytokines produced by T cells and antigen-presenting cells modulate the function of MSCs leading to: (i) release of immunosuppressive factors; (ii) altered expression of surface molecules; and (iii) production of growth factors. These factors/molecules are crucial components for immunoregulation and tissue repair by MSCs.
In addition to tissue repair, MSCs may have potential in suppressing uncontrolled immune responses, providing in situ negative regulation during the inflammatory response. It is thought that MSC-mediated immunosuppression occurs in the microenvironment surrounding the MSCs: the inflammatory factors produced during the immune response act to turn on the immunosuppressive capacity of MSCs [23,24]. Therefore, the inflammatory status in the particular microenvironments associated with specific diseases will probably need to be considered when developing novel therapeutic strategies involving MSCs.
MSCs are not spontaneously immunosuppressive
Initial studies on the immunomodulatory effects of MSCs have produced conflicting data: immunosuppression was evident in vivo in both animals and humans with immune disorders [2,7,25,26], but it has not always been detected in some in vivo experimental settings. For example, MSCs have been found to be effective in suppressing excessive immune responses, such as graft-versus host disease (GvHD) and systemic lupus erythematosus (SLE) in both animal models and humans [23,25,27]. By contrast, several other studies have found that MSCs are unable to prolong graft survival or suppress GvHD in vivo, although they do suppress lymphocyte proliferation to some extent in vitro [28,29]. Furthermore, co-transfer of MSCs with allogeneic bone marrow transplantation in sublethally irradiated mice, unexpectedly decreased engraftment of donor cells[30], and combined administration of MSCs and cyclosporine A (CsA), a universally used immunosuppressant, actually accelerated graft rejection [29]. These studies would seem to indicate that MSCs are immunosuppressive, but not under all conditions. As used in different experimental settings, MSCs are usually heterogeneous cell populations. They are isolated from different tissues and used at different passages upon in vitro expansion. It has been reported that the phenotype, differentiation potential, and gene expression of MSCs change significantly with in vitro passage [31]. In a rat ischemia/reperfusion injury model, MSCs with high passage numbers adversely affected myocardial protection in isolated rat hearts [32]. Furthermore, considering the complexity of the microenvironment at damaged tissue sites, the different therapeutic effects of MSCs may also be due to the specific microenvironment that they encounter.
To tease out these possibilities, one study has used clones of mouse MSCs (comprising a homogeneous cell population) that were generated from mouse bone marrow [33]. To test whether their immunosuppressive activity is intrinsic, these MSC clones were cultured with T cell blasts or T cell hybridomas, neither of which produces inflammatory cytokines in the absence of antigen receptor stimulation. No immunosuppression was observed unless the MSCs were pretreated with interferon (IFN)-γ, together with TNF-α or IL-1 [23,24]. This result is consistent with several studies indicating that priming by inflammatory cytokines is essential for MSC-mediated immunosuppression [24,34–38]. Thus, MSCs need to be ‘licensed’ to become immunosuppressive. Interestingly, MSCs can participate in antigen presentation if exposed to a narrow window of low levels of IFN-γ through upregulation of MHC-II, whereas high concentrations of IFN-γ and other inflammatory factors such as TGF-β suppress MHC-II expression. These data further indicate the importance of the local inflammatory conditions in regulating the plasticity of MSCs [39–41]. Therefore, MHC-II expression on MSCs should be considered when establishing protocols for allogeneic MSC expansion for clinical purposes due to the involvement of MHC-II in allorejection.
