Abstract
Aims
Nitric oxide (NO) deficiency contributes to chronic kidney disease progression. Nebivolol, a beta adrenergic receptor antagonist, may enhance endogenous NO. Here, we investigated whether nebivolol attenuates hypertension and renal injury after 5/6 ablation/ infarction (A/I). Efficacy was compared to the AT1 receptor antagonist olmesartan.
Main Methods
Kidney disease and hypertension were induced by right kidney ablation and ~2/3 infarction of the left kidney. Rats were treated orally with vehicle (Placebo), nebivolol (5 mg/kg b.i.d.), or olmesartan (2.5 mg/kg/day) for 6 weeks after A/I.
Key Findings
With placebo, glomerular sclerosis and tubulointersititial fibrosis developed with increased blood pressure and proteinuria, and a fall in NOx excretion. Olmesartan prevented these changes, but Nebivolol had no effect on these measures but lowered heart rate. Neither treatment reduced systemic oxidative stress (urinary hydrogen peroxide and TBARS). Compared to controls, renal cortex abundance of nNOSα decreased and nNOSβ increased in rats after 5/6 A/I, with no changes in eNOS. Neither treatment restored nNOSα; however, both reduced nNOSβ. Activity of DDAH was decreased by 5/6 A/I but restored by both treatments despite no increase in DDAH protein abundance. Kidney cortex abundance of manganese SOD fell after 5/6 A/I and was restored by treatment with olmesartan but not nebivolol. Extracellular and copper/zinc SOD abundances were not changed.
Significance
In conclusion, nebivolol showed no benefit after 6 weeks in rapidly progressing, ANG II-dependent 5/6 A/I model of chronic kidney disease. This contrasts to the protection seen with 6 months nebivolol in the slowly progressing 5/6 ablation model.
Keywords: Olmesartan, glomerular sclerosis, nitric oxide, proteinuria, hypertension
Introduction
Production of nitric oxide (NO) is decreased in chronic kidney disease (CKD) and this contributes to cardiovascular risk and to further progression of kidney damage (Baylis, 2008). Interventions that can restore NO production are likely to reduce the cardiovascular complications of CKD as well as slow the rate of progression of renal injury. In addition to its β-adrenergic blocking action, Nebivolol enhances NO production/ activity by several mechanisms (Ignarro, 2004) which make Nebivolol a potential novel therapeutic for use in treatment of CKD. While the NO stimulatory actions of Nebivolol are still not completely understood, there is evidence that Nebivolol exerts antioxidant actions by inhibition of NADPH oxidase (Ignarro, 2004;Mollnau et al., 2003; Oelze et al., 2006; Garbin et al., 2008; Whaley-Connell et al., 2009) which may prevent NOS uncoupling. In addition, previous studies have shown that Nebivolol increases the abundance and phosphorylation of endothelial nitric oxide synthase (eNOS) (Ignarro, 2004; Georgescu et al., 2005; de Nigris et al., 2008; Reidenbach et al., 2007; Gauthier et al., 1998; Rozec et al., 2009 Dessy et al., 2005) and Nebivolol activates inducible NOS in the heart (Maffei et al., 2007). Nebivolol may also enhance NO production via prevention of the accumulation of the endogenous NOS inhibitor, asymmetric dimethylarginine (ADMA) (Garbin et al., 2007, Oguz et al., 2007, Sen et al., 2009, Pasini et al., 2008).
The blood pressure lowering effects of Nebivolol have been widely studied, and there is some information regarding the effects of this agent in models of CKD. Nebivolol exerts short (4 weeks) and long (6 months) term renal protection in the 5/6 nephrectomy (Nx) model (Gschwend et al, 2009; Pires et al., 2007), the transgenic Ren 2 rat (Whaley-Connell et al., 2009; Hayden et al., 2010), high-salt fed spontaneously hypertensive rats (Varagic et al., 2010), and the zucker obese rat (Habibi et al., 2011; Toblli et al., 2011). In addition, we recently reported that nebivolol is protective against hypertension and CKD during chronic NOS inhibition with L-NAME, (Moningka et al., 2012). In many of these studies, the reno-protective effect is associated with preserved NO and/or diminished oxidative stress.
The 5/6 renal ablation/ infarction model (5/6 A/I) of CKD and hypertension is also associated with reduced renal NOS activity and a decline in renal neuronal NOS abundance that is correlated with the degree of glomerular sclerosis (Szabo et al., 2003). We proposed that restoration of the renal NO system in this model would improve renal injury and delay the progression of CKD. Since Nebivolol has been shown to increase NO production, the goal of the current study was to investigate the impact of Nebivolol on the development of hypertension and renal injury and the determinants of renal NO production in this severe and rapidly progressing model of CKD. Since chronic RAS inhibition is used clinically for control of CKD progression and hypertension (Norris and Vaughn, 2003; Chobanian et al, 2003), we also compared the actions of Nebivolol to those of Olmesartan, an angiotensin type 1 (AT1) receptor antagonist.
Methods
Studies were performed in 31 male Sprague Dawley (SD) rats (obtained from the Dublin, VA facility of Harlan Sprague Dawley) at 10–12 weeks of age. All experiments were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved and monitored by the University of Florida Institutional Animal Care and Use Committee. Animals were housed under conditions of constant temperature and humidity and exposed to a 12:12-h light-dark cycle. Rats were allowed ad lib drinking water and rat chow during acclimatization to the animal facility (~ 1 week). Some of the rats were implanted with telemetry transmitters (Data Sciences, St. Paul, MN) for the measurement of blood pressure in conscious, freely moving rats. Rats were anesthetized with isoflurane (IsoFlo; Abbott Laboratories, North Chicago, IL), and telemetry transmitters were implanted in the left femoral artery with the transmitter housed in the abdomen and attached to the abdominal wall. Rats were allowed to recover from surgery and returned to individual housing for 7 to 10 days before initiation of data acquisition. Mean arterial pressure (MAP) and heart rate (HR) were recorded continuously over a 24h period for 5 min every 30 min at baseline and at 2 week intervals.
Rats were introduced to daily aliquots of ½ tsp chocolate pudding (future vehicle for Nebivolol delivery, Hunts Snack Pack, Con-Agra Foods), and intake was monitored. Occasional rats that were reluctant to eat the pudding were excluded from further study, and all rats included in the study consumed the full amount of pudding.
One day before baseline (and subsequent) metabolic cage measurements, rats were placed on a low nitrate, otherwise normal and complete diet (ICN AIN 76C, MP Biomedical, Solon, OH). Rats were placed in the metabolic cage for 16 h during which free access to distilled drinking water was allowed but food was withheld. Rats then underwent 5/6 A/I surgery, conducted under full sterile conditions. Animals were anesthetized with isoflurane, the left and right flank were shaved and an incision was made under the floating rib, and the left kidney was exposed. Using 6-0 silk, branches of the renal artery were ligated to achieve a two-third infarction of the left kidney. The right kidney was removed after ligation of the renal vein and artery. One day later, rats were randomized to one of the 3 following groups: Placebo (P), in which rats received drinking water containing 0.1% sodium bicarbonate and 5% ethanol (vehicle for Olmesartan) and 2 × daily aliquots of ½ tsp of chocolate pudding (n=7); Nebivolol (N), in which rats received drinking water containing 0.1% sodium bicarbonate and 5% ethanol and 2 × daily aliquots of ½ tsp of chocolate pudding each containing 5 mg/kg of Nebivolol (Forest Pharmaceuticals, Inc., to deliver a total daily dose of 10 mg/kg/day) (n=10); Olmesartan (O) in which rats received water containing 25mg/L Olmesartan (LGM Pharma, Inc. or Topharman Shanghai Co. Ltd.) in 0.1% sodium bicarbonate and 5% ethanol to deliver 2.5 mg/kg/day and 2 × daily aliquots of ½ tsp chocolate pudding (n=9). Water and pudding consumption was monitored daily to ensure that rats were ingesting the correct drug doses. MAP and HR were measured by telemetry in 5/7 rats in the P group; in 6/10 rats in the N group and in 5/9 rats in the O group. An additional 5 age-matched SD rats were studied as an untreated control group, without telemetry.
Body weight (BW) was measured at baseline and then 2–3 times weekly, and 16h metabolic cage measurements were performed at baseline and week 5–6 of study for overnight urine collection. On these days, the nebivolol was given as a single dose (10mg/kg/day), 8 h before rats were placed in the metabolic cage, to prevent interference with the NOx measurements. These urine samples were stored in aliquots at −80°C for later analysis.
After the final measurement, rats were anesthetized with isoflurane, a terminal blood sample (~3ml) was taken via the abdominal aorta and then centrifuged, and plasma was stored in aliquots at −80°C for later analysis. The vasculature was perfused with cold PBS, the left kidney removed and weighed, a midcoronal section was cut and placed in 10% buffered formalin for pathology. The cortex was isolated from the remaining kidney tissue, flash frozen in liquid nitrogen, and stored at − 80°C for later analysis.
Chemical Analyses
Plasma and urine creatinine were measured by HPLC (Sasser et al, 2010), and urine NOx concentration was determined by Griess assay, as previously described (Suto et al, 1995). Urine protein was measured by the Bradford assay. Urinary excretion of thiobarbituric acid reactive substances (TBARS) was measured using the OxiTek TBARS assay kit (Zeptometrix), and urinary H2O2 excretion was measured using Amplex Red (Molecular Probes),
Western blotting
Protein abundances were detected using Western blotting, as previously described (Xiao et al, 2001). Briefly, samples (200 µg of kidney cortex) were separated by electrophoresis, transferred (1 hr, 0.46 Amps) to Hy-bond nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). Immediately following the transfer, the membranes were stained for 10 minutes with Ponceau S, washed 3 times with water, and then photographed using the Bio-Rad Versa Doc Imaging System. The membranes were then probed for specific proteins as shown in Table 1. Bands of interest were visualized using enhanced chemiluminescence reagent and quantified by densitometry (VersaDoc imaging system and Quantity One Analysis software, Bio-Rad) as integrated optical density (IOD) after subtraction of background. To quantitate total protein loaded per lane, we used Bio-Rad Quantity One software to determine density of the Ponceau S stain over the entire lane. The density of the specific band of interest (IOD) was then divided by the density of the Ponceau S stain for the lane to correct for any variations in total protein loading. This value was then factored by a standard positive control used to correct for any variations between membranes (2 membranes were used for each protein). Protein abundance is represented as IOD/Ponceau Red/positive control (PC).