These results raise several points that should be considered when testing the use of MSCs clinically. First, the schedule of administration of MSCs may be important. Based on the ‘licensing’ theory, MSCs are most probably effective when administered after the onset of inflamma-tory diseases. Supporting this, in a mouse GvHD model, MSC administration on the same day as bone marrow transplantation (BMT) had no protective effect [28], whereas administration 3, 8 or 20 days after BMT significantly suppressed the progression of GvHD and lessened the related symptoms [23,42]. Additionally, several reports have indicated that after disease stabilization, the therapeutic efficacy of MSCs is not as apparent in mouse GvHD and experimental autoimmune encephalomyelitis (EAE) models [43,44]. This is not surprising because the levels of inflammatory cytokines in recipients with stabilized disease may be insufficient to elicit the immunomodulatory effect of MSCs. In the mouse GvHD model, multiple administrations of MSCs are often utilized to sustain and prolong their inhibitory effect, and also probably enhance the exposure of MSCs to released inflammatory cytokines, thus conferring more beneficial effects [23,44]. Second, pretreatment of MSCs with inflammatory cytokines may enhance potential therapeutic efficacy. It has been reported that IFN-γ-pretreated MSCs could protect 100% of mice from GvHD-induced death [42]. Although there is some discrepancy in the effects of IFN-γ pretreatment on MSC-mediated immunosuppression among different models, such as EAE in mice [45], various studies have indicated that pretreatment with inflammatory cytokines can enhance the therapeutic effect of MSCs in animal models of colitis and acute myocardial ischemia/reperfusion injury [46,47]. Finally, it is worth considering the combined use of immunosuppressive drugs with MSCs. A recent study has shown that the immunosuppressive drug mycophenolate mofetil (MMF) significantly enhances the effect of MSCs in promoting the survival of allogeneic heart grafts in a mouse model, whereas other immunosuppressive drugs such as CsA diminishes this effect. This discrepancy may be explained by the fact that CsA inhibits IFN-γ production, whereas MMF does not [48].
Mechanisms associated with inflammatory stimuli
What are the mechanisms by which MSCs inhibit the immune response after being activated by inflammatory factors? Several molecules, most of which are induced by inflammatory cytokines, have been reported to be involved in MSC-mediated immunosuppression (Figure 1). MSCs have been suggested to inhibit many kinds of immune cells, including T cells, B cells, dendritic cells, and natural killer (NK) cells in vitro and in vivo [49–53]. Here, we consider how MSC-derived factors may contribute to immunosuppression.
NO
NO in high concentrations is known to inhibit immune responses through mechanisms that remain largely unidentified [54]. Murine MSCs express inducible NO synthase (iNOS) upon stimulation by the inflammatory cytokines IFN-γ in combination with either TNF-α or IL-1 [23,55]. The high concentration of NO leads to suppression of signal transducer and activator of transcription (STAT)5 phosphorylation in T cells and induction of immune cell apoptosis in vitro [55]. In vivo, suppression of iNOS activity by either chemical inhibitors or genetic ablation in MSCs largely reverses the therapeutic effect of MSCs in mouse models of GvHD and delayed-type hypersensitivity responses, as well as in the rat heart allograft transplantation model [23,56,57]. A recent study has shown that MSCs are the critical mediators of Mycobacterium tuberculosis-induced immunosuppression in an NO-dependent manner in vitro and in vivo, suggesting a function of the MSC–NO axis in endogenously induced immunosuppression [58]. Intriguingly, fibroblastic reticular cells (FRCs) and lymphatic endothelial cells (LECs), which are the stromal cells in lymphoid organs, have also been shown to play a major role in controlling the activated T cell pool in lymph nodes through induction of iNOS by IFN-γ and TNF-α stimulation, suggesting a general role of NO-mediated immunoregulation shared by distinct types of stromal cells [59].
NO is a highly labile oxidative species and therefore can act only in close proximity to the cells producing it [60]. The biological activity of NO diminishes markedly over a distance of a few cell diameters [61]. This explains why MSC-mediated immunosuppression was largely abrogated when separated by a semi-permeable membrane in some investigations [23]. How then are MSCs immunosuppressive? After stimulation with inflammatory cytokines, MSCs produce large amounts of chemokines and adhesion molecules, especially the CXCR3 and CCR5 ligand chemokines, and the adhesion molecules, intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 [23,62]. Through the synergistic action of these chemokines and adhesion molecules, immune cells accumulate in close proximity to the MSCs, where the high concentration of secreted NO can suppress the immune cells.
Indoleamine 2,3-dioxygenase (IDO)
Recently, the role of IDO in MSC-mediated immunoregulation has been demonstrated in the suppression of various immune cell populations, including T cells and NK cells [34,35,52]. IDO catalyzes the rate-limiting step in the degradation of tryptophan, an essential amino acid, along the kynurenine pathway. The resulting reduction in local tryptophan concentration and the production of tryptophan metabolites that are immunomodulatory are thought to contribute to the immunosuppressive effects of IDO-expressing cells [63]. IDO-mediated immunosuppression/immune tolerance has been extensively studied in dendritic cells. IDO expression is not constitutive, but inducible by stimulation with IFN-γ, and further promoted by other inflammatory cytokines, such as TNF-α or IL-1 [24,35].