Table 1.
Western blotting conditions and antibodies used.
| Protein | % Acrylamide Gel |
Electrophoresis Conditions |
Primary Antibody / Dilution |
Secondary Antibody / Dilution |
|---|---|---|---|---|
| nNOS α | 6 | 35 mA, 2.5 hr | Santa Cruz, 1:50 | goat anti-mouse (BioRad 170-6516), 1:3000 |
| nNOS β | 6 | 35 mA, 2.5 hr | Thermo Scientific, formerly ABR PA1-033, 1:500 | goat anti-rabbit (BioRad 170-6515), 1:3000 |
| eNOS | 7.5 | 35 mA, 2.5 hr | BD Transduction, 1:250 | goat anti-mouse (BioRad 170-6516), 1:2000 |
| p22phox | 12 | 140 V, 65 min | Santa Cruz sc-11712, 1:50 | donkey anti-goat (Santa Cruz sc-2020),1:2000 |
| ecSOD | 12 | 140 V, 65 min | Abcam ab21974, 1:250. | goat anti-rabbit (BioRad 170-6515), 1:2000 |
| MnSOD | 12 | 140 V, 65 min | Stressgen SOD-111, 1:2000. | goat anti-rabbit (BioRad 170-6515), 1:2000 |
| CuZn SOD | 12 | 140 V, 65 min | Stressgen SOD-101, 1:2000. | goat anti-rabbit (BioRad 170-6515), 1:2000 |
| PRMT-1 | 12 | 140 V, 65 min | Millipore 07-404, 1:2000 | goat anti-rabbit (BioRad 170-6515), 1:2000 |
| DDAH-1 | 12 | 140 V, 65 min | Santa Cruz sc-26068, 1:250 | donkey anti-goat (Santa Cruz sc-2020),1:2000 |
| DDAH-2 | 12 | 140 V, 65 min | Santa Cruz sc-32859, 1:250 | donkey anti-goat (Santa Cruz sc-2020),1:2000 |
| Nitrotyrosine | 7.5 | 50 mA, 2.5 hr | Upstate, 05-233, 1:500 | goat anti-mouse (BioRad 170-6516), 1:2000 |
Histology
After fixation in 10% buffered formalin the kidney section was blocked in paraffin wax and 5 µ thick sections were cut and stained with periodic acid schiff with hematoxylin/ eosin counterstain. Sections were examined, blind, for the level of glomerular sclerosis and tubulointerstitial fibrosis. Up to 100 glomeruli were scored as follows: 0 = healthy glomeruli, +1=<25% damage, +2=25% to 50% damage, +3=51% to 74% damage, and +4=>75% damage. A glomerulosclerosis index score was calculated using the following equation: (#of+1)+ 2(#of+2)+ 3(#of+3)+ 4(#of+4)/total glomeruli observed as described previously (Sasser 2011). Tubulointerstitial fibrosis was scored as 0=none, 1=10%, 2=10–25%, 3=25–50%, 4=50–75%, 5=75–100% as described (Moningka, 2011).
Statistical Analysis
Data are presented as mean ±SEM. Repeated measures two-way ANOVA with Bonferroni post test was used for comparing the effects of both treatment and time on BW, MAP, UProtV and UNOxV. One-way ANOVA with Bonferroni correction was used for 4 group comparisons for all normally distributed data, and Kruskal Wallis one way analysis was used for histological analysis.
Results
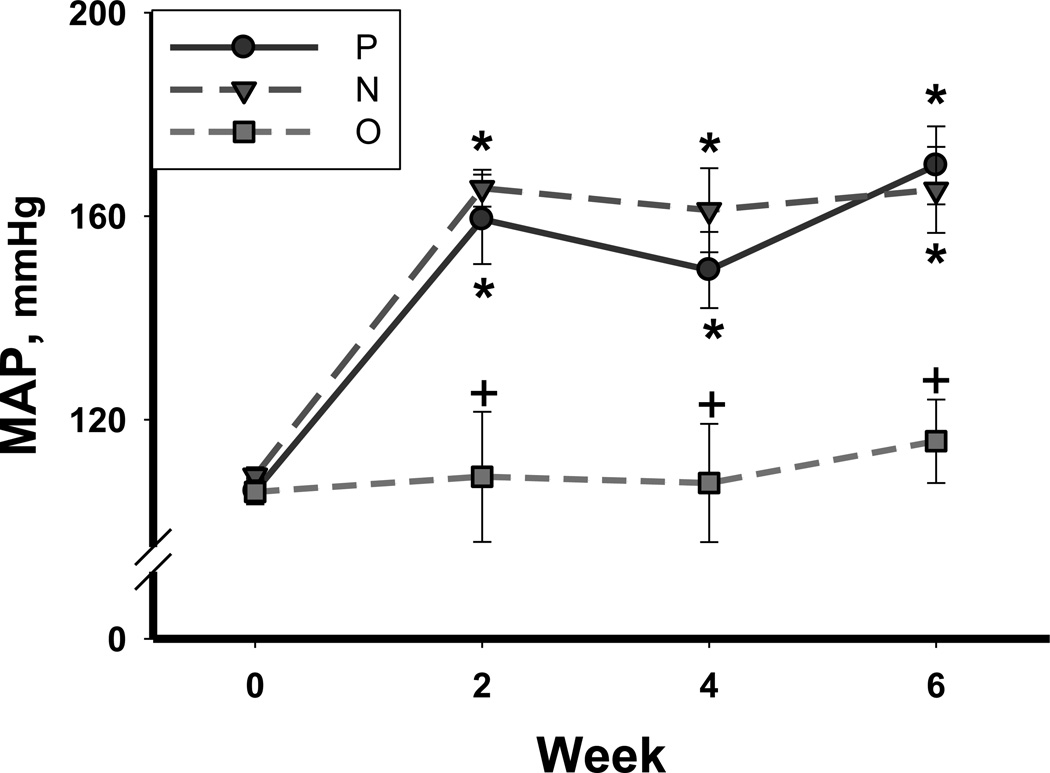
In the placebo treated 5/6 A/I rats (P), only 4/7 completed the 6 week observation period; 2 animals were sacrificed at week 4, and 1 at week 5, due to rapid deterioration. All rats given N or O survived until the 6th week. As shown in Table 2, the BW was similar in all groups at baseline and only N treated rats showed an increase in body weight at the 6th week of treatment. MAP was measured by telemetry in some rats in each group. As shown in Figure 1, MAP at baseline (week 0) was normal and similar in all rats (108±1 mmHg). MAP rapidly increased after 5/6 A/I in P and N treated rats and remained higher than baseline throughout the study (p<0.001). There was no change in MAP over the 6 week period in the O treatment group (Figure 1), and O treated rats had a lower MAP compared to P throughout the treatment (p≤0.05). Heart rate was significantly lowered by treatment with N (p<0.05) but was not changed by O (Table 2).
Table 2.
Body weight, Heart Rate, Creatinine Clearance, Plasma Creatinine, and urinary excretion rate of Protein, NO metabolites (NOx), Hydrogen Peroxide (H2O2), and thiobarbituric acid reactive substances (TBARS) excretion in Placebo, Nebivolol, and Olmesartan treated rats. n=6–10.
| Body weight (g) | Heart Rate (beats/ min) |
Creatinine Clearance (ml/min) |
Plasma Creatinine (mg/dl) |
Urinary Protein Excretion (mg/day) |
Urinary NOx Excretion (µmol/day) |
Urinary H2O2 Excretion (µmol/day) |
Urinary TBARS Excretion (nmol/day) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 6 | Week 6 | Week 6 | Week 6 | Baseline | Week 6 | Baseline | Week 6 | Week 6 | Week 6 | |
| Placebo | 377±14 | 374±10 | 339±17 | 2.1±0.8 | 1.0±0.3 | 24±6 | 307±121* | 2.0±0.4 | 0.5±0.3* | 0.14±0.03 | 113±25 |
| Nebivolol | 343±5 | 75±9* | 301±3* | 1.3 ± 0.7 | 0.7±0.1 | 28±4 | 213±36* | 2.5±0.4 | 1.1±0.3* | 0.20±0.01 | 121±19 |
| Olmesartan | 354±16 | 374±11 | 336±2 | 2.1 ± 0.4 | 0.6±0.1 | 21±4 | 84±16*+ | 2.3±0.2 | 1.9±0.7 | 0.14±0.03 | 83±5 |
denotes p<0.05 vs respective baseline,
denotes p<0.05 vs Placebo at same time point.
Figure 1.
Mean arterial pressure (MAP) measured by telemetry at baseline (time 0) and at 2, 4, and 6 weeks after 5/6 A/I surgery in placebo treated (P; solid circles), Nebivolol treated (N; inverted triangle), and Olmesartan treated (O; square) rats. n=6–10. * denotes p<0.05 vs respective baseline, + denotes p<0.05 vs placebo at same time point.