In an effort to elucidate the molecular mechanisms underlying immunosuppression by MSCs in different species, MSCs from humans, monkeys and mice have been compared. A species variation in MSC-mediated immunosuppression was discovered: mouse MSCs utilized NO, whereas human and monkey MSCs used IDO as their respective immunosuppressive molecules [24,34,35]. Therefore, in selecting animal models of human disease, the distinct mechanisms of MSC-mediated immune modulation must be considered. It is important to point out that tryptophan depletion or the production of tryptophan metabolites by the action of IDO would also be expected to act only locally [63], because the total body pool of tryptophan would not be depleted significantly. The role of localized tryptophan depletion and accumulated metabolites in immunosuppression remains speculative. Nevertheless, as with mouse MSCs, human MSCs also secrete several leukocyte chemokines that serve to attract immune cells into proximity with MSCs, which may serve to amplify their effects [24].
Prostaglandin E2 (PGE2)
PGE2 is another inflammatory stimulus-induced, immunosuppressive molecule produced by MSCs. PGE2 has been reported to mediate MSC-mediated suppression of T cells, NK cells and macrophages [52,64,65]. In an experimental mouse arthritis model, IL-6-dependent PGE2 production was found to play a major role in the therapeutic effect of MSCs [66]. More importantly, PGE-2 has been shown to educate macrophages in vivo to develop an IL-10-overexpressing phenotype, contributing to the attenuation of sepsis by MSCs in mice [65]. It is worth noting that the effect of PGE2 in MSC-mediated immunoregulation is always exerted in combination with other immunosuppressive molecules. In mouse MSCs, both the PGE2 inhibitor, indomethacin, and NO inhibitor, nitro-L-arginine methyl ester (L-NAME), have been found to reverse the inhibitory effect of MSCs on T cell proliferation, although their effects are not additive [55]. With human MSCs, PGE2 has been found to act with IDO to alter T cell proliferation, as well as proliferation, cytotoxicity and cytokine production by NK cells [52,67]. Considering that PGE2 is a crucial component of the inflammatory microenvironment, further investigation of its role in MSC-mediated immunomodulation will provide crucial information for better understanding of the biology of MSCs.
TSG6
TSG6 is an anti-inflammatory protein highly expressed in a variety of cells in patients with different inflammatory or autoimmune disorders, which is inducible by a subset of inflammatory cytokines such as TNF-α and IL-1 [68]. In a mouse myocardial infarction model, it has been demonstrated that TSG6 is one of the most upregulated proteins in human MSCs in response to the injury to the lung produced by microembolization of the infused cells, playing a crucial role in the suppression of inflammatory response, reduction of infarct size, and improvement of cardiac function [19]. More recently, TSG6 produced by human MSCs has been found to alleviate zymosan-induced mouse peritonitis through reduction of Toll-like receptor (TLR)2/NF-κB signaling in resident macrophages [69]. Whether TSG6 acts alone or works together with other inflammatory stimuli-inducible factors such as IDO, NO or PGE2 at different phases of inflammation requires further investigation.
CCL2 chemokine
A role for metalloprotease-cleaved CCL2, which acts as an antagonist to its cognate receptor CCR2, has been described for MSC-mediated immunosuppression in an EAE model [70]. MSCs deficient in CCL2 exhibit a much lower capacity to inhibit IL-17 secretion by activated T cells, and therefore lose their protective effect against EAE [70]. The effects of cleaved CCL-2 are not limited to unrestrained IL-17 production by T cells; it also suppresses the production of immunoglobulin by plasma cells through inactivation of STAT3 and induction of paired box protein 5 (PAX5) [71]. Moreover, during bacterial infection, the stimulation of TLRs leads MSCs to produce CCL-2, which induces monocyte emigration from bone marrow to the periphery [72], although it is not known whether this monocyte mobilization is associated with immunosuppression or immune promotion.
IL-10
Although IL-10 has been implicated in MSC-mediated immunosuppression, direct IL-10 production by MSCs has not been demonstrated. Instead, contact with MSCs has been found to induce IL-10 production in antigen-presenting cells such as dendritic cells or monocytes [64,73]. Additionally, IL-10 has been reported to be associated with induction of other immunosuppressive factors such as nonclassic HLA class I molecule HLA-G [74].
Other mediators
In addition to the above molecules, several additional mediators are produced by MSCs or other adult stem/progenitor cells upon inflammatory stimulation, such as the inhibitory surface protein programmed death ligand 1 (PD-L1) [36], heme oxygenase-1 (HO-1) [37], leukemia inhibitory factor (LIF) [75], IL-6 [76], galectins [77], TGF-β and regulatory T cells [74,78]. Their modes of action in MSC-mediated immunosuppression require further investigation.
In summary, several inflammatory-factor-inducible, immunosuppressive mediators produced by MSCs have been identified in various in vivo disease models and in vitro experimental settings. It is likely that the importance of any specific mediator varies depending upon the local microenvironment. Therefore, the microenvironments that ‘license’ MSCs are complex. It is also likely that the therapeutic benefits of MSCs may be exerted through the synergistic action of several factors. Further investigation into the molecular mechanisms that govern the immunosuppressive properties of MSCs in particular microenvironments will provide information for better clinical application of MSCs.
Potential clinical applications of MSCs
As a result of their extensive proliferative capacity, it is possible to produce relatively large numbers of MSCs for potential clinical applications from a single bone marrow aspirate, a segment of umbilical cord, or a small piece of excised adipose tissue. In animal models, MSCs have been extensively studied in disease models such as GvHD, EAE, collagen-induced arthritis (CIA), inflammatory bowel disease (IBD), type 1 diabetes, and systemic lupus erythematosus (SLE) [27,42,45,79–81]. Although most reports have shown effects of MSCs on T helper (Th)1-driven diseases, some recent studies have revealed that they could also efficiently control Th2-type diseases, such as a mouse model of asthma, in which MSC effects involve TGF-β and regulatory T cells [82,83]. It should be noted that the efficacy of MSC treatment in animal models has been variable. In several studies, MSC treatment has resulted in significant improvement, whereas in others, little or no benefit has been seen, and in some studies, treatment has actually resulted in a worse outcome [84,85]. For example, in a mouse model of CIA, the initial study to utilize MSCs in CIA treatment did not demonstrate any benefits from intravenous MSC administration, in contrast to the significant improvements of arthritis-induced tissue damages observed in other studies following a single intraperitoneal injection of MSCs [86,87]. Similarly, in a mouse model of SLE using MPR/lpr mice and (NZB × NZW)F1 mice, conflicting data have also been obtained: treatment with allogeneic BM-derived MSCs actually exacerbated the disease progression in mice, with more severe histopathological characteristics in the kidney, increased proteinuria, and elevated autoantibody level [88], whereas other recent studies have shown beneficial effects from MSCs in the treatment of SLE in both mouse models and human clinical trials [27]. In the mouse model of GvHD, as described above, diverse results were reported as well [28,60,65]. By analyzing these conflicting results, many parameters in experimental design, including MSC administration dose, schedule, and routes, as well as the specific combinations with the traditional immunosuppressive drugs, should be carefully considered.
Allogeneic human MSCs have been utilized in the treatment of different human diseases including GvHD, type 1 diabetes, acute myocardial infarction, multiple sclerosis, Crohn's disease and SLE [25,26,89]. Although no apparent difference in therapeutic efficacy has been found between autologous and allogeneic sources of MSCs in animal models [90,91], it is not clear whether autologous and allogeneic MSCs may differ in humans. There are over 100 clinical trials with MSCs registered on clinicaltrials.-gov. While a majority of these trials are not completed, the available data, most of which are derived from GvHD treatment, are not conclusive, which suggests the need for larger trials with MSCs. In treating refractory, severe acute GvHD, an initial study has shown that third-party haploidentical MSCs could reduce the inflammation associated with GvHD [26]. In a Phase II multicenter study of 55 steroid refractory GvHD patients, >50% of the patients had a complete response, with a lower transplantation-related mortality 1 year after MSC infusion and a higher overall survival 2 years after hematopoietic stem cell (HSC) transplantation [60]. In 2009, Osiris Therapeutics reported their preliminary results of a phase III double-blind, placebo-controlled trial of human MSCs for steroid-refractory GvHD. Although the patients gained statistically significant improvements in inhibition of systemic inflammation in gut and liver GvHD, no improvements were found in skin GvHD and the primary endpoint was not reached [92]. Thus, although promising, a definite conclusion on the efficacy of MSCs in the treatment of GvHD cannot yet be drawn. An in depth understanding of the mechanisms of MSC therapy and the interaction between MSCs and inflammatory tissue microenvironments should lead to design of better protocols for MSC-based therapies.