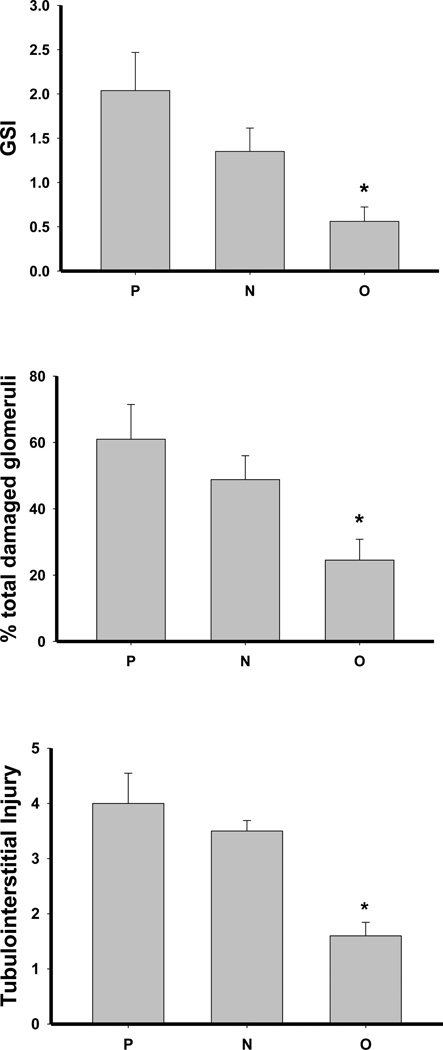
No differences were observed in creatinine clearance or plasma creatinine concentration among the 5/6 A/I groups (Table 2). There was significant glomerular sclerosis and tubulointersititial fibrosis in placebo treated rats, and while this was not lowered with Nebivolol treatment, Olmesartan substantially reduced both glomerular and interstitial injury (Figures 2–3). At baseline, the urine protein excretion was low and similar in all groups and was elevated at week 5–6 in all groups; however, proteinuria at 6 weeks was lower in O vs P (p<0.05, Table 2). The 24h UNOxV was similar in all groups at baseline and fell significantly at week 5– 6 in P and N treated rats but not in O treated rats (Table 2).
Figure 2.
Glomerular sclerosis index (GSI, A), percent of glomeruli showing any degree of damage (B), and tubulointerstitial injury (C) in placebo treated (P), Nebivolol treated (N), and Olmesartan treated (O) rats. n=6–10. * denotes p<0.05 vs P.
Figure 3.
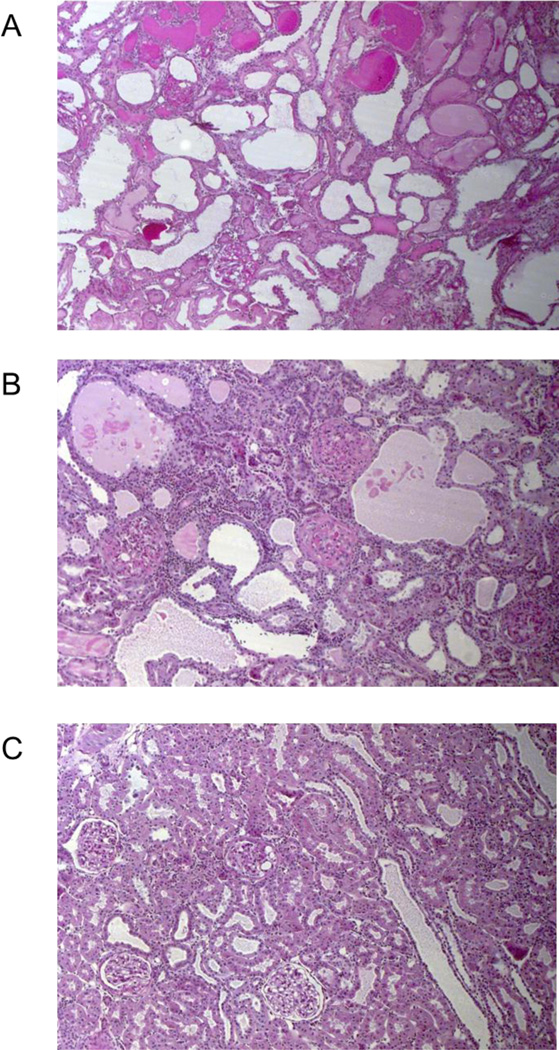
Panels A–C show low power (100×) views of the kidney cortex of rats after ~ 6 weeks of 5/6 AI for (A) placebo (untreated), (B) Nebivolol treated and (C) Olmesartan treated rats. Panel A shows loss of the normal tubulo-interstitial organization, areas of dilated tubules and several casts; Panel B also shows considerable tubulo-interstitial injury with some dilated tubules, areas of tubule atrophy and many infiltrating inflammatory cells; Panel C shows a fairly normal tubulo-interstitium with one cast on the far left of the field.