Unlike embryonic stem cells, MSCs do not have the potential to form teratomas and are not associated with the complex ethical issues that accompany ESC therapy, as MSCs do not involve the destruction of human embryos. Several studies have provided important information about the safety of MSC-based therapy. In a series of clinical trials, MSC administration has been shown to be a clinically feasible and relatively safe procedure without major adverse effects in patients with multiple sclerosis, amyotrophic lateral sclerosis (ALS), and GvHD [60,93,94]. Recent reports have revealed that MSCs do not induce either tumorigenesis or infections after follow-up between 5 and 137 (mean 75) months in 41 patients receiving MSCs for cartilage repair [95]. However, it appears that MSCs may not be completely free from malignant potential: several studies have shown that MSCs can form spontaneous malignant tumors after their in vivo infusion in animal models, with multiple chromosomal aberrations found in the transformed cells [96,97]. Additionally, MSCs have a potential to enhance tumor development, due to their immunosuppressive activities or to their conversion into tumor-associated fibroblasts, as has been shown in mouse carcinoma models [38,98]. In clinical trials, co-transplantation of MSCs with HSCs was associated with a higher recurrence rate in hematological malignancy patients [99]. Therefore, there is a continued need for further studies in this regard. To date, governing standards and recommendations to guide the clinical application of MSCs have not been established in any country.
Concluding remarks
Emerging data suggest that MSCs have an immunomodulatory function and thus these cells hold potential for clinical applications for regenerative therapy. Based on the current data on the orchestrated actions of inflamma-tory cytokines, chemokines, and effector molecules in MSC-mediated immunosuppression, it is apparent that interactions between immune cells and MSCs in the tissue microenvironment can modulate MSC function and there fore may contribute to the outcome of MSC-based therapy in any individual patient (Figure 2). It is important to achieve a better understanding of these mechanisms, and there are still many questions (Box 1). Further elucidation of the molecular mechanisms underlying the finely regulated interactions between MSCs and inflammation will be crucial for developing potential therapeutic applications of MSC-based therapies and for improving our understanding of pathological processes in general.
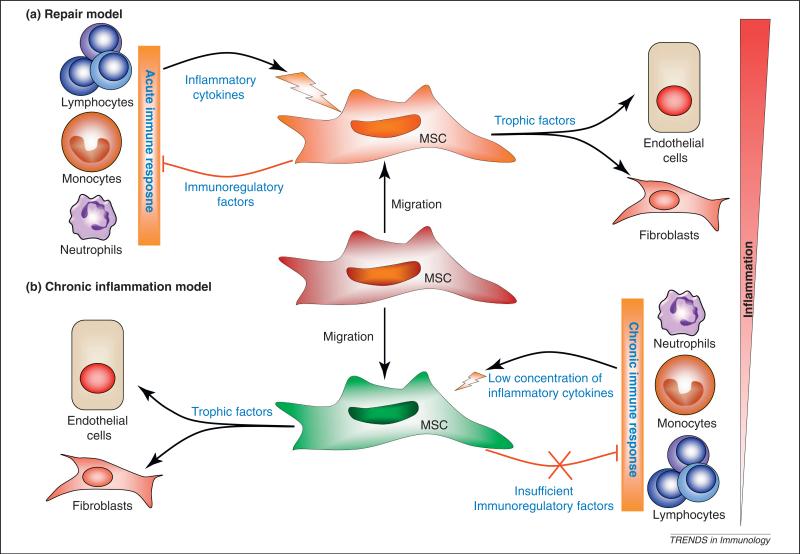
Figure 2.
Role of MSCs in tissue repair and chronic inflammation. Recent studies on MSC-mediated immunoregulation suggest that MSCs are recruited to sites of tissue damage and activated by local inflammatory cytokines produced by activated immune cells. MSC activation leads to the production of immunoregulatory factors and trophic factors. Depending on the types of immune responses (acute vs chronic inflammation), MSCs may either attenuate the inflammatory response and lead to repair of the damaged tissue, or maintain a persistent chronic inflammatory response, leading to fibrosis and deformation of tissue architecture.
Box 1. Unanswered questions.
What is the immunoregulatory role of MSCs in situ?
How do chemokines participate in tissue repair?
Why do MSCs from different species differentially utilize iNOS and IDO?
Does inflammation play a role in MSC-mediated tissue repair?
Is there a role for iNOS or IDO in MSC-mediated tissue repair?
Do inflammatory cytokines affect the multipotency and differentiation of MSCs?
Is there variation in the immunoregulatory effects of MSCs derived from different tissues?
Is there a correlation between multipotency and immunomodulation among MSCs derived from different tissues?
Can the changes induced by inflammatory cytokine-stimulated MSCs be reversed?
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (2010CB945600, 2011DFA30630, 2009ZX09503024), Scientific Innovation Project of the Chinese Academy of Science (KSCX1-YW-22, KSCX2-YW-R-245, XDA01040000), and the National Science and Technology Project of China (31010103908), and grants from the National Institutes of Health of the United States of America (DE014913, DE019932, and GM866889). We would also like to thank the Robert Wood Johnson Foundation (67038) for their support of the Child Health Institute of New Jersey.
References
- 1.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 2.Uccelli A, et al. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning i and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Huss R. Isolation of primary and immortalized CD34-hematopoietic and mesenchymal stem cells from various sources. Stem Cells. 2000;18:1–9. doi: 10.1634/stemcells.18-1-1. [DOI] [PubMed] [Google Scholar]
- 6.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 7.Bartholomew A, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 8.Savill J, et al. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 10.Luster AD, et al. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 11.Eming SA, et al. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 12.Krysko DV, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13:2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, et al. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J. Cell. Mol. Med. 2010;14:1494–1508. doi: 10.1111/j.1582-4934.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki M, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnecchi M, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 18.Timmers L, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6:206–214. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roddy GW, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 21.Meirelles LS, et al. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Crisostomo PR, et al. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 23.Ren G, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Ren G, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 26.Le Blanc K, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudres M, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J. Immunol. 2006;176:7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 29.Inoue S, et al. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589–1595. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]
- 30.Nauta AJ, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner W, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crisostomo PR, et al. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 33.Xu G, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240–248. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 34.Meisel R, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 35.Krampera M, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 36.Sheng H, et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- 37.Mougiakakos D, et al. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826–4835. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- 38.Djouad F, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 39.Chan JL, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang KC, et al. Down-regulation of MHC II in mesenchymal stem cells at high IFN-gamma can be partly explained by cytoplasmic retention of CIITA. J. Immunol. 2008;180:1826–1833. doi: 10.4049/jimmunol.180.3.1826. [DOI] [PubMed] [Google Scholar]
- 41.Romieu-Mourez R, et al. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J. Immunol. 2007;179:1549–1558. doi: 10.4049/jimmunol.179.3.1549. [DOI] [PubMed] [Google Scholar]
- 42.Polchert D, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zappia E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 44.Yanez R, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 45.Rafei M, et al. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol. Ther. 2009;17:1799–1803. doi: 10.1038/mt.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duijvestein M, et al. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549–1558. doi: 10.1002/stem.698. [DOI] [PubMed] [Google Scholar]