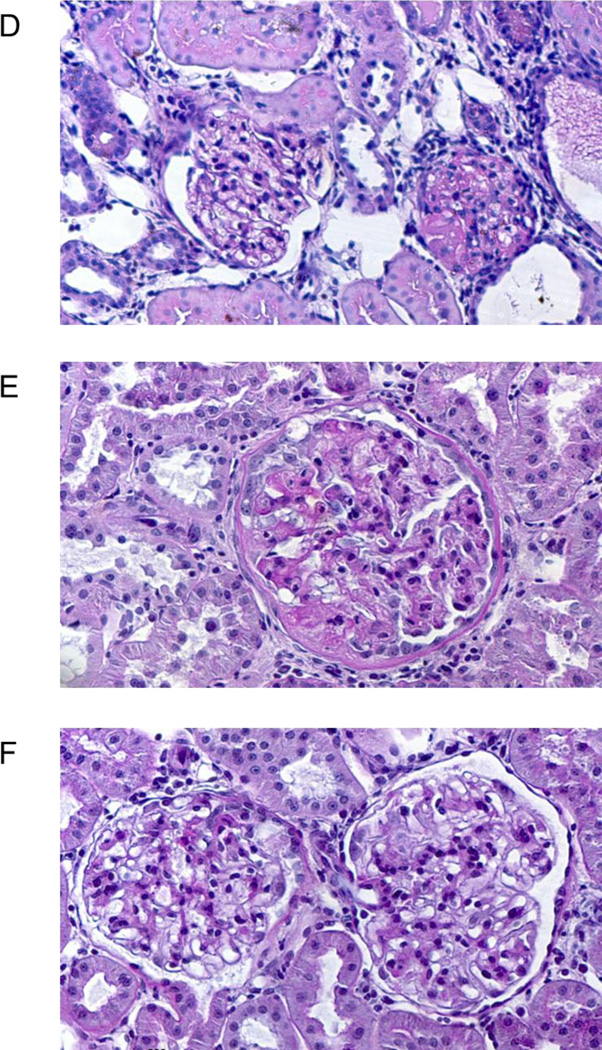
Panels D–F show high power (400×) views of the glomeruli in the 3 groups. Panel D shows a relatively normal glomerulus on the right and on the left, a glomerulus with ~75% sclerosis , from a placebo kidney; Panel E shows an enlarged glomerulus with ~ 50% glomerular sclerosis, thickening of Bowman’s capsule and an area of adhesion of the tuft to the to the capsule as well as bleb formation (at 11 o’clock), from a nebivolol treated kidney; Panel F shows 2 fairly normal glomeruli with mesangial expansion in the lower right quadrant of the left glomerulus.
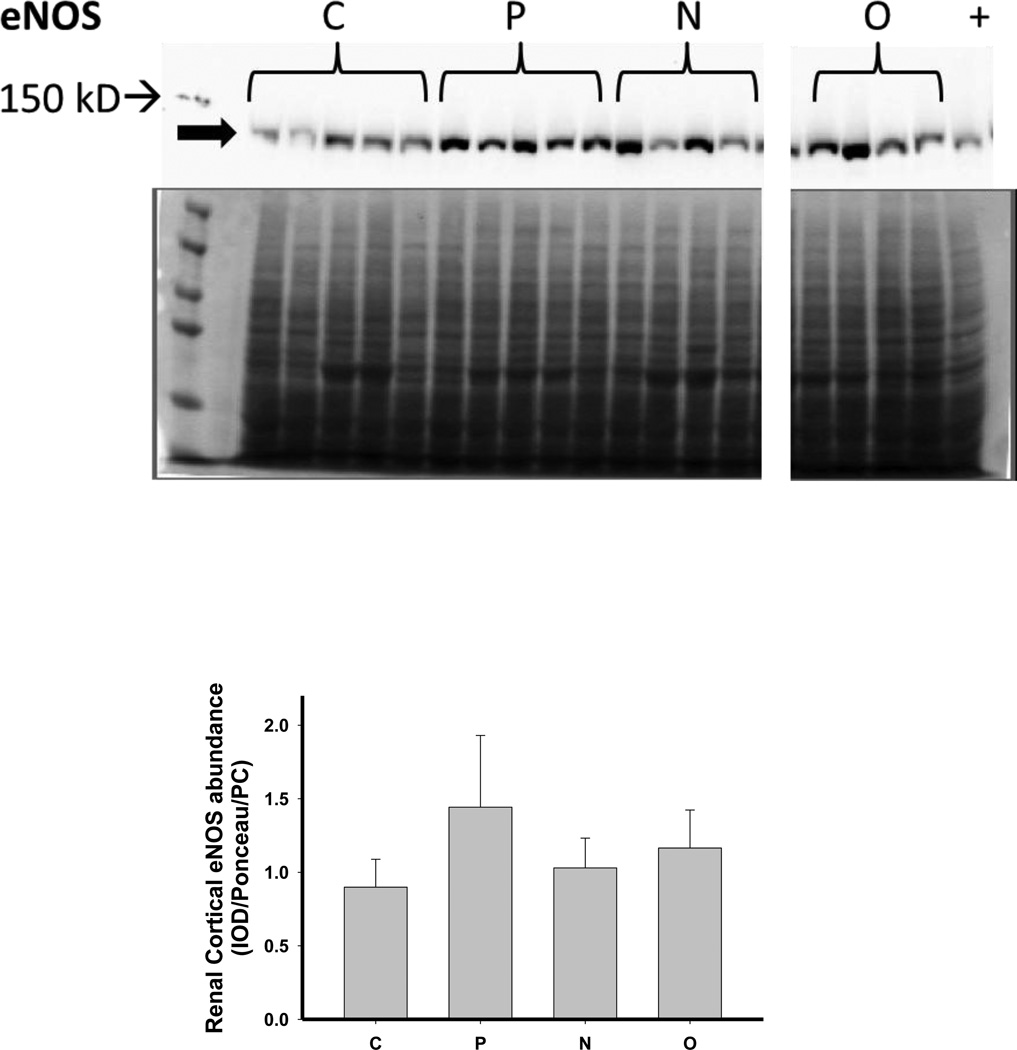
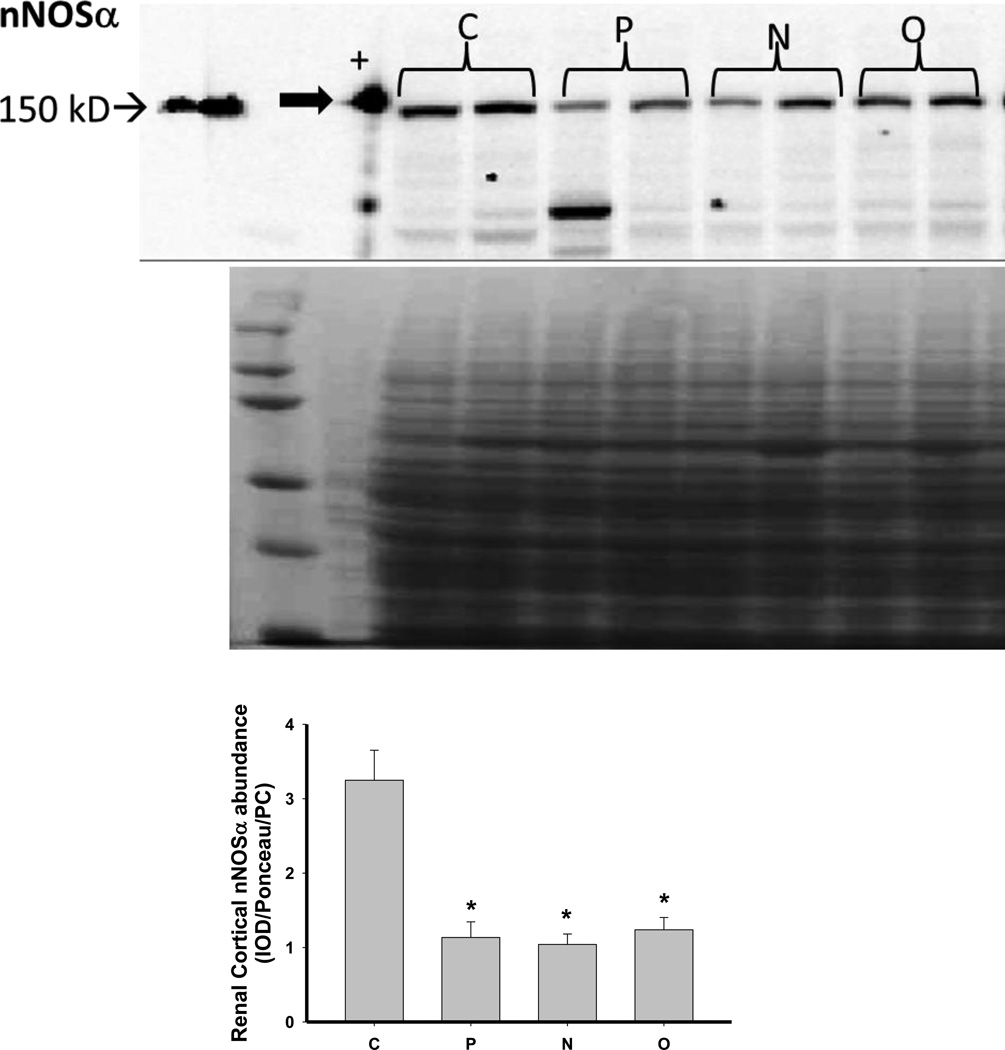
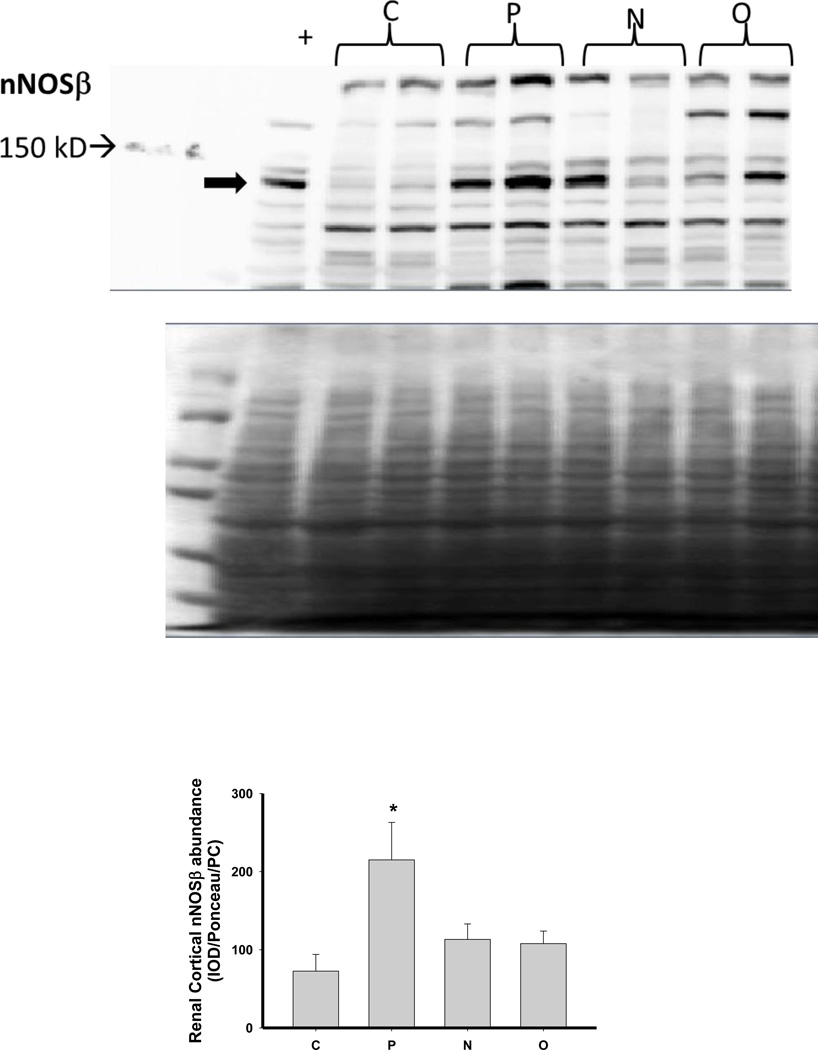
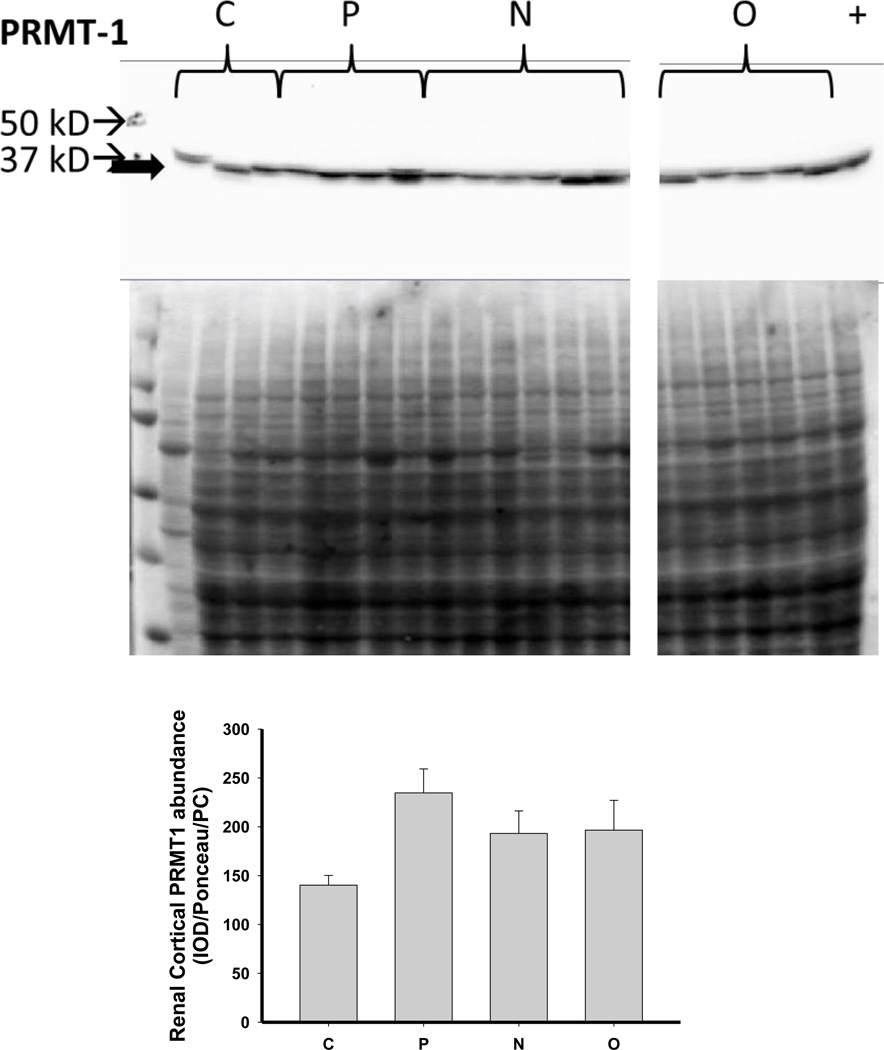
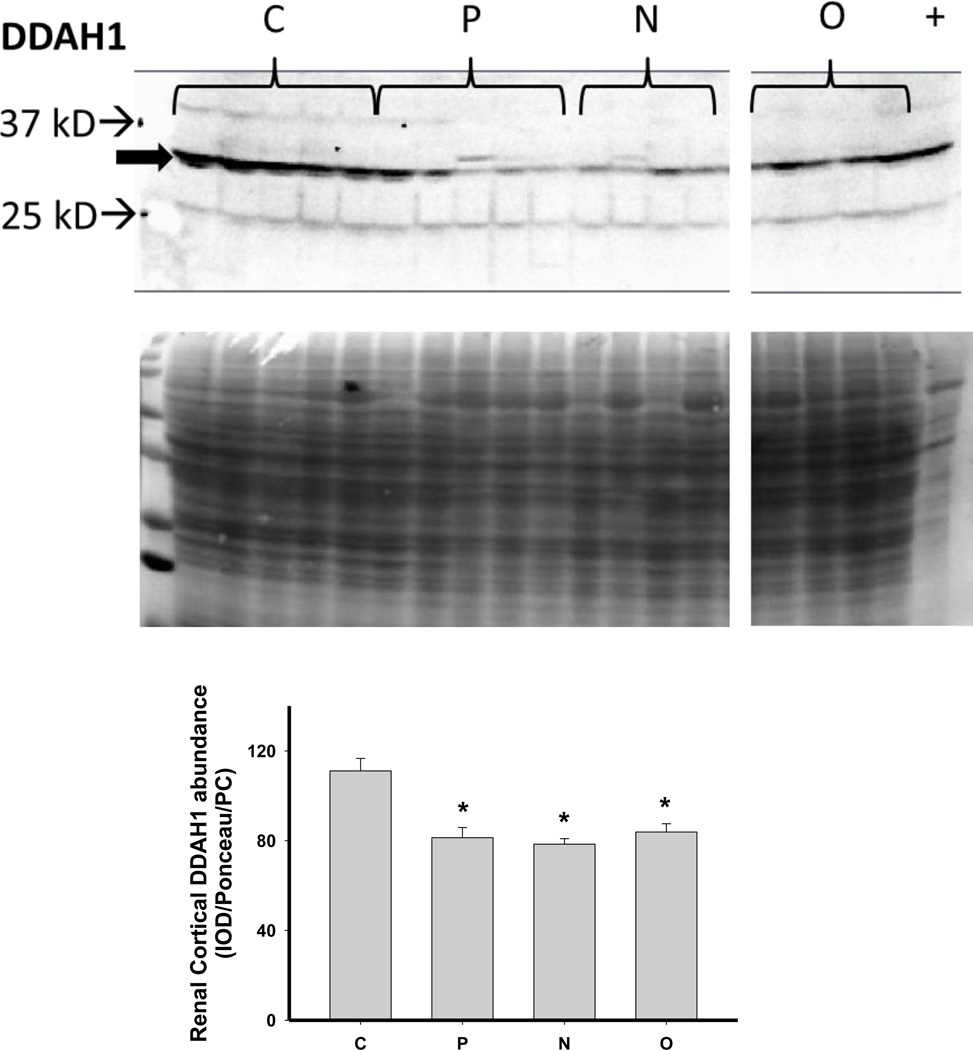
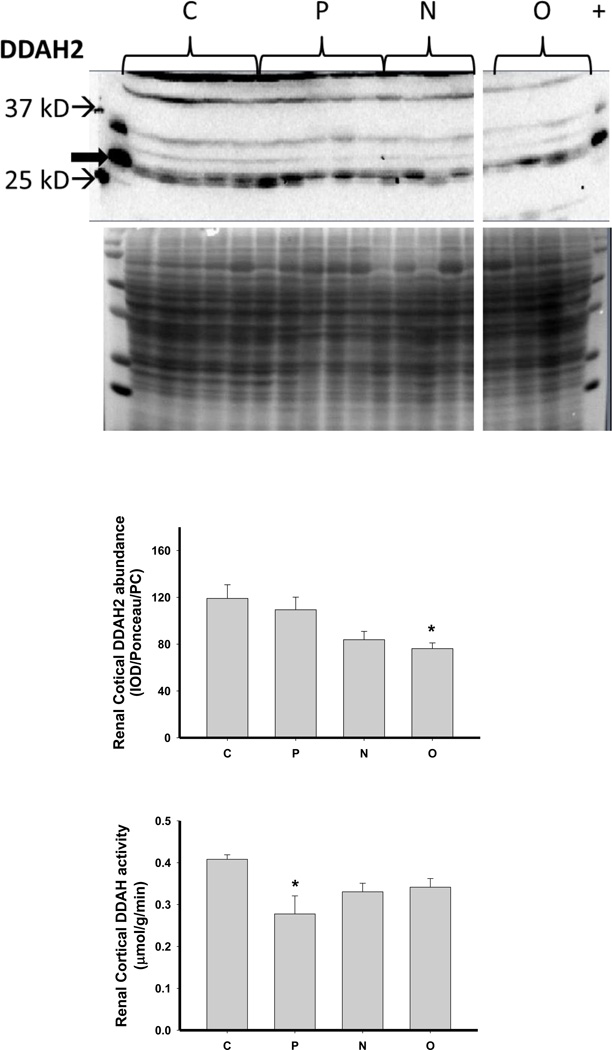
We next investigated the impact of 5/6 A/I and the two treatments on indices of renal cortical NO and oxidant/ antioxidants in comparison with normal control (C) age-matched rats. As shown in Figure 4, 5/6 A/I had no impact on eNOS protein abundance, while nNOSα was markedly decreased and nNOSβ protein abundance was increased compared to C (p<0.05). Neither of the treatments (N nor O) restored nNOSα abundance in the kidney cortex; however, both N and O reduced nNOSβ protein abundance to control levels. We next examined the expression of the enzymes responsible for the generation and degradation of the NOS inhibitor ADMA. PRMT-1 abundance in the renal cortex was not altered by 5/6 A/I or either treatment (Figure 5). DDAH1 abundance was decreased by 5/6 A/I, and this decline persisted with N and O. While DDAH2 abundance was unaffected by 5/6 A/I, DDAH2 was lower in 5/6 A/I rats treated with O. Despite the fact that DDAH abundance was not increased by the treatments, the fall in DDAH activity after 5/6 A/I (p=0.02) was restored ~ to control levels by treatment with both N or O.
Figure 4.
Representative Western blots, representative images of total protein loading (ponceau red staining), and protein abundance (factored for total protein loaded and a positive control) in kidney cortex of endothelial nitric oxide synthase (eNOS), neuronal NOS (nNOS)α and nNOSβ in placebo treated (P), Nebivolol treated (N), and Olmesartan treated (O) rats 6 weeks after 5/6 renal ablation/infarction and age-matched control (C) rats. n=6–10. * denotes p<0.05 vs C.
Figure 5.
Representative Western blots, representative images of total protein loading (ponceau red staining), and protein abundance (factored for total protein loaded and a positive control) in kidney cortex of the protein methyltransferase 1 (PRMT1) and the dimethylarginine dimethylaminohydrolase (DDAH) isoforms 1 and 2 and DDAH activity in the renal cortex of placebo treated (P), Nebivolol treated (N), and Olmesartan treated (O) rats 6 weeks after 5/6 renal ablation/infarction and age-matched control (C) rats. n=6–10. * denotes p<0.05 vs C.