- 47.Herrmann JL, et al. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 48.Eggenhofer E, et al. Features of synergism between mesenchymal stem cells and immunosuppressive drugs in a murine heart transplantation model. Transpl. Immunol. 2011;25:141–147. doi: 10.1016/j.trim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Krampera M, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 50.Corcione A, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 51.Jiang XX, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 52.Spaggiari GM, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 53.Chiesa S, et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 55.Sato K, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 56.Chabannes D, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 57.Lim JH, et al. Immunomodulation of delayed-type hypersensitivity responses by mesenchymal stem cells is associated with bystander T cell apoptosis in the draining lymph node. J. Immunol. 2010;185:4022–4029. doi: 10.4049/jimmunol.0902723. [DOI] [PubMed] [Google Scholar]
- 58.Raghuvanshi S, et al. Mycobacterium tuberculosis evades host immunity by recruiting mesenchymal stem cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21653–21658. doi: 10.1073/pnas.1007967107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lukacs-Kornek V, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 2011;12:1096–1104. doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ignarro LJ, et al. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porterfield DM, et al. Proteins and lipids define the diffusional field of nitric oxide. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L904–L912. doi: 10.1152/ajplung.2001.281.4.L904. [DOI] [PubMed] [Google Scholar]
- 62.Ren G, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 64.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 65.Nemeth K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bouffi C, et al. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matysiak M, et al. Immunoregulatory function of bone marrow mesenchymal stem cells in EAE depends on their differentiation state and secretion of PGE2. J. Neuroimmunol. 2011;233:106–111. doi: 10.1016/j.jneuroim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Wisniewski HG, Vilcek J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997;8:143–156. doi: 10.1016/s1359-6101(97)00008-7. [DOI] [PubMed] [Google Scholar]
- 69.Choi H, et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-{kappa}B signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rafei M, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J. Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 71.Rafei M, et al. Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood. 2008;112:4991–4998. doi: 10.1182/blood-2008-07-166892. [DOI] [PubMed] [Google Scholar]
- 72.Shi C, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang SH, et al. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp. Mol. Med. 2009;41:315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Selmani Z, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 75.Cao W, et al. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity. 2011;35:273–284. doi: 10.1016/j.immuni.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 76.Djouad F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 77.Sioud M, et al. Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int. J. Oncol. 2011;38:385–390. doi: 10.3892/ijo.2010.869. [DOI] [PubMed] [Google Scholar]
- 78.Di Nicola M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez MA, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 80.Fiorina P, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J. Immunol. 2009;183:993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Q, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nemeth K, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc. Natl. Acad. Sci. U.S.A. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–531. doi: 10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 84.Djouad F, et al. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nature reviews. Rheumatol. 2009;5:392–399. doi: 10.1038/nrrheum.2009.104. [DOI] [PubMed] [Google Scholar]
- 85.Sensebe L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87:S49–S53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- 86.Augello A, et al. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 87.Djouad F, et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–1603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 88.Youd M, et al. Allogeneic mesenchymal stem cells do not protect NZBxNZW F1 mice from developing lupus disease. Clin. Exp. Immunol. 2010;161:176–186. doi: 10.1111/j.1365-2249.2010.04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaplan JM, et al. Immunomodulatory activity of mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2010;1:2. doi: 10.2174/157488811797904353. [DOI] [PubMed] [Google Scholar]
- 90.Planka L, et al. Allogeneic and autogenous transplantations of MSCs in treatment of the physeal bone bridge in rabbits. BMC Biotechnol. 2008;8:70. doi: 10.1186/1472-6750-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolf D, et al. Regenerative capacity of intravenous autologous, allogeneic and human mesenchymal stem cells in the infarcted pig myocardium-complicated by myocardial tumor formation. Scand. Cardiovasc. J. 2009;43:39–45. doi: 10.1080/14017430802100280. [DOI] [PubMed] [Google Scholar]
- 92.Allison M. Genzyme backs Osiris, despite Prochymal flop. Nat. Biotechnol. 2009;27:966–967. doi: 10.1038/nbt1109-966. [DOI] [PubMed] [Google Scholar]
- 93.Karussis D, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prasad VK, et al. Efficacy and safety of icultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol. Blood Marrow Transplant. 2011;17:534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 95.Wakitani S, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J. Tissue Eng. Regen. Med. 2011;5:146–150. doi: 10.1002/term.299. [DOI] [PubMed] [Google Scholar]
- 96.Prockop DJ, et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12:576–578. doi: 10.3109/14653249.2010.507330. [DOI] [PubMed] [Google Scholar]
- 97.Jeong JO, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011;108:1340–1347. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spaeth EL, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ning H, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–599. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]