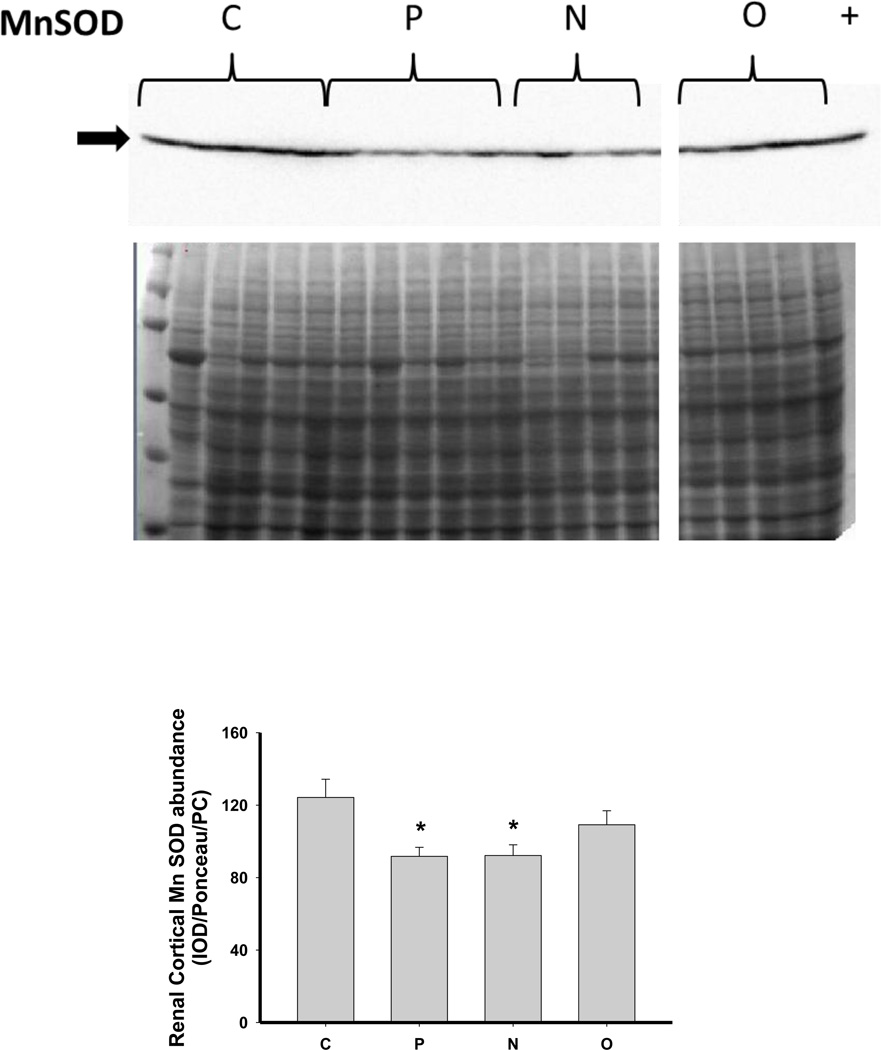
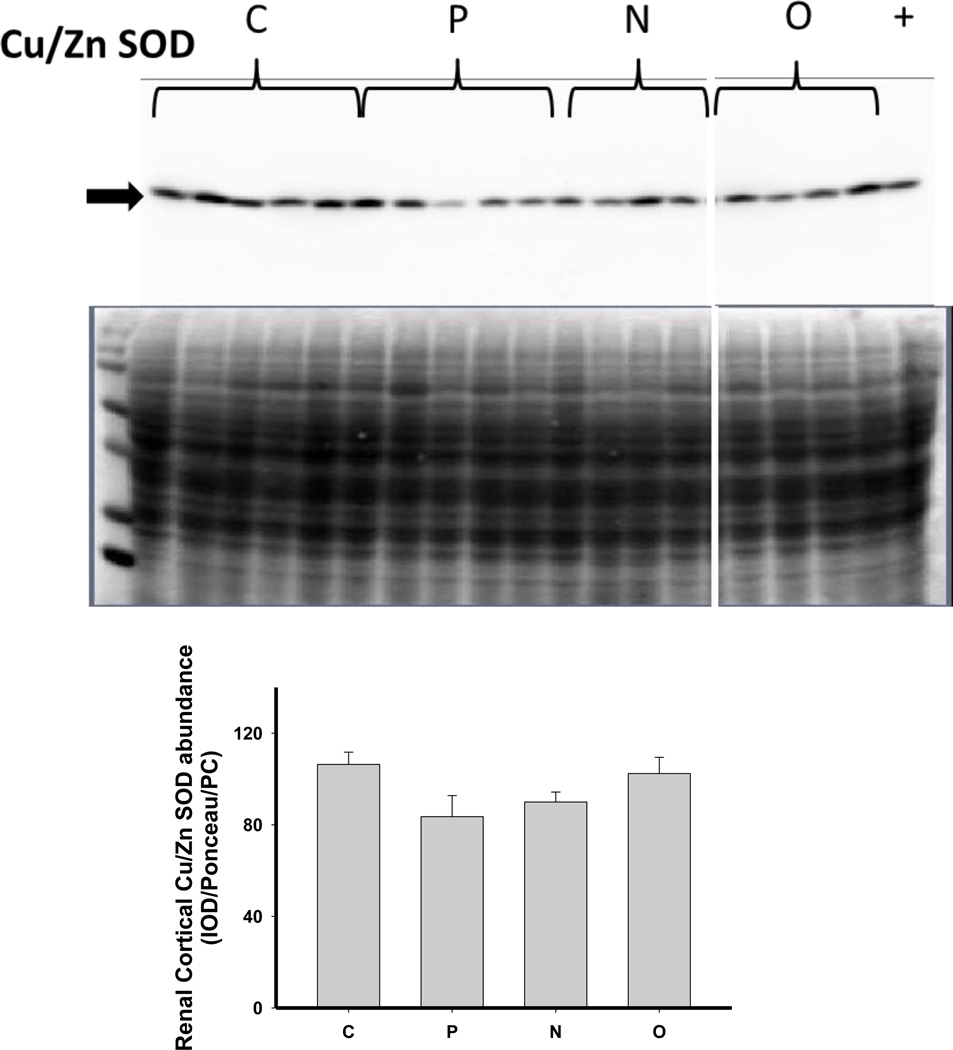
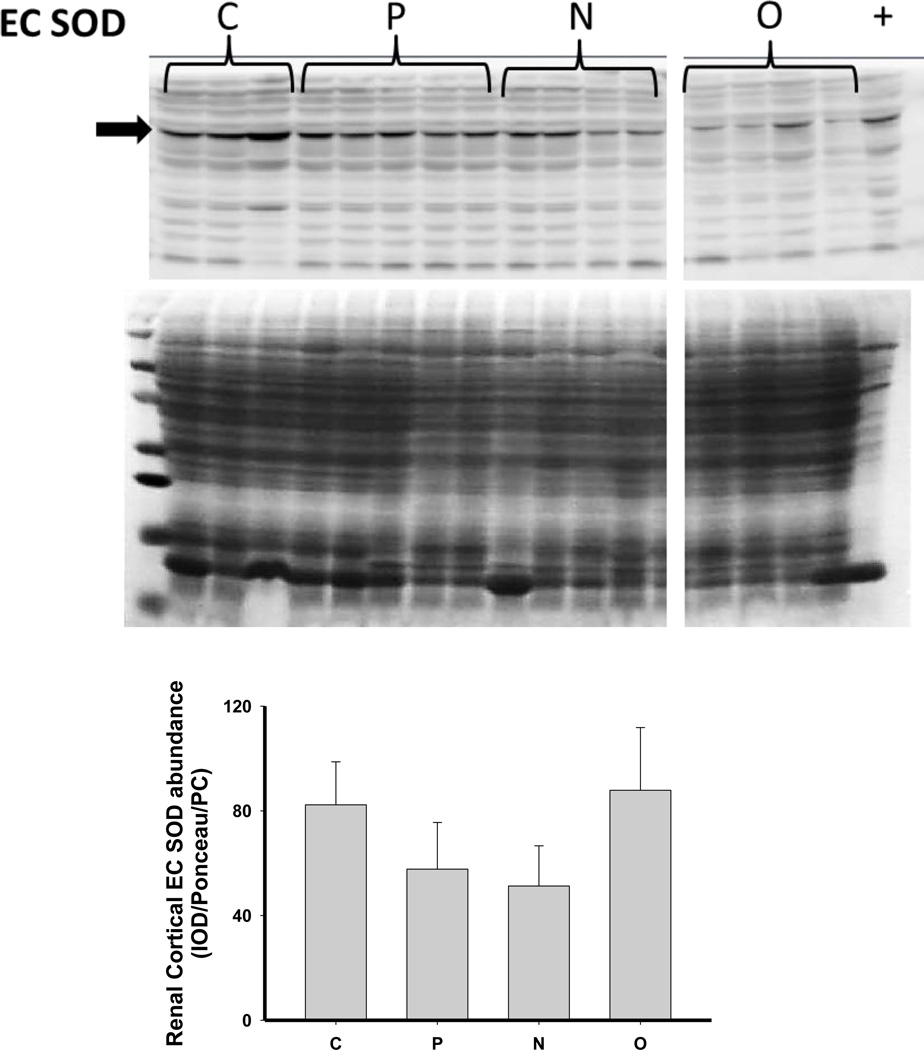
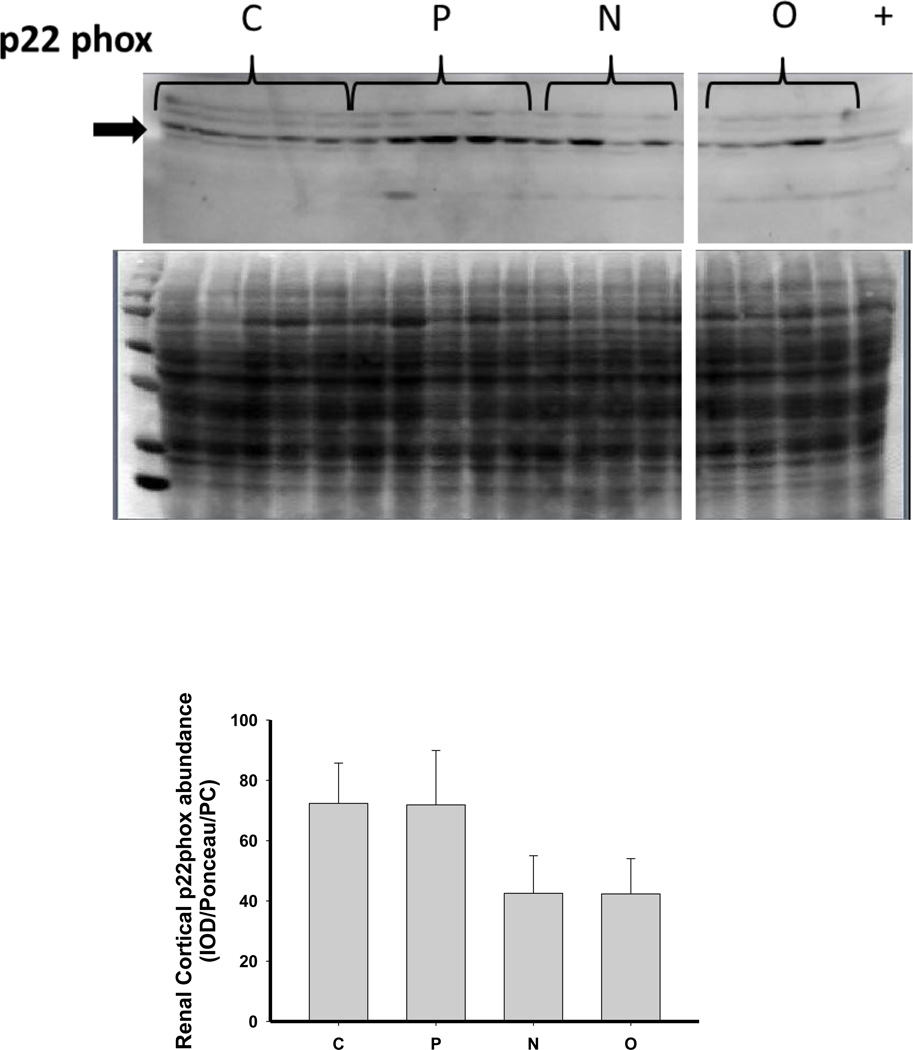
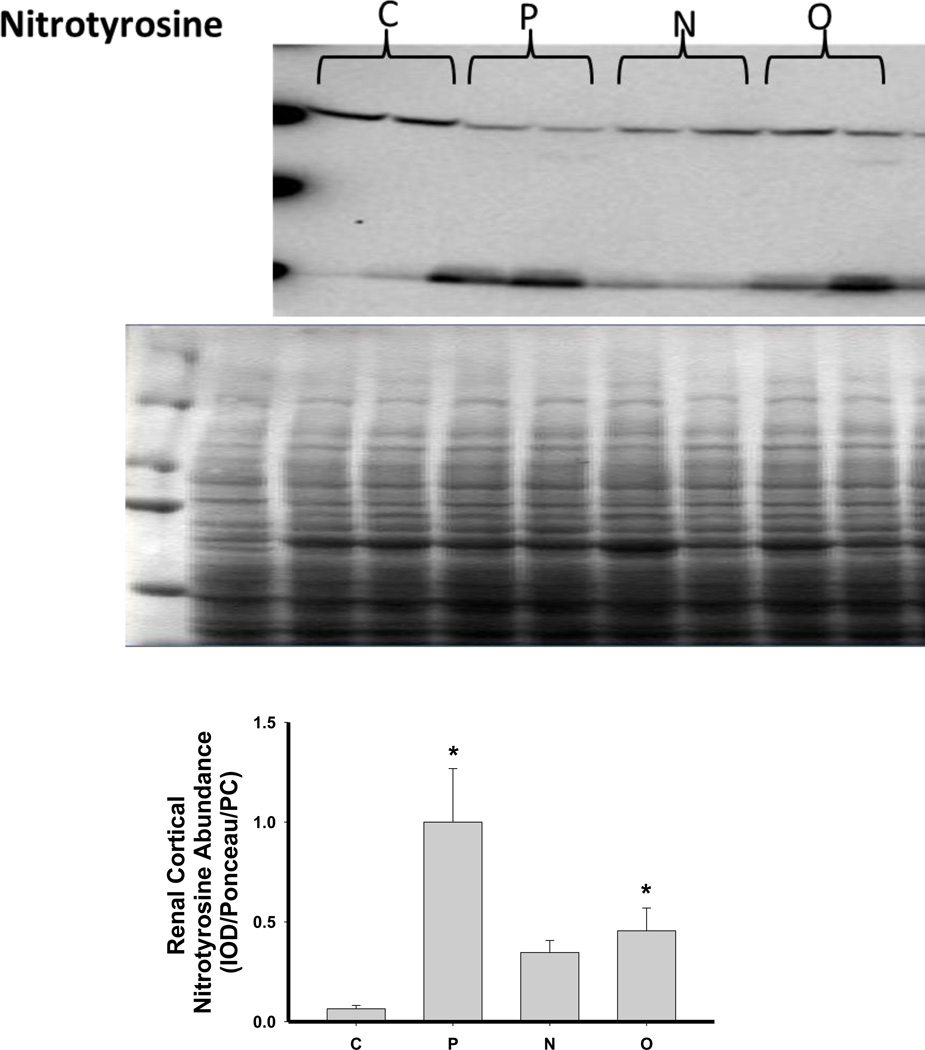
As shown in Figure 6, there was no change in the expression of extracellular superoxide dismutase (ecSOD) or copper/zinc SOD (Cu/Zn SOD) after 5/6 A/I or with either treatment. Renal cortical abundance of manganese SOD (mnSOD) fell after 5/6 A/I, and this was restored by treatment with O but not by N. The NADPH oxidase subunit p22phox was also unchanged vs. control, but the renal cortical abundance of nitrotyrosine, a marker of peroxynitrite formation, was increased after 5/6 A/I (Figure 6). While levels of nitrotyrosine appeared lower in the treated groups, this did not reach statistical significance. Urinary excretion of hydrogen peroxide and TBARS was not altered by either N or O treatment (Table 2), and all groups exhibited greater excretion of peroxide and TBARS than historical control groups (peroxide: 0.03±0.01 µmol/day, TBARS: 55±4 nmol/day, Sasser et al., 2011).
Figure 6.
Representative Western blots, representative images of total protein loading (ponceau red staining), and protein abundance (factored for total protein loaded and a positive control) in kidney cortex of extracellular superoxide dismutase (EC SOD), copper zinc (CuZn; cytosolic) SOD, manganese (Mn; mitochondrial) SOD, the p22phox subunit of NADPH oxidase, and nitrotyrosine in placebo treated (P), Nebivolol treated (N), and Olmesartan treated (O) rats 6 weeks after 5/6 renal ablation/infarction and age-matched control (C) rats. n=6–10. * denotes p<0.05 vs C.
Discussion
These studies show that Nebivolol has no protective action against renal injury, proteinuria or hypertension in the rapidly progressing 5/6 A/I model of CKD. Furthermore, Nebivolol did not alter measures of NO production or oxidative stress in rats with 5/6 A/I. Nebivolol was, however, effective in lowering heart rate, confirming delivery of the drug. In contrast, the angiotensin type 1 receptor (AT1) blocker, Olmesartan, lowered blood pressure, reduced renal injury and preserved nitric oxide production in rats with 5/6 A/I despite having no effect on the measured indices of increased oxidative stress.
The preclinical studies with Nebivolol in CKD have been quite impressive. Nebivolol treatment reduced renal injury and oxidative stress in the transgenic Ren 2 rat (Whaley-Connell et al., 2009; Hayden et al., 2010), the zucker obese rat (Habibi et al., 2011) and in high-salt fed spontaneously hypertensive rats (Varagic et al., 2010), and these improvements were associated with increases in renal endothelial or neuronal NOS expression. In addition, we recently reported that Nebivolol is protective against both the hypertension and CKD produced by chronic NOS inhibition with L-NAME, with an associated reduction in oxidative stress (Moningka et al., 2012). These previous studies highlight an important role of maintaining the NO / oxidative stress balance in the therapeutic actions of Nebivolol.Our previous studies in the 5/6 A/I rat model of CKD indicated that loss of NO contributes to the hypertension and progression of CKD (Erdely et al., 2003; Szabo et al., 2003), and we therefore anticipated that Nebivolol would be protective in this form of CKD via NO preservation / restoration. In the present study, however, Nebivolol was without any antihypertensive effect and did not prevent the progression of kidney damage in rats with 5/6 A/I. It is unlikely that this lack of efficacy was due to inadequate dosing of the drug. These rats were treated orally with Nebivolol exactly as in our previous study using the NOS inhibition model of hypertension (Moningka, 2012) where we saw a profound protective effect of Nebivolol. In the chronic NOS inhibited rats receiving Nebivolol we also saw a reduction in heart rate, consistent with beta blockade, and we observed a similar fall in heart rate in the 5/6 AI rats given Nebivolol in this study. The lack of effect of Nebivolol was particularly unexpected since other groups have shown beneficial effects of this agent in a different model of 5/6 renal mass reduction, 5/6 Nx (Gschwend et al., 2009, Pires et al., 2007). The 5/6 Nx model involves surgical removal of the right and 2/3rds of the left kidney which produces slowly progressing kidney damage and delayed, mild hypertension, compared to the 5/6 A/I model, despite similar reduction in nephron number (Griffin et al, 1994). In rats with 5/6 Nx, studied over ~15 weeks, we previously reported that the antioxidant vitamin E reduces NADPH-dependent superoxide production and fibrotic injury (Tain et al., 2007), implicating oxidative stress in the pathogenesis of this form of CKD. Short-term Nebivolol treatment (4 weeks) reduced albuminuria in 5/6 Nx rats without lowering blood pressure or preserving renal function (Gschwend et al., 2009). In a long-term (6 month) study in rats with 5/6 Nx, Nebivolol reduced glomerular sclerosis and interstitial fibrosis (Pires et al., 2007). This protection was not primarily due to lowering the blood pressure since atenolol had similar antihypertensive actions but exerted much less protection. The majority of the protective effect appeared to be via enhanced NO and diminished oxidative stress (Pires et al., 2007).
The renal injury and the progression of hypertension is much more severe with 5/6 A/I vs. 5/6 Nx model and the fundamental difference between the models is the presence of the infarcted 2/3rds of the left kidney in the 5/6AI rats. This infarcted tissue is thought to release local and circulating agents which cause severe hypertension and accelerate the CKD progression. As reviewed by Griffin and colleagues (Griffin et al, 1994), an early increase in plasma renin activity (PRA) occurs but later PRA falls to normal although several groups suggest that activation of the intrarenal renin angiotensin system (RAS) sustains the hypertension and CKD progression; nevertheless the precise pathogenesis of this CKD model remains unclear. One thing that is clear, however, is that blockade of the RAS in 5/6 AI provides superior protection vs other, equivalent antihypertensive interventions, at least in part via reduction in glomerular blood pressure (Anderson et al., 1988).
In the present study, Nebivolol was not able to overcome the progression of disease in these 5/6 AI rats or show the same benefits as RAS inhibition with AT1 receptor blockade; a finding in sharp contrast to our earlier observations in the chronic NOS inhibition model where both Nebivolol and AT1 blockade were highly protective (Moningka et al, 2012). One difference between the 5/6 AI and chronic NOSI models is that the primary event with chronic NOS inhibition is loss of NO production which Nebivolol was able to reverse, whereas this is a secondary event in 5/6 AI, perhaps driven by activation of RAS, and is unresponsive to Nebivolol. Although we did not directly measure renal NOS activity in the present study, we observed no effect of Nebivolol on urinary NOx excretion (an index of systemic NO production), whereas Olmesartan did prevent the fall in total NO bioavailability seen in the placebo group.
A number of factors could contribute to the fall in NO production with 5/6 AI. We previously reported a shift in the distribution of the nNOS isoforms in the 5/6 A/I kidney, with loss of renal cortex nNOSα and an increase in nNOSβ (Smith et al., 2009); a similar pattern was observed here in the placebo group. We previously found similar changes in renal cortical nNOS in rats with chronic allograft nephropathy and 5/6 Nx (Tain et al., 2007; Tain et al., 2008; Tain et al., 2011). In the present study, however, neither Nebivolol nor Olmesartan had any effect on the fall in nNOSα in the kidney cortex although both prevented the rise in nNOSβ. In contrast to nNOS, renal cortex eNOS abundance varies according to the stage and model of CKD (Baylis, 2008), and in this study there was no effect on eNOS in placebo, Nebivolol or Olmesartan treated rats vs normal controls.
In addition to falls in NOS enzyme abundance, there are other potential causes of NO deficiency, including increased circulating levels of the endogenous NOS inhibitor ADMA. Increased ADMA can occur due to increased production (via methylation of arginine incorporated into proteins and subsequent proteolysis to release free ADMA) or by reduced ADMA catabolism by DDAH. Renal DDAH activity is a major contributor to plasma ADMA levels (Baylis, 2008). In vitro, Nebivolol stimulates NO production via its ability to stimulate DDAH abundance/ activity in endothelial cells and therefore reduce ADMA levels (Garbin et al., 2007). Plasma ADMA levels are elevated in patients with essential hypertension (Pasini et al., 2008) and in the spontaneously hypertensive rat (Wang et al., 2011) compared to normotensive controls. Sera from Nebivolol treated hypertensives increased the abundance of the DDAH2 isoform in cultured endothelial cells (Pasini et al., 2008). In the spontaneously hypertensive rat, Nebivolol lowered plasma ADMA while enhancing NO production and reduced aortic PRMT1 and increased DDAH2 levels (Wang et al., 2011). In the present study, we were unable to measure plasma ADMA due to technical reasons. Neither 5/6 A/I nor the treatments had an effect on renal cortex abundance of PRMT-1. Renal cortex DDAH activity was decreased in this model and both Nebivolol and Olmesartan reversed this although DDAH activity was not predicted by abundance of the DDAH isoforms. These data suggest that the activity of the renal DDAH enzymes is regulated by posttranslational mechanisms.
Another factor that reduces NO bioavailability is oxidative stress, and CKD is associated with increased oxidative stress. Previous studies in humans and experimental animals have demonstrated an antioxidant effect of Nebivolol (Ignarro, 2004; Mollnau et al., 2003; Oelze et al., 2006; Whaley Connell et al., 2009, Varagic et al., 2010; Toblli et al., 2011; Moningka et al., 2012); therefore, we also examined the effect of Nebivolol on measures of systemic and renal oxidative stress and renal cortex antioxidant enzyme abundance. The 5/6 A/I model was associated with an increase in nitrotyrosine immunoreactivity and a decrease in mnSOD abundance in the renal cortex vs. control. In addition, the excretion of both hydrogen peroxide and TBARS, a marker of lipid peroxidation, was elevated compared to historical controls (Sasser, 2011). These data confirm that the 5/6 A/I model of CKD does undergo increased oxidative stress, which may contribute to the pathogenesis of the disease in these rats. While Nebivolol did not restore mnSOD abundance, it did prevent the rise in renal nitrotyrosine staining which is suggestive that renal oxidative stress was reduced by Nebivolol, perhaps explaining the restoration of renal DDAH activity. In contrast, neither Nebivolol nor Olmesartan reduced hydrogen peroxide or TBARS excretion suggesting that neither agent affects these measures of systemic oxidative stress in this model of CKD.
Conclusion
In conclusion, this study shows that olmesartan is superior to Nebivolol treatment in the rapidly progressing 5/6 A/I model of chronic kidney disease. While we cannot determine from these studies whether the effect of olmesartan on renal injury was secondary to the blood pressure reduction or was specific to the blockade of the renal AT1 receptor, the earlier studies by Anderson et al (1988) suggest that reduction of glomerular blood pressure is the key event in protecting the kidney. It is clear that olmesartan was more effective in restoring NO bioavailability in this model of CKD, suggesting that here the loss of NO was a secondary event in the pathogenesis. While Nebivolol is effective in treating many forms of experimental hypertension and mild to moderate CKD, including the 5/6 Nx model, it was ineffective in the 5/6 A/I model of severe hypertension and rapidly progressing renal disease. It is unclear why Nebivolol was ineffective in 5/6 A/I although the lack of therapeutic action was associated with a failure to restore systemic NO production.
Acknowledgements
The authors thank Harold Snellen and Bruce Cunningham for technical assistance. This study was supported by a contract from Forest Research Institute and by NIH grant R01DK056843 to C.B. N.C.M. and J.M.S. were supported by NIH Institutional Training Grants T32DK076541 and T32HL083810, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Parts of this work were presented in abstract form at the 2011 Experimental Biology Conference. FASEB J, 25: 839.13, 2011 and FASEB J, 25:839.6, 2011.
References
- Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. Journal of Clinical Investigation. 1986;77:1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis C. Nitric oxide deficiency in chronic kidney disease. Gottschalk Lecture 2007. Am J Physiol. 2008;294:F1–F9. doi: 10.1152/ajprenal.00424.2007. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- de Nigris F, Mancini FP, Balestrieri ML, Byrns R, Fiorito C, Williams-Ignarro S, Palagiano A, Crimi E, Ignarro LJ, Napoli C. Therapeutic dose of nebivolol, a nitric oxide-releasing beta-blocker, reduces atherosclerosis in cholesterol-fed rabbits. Nitric Oxide. 2008;19:57–63. doi: 10.1016/j.niox.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Dessy C, Saliez J, Ghisdal P, Daneau G, Lobysheva II, Frérart F, Belge C, Jnaoui K, Noirhomme P, Feron O, Balligand JL. Endothelial beta3-adrenoreceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third-generation beta-blocker nebivolol. Circulation. 2005;112:1198–1205. doi: 10.1161/CIRCULATIONAHA.104.532960. [DOI] [PubMed] [Google Scholar]
- Erdely A, Wagner L, Muller V, Szabo A, Baylis C. Protection of Wistar-Furth rats from chronic renal disease is associated with maintained renal nitric oxide synthase. J Am Soc Nephrol. 2003;14:2526–2533. doi: 10.1097/01.asn.0000086476.48686.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbin U, Fratta Pasini A, Stranieri C, Manfro S, Mozzini C, Boccioletti V, Pasini A, Cominacini M, Evangelista S, Cominacini L. Effects of nebivolol on endothelial gene expression during oxidative stress in human umbilical vein endothelial cells. Mediators Inflamm. 2008;2008:367590. doi: 10.1155/2008/367590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbin U, Pasini AF, Stranieri C, Manfro S, Boccioletti V, Cominacini L. Nebivolol reduces asymmetric dimethylarginine in endothelial cells by increasing dimethylarginine dimethylaminohydrolase 2 (DDAH2) expression and activity. Pharmacol Res. 2007;56:515–521. doi: 10.1016/j.phrs.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Georgescu A, Pluteanu F, Flonta ML, Badila E, Dorobantu M, Popov D. The cellular mechanisms involved in the vasodilator effect of nebivolol on the renal artery. Eur J Pharmacol. 2005;508:159–166. doi: 10.1016/j.ejphar.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–2031. doi: 10.1681/ASN.V4122023. [DOI] [PubMed] [Google Scholar]
- Gschwend S, Haug MB, Nierhaus M, Schulz A, Vetter R, Kossmehl P, Orzechowski HD, Scholze J, Rothermund L, Kreutz R. Short-term treatment with a beta-blocker with vasodilative capacities improves intrarenal endothelial function in experimental renal failure. Life Sci. 2009;85:431–437. doi: 10.1016/j.lfs.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Habibi J, Hayden MR, Sowers JR, Pulakat L, Tilmon RD, Manrique C, Lastra G, Demarco VG, Whaley-Connell A. Nebivolol attenuates redox-sensitive glomerular and tubular mediated proteinuria in obese rats. Endocrinology. 2011;152:659–668. doi: 10.1210/en.2010-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MR, Habibi J, Whaley-Connell A, Sowers D, Johnson M, Tilmon R, Jain D, Ferrario C, Sowers JR. Nebivolol attenuates maladaptive proximal tubule remodeling in transgenic rats. Am J Nephrol. 2010;31:262–272. doi: 10.1159/000278757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ. Experimental evidences of nitric oxide-dependent vasodilatory activity of nebivolol, a third-generation beta-blocker. Blood Press Suppl. 2004;1:2–16. [PubMed] [Google Scholar]
- Ignarro LJ. Different pharmacological properties of two enantiomers in a unique beta-blocker, nebivolol. Cardiovasc Ther. 2008;26:115–134. doi: 10.1111/j.1527-3466.2008.00044.x. [DOI] [PubMed] [Google Scholar]
- Maffei A, Di Pardo A, Carangi R, Carullo P, Poulet R, Gentile MT, Vecchione C, Lembo G. Nebivolol induces nitric oxide release in the heart through inducible nitric oxide synthase activation. Hypertension. 2007;50:652–656. doi: 10.1161/HYPERTENSIONAHA.107.094458. [DOI] [PubMed] [Google Scholar]
- Mollnau H, Schultz E, Daiber A, Balder S, Oelze M, August M, Wendt M, Walter U, Geiger C, Agrawal R, Kleschyov AL, Meinertz T, Munzel T. Nebivolol prevents vascular eNOS uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells. Arterioscler Thromb Vasc Biol. 2003;23:615–621. doi: 10.1161/01.ATV.0000065234.70518.26. [DOI] [PubMed] [Google Scholar]
- Moningka NC, Sasser JM, Croker B, Carter C, Baylis C. Protection against age-dependent renal injury in the F344xBrown Norway male rat is associated with maintained nitric oxide synthase. Mech Ageing Dev. 2011;132:1–7. doi: 10.1016/j.mad.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moningka N, Tsarova T, Sasser J, Baylis C. Protective actions of nebivolol on chronic nitric oxide synthase inhibition-induced hypertension and chronic kidney disease in the rat: a comparison with angiotensin II receptor blockade. Nephrol Dial Transplant. 2012 doi: 10.1093/ndt/gfr449. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K, Vaughn C. The role of renin-angiotensin-aldosterone system inhibition in chronic kidney disease. Expert Rev Cardiovasc Ther. 2003;1:51–63. doi: 10.1586/14779072.1.1.51. [DOI] [PubMed] [Google Scholar]
- Oelze M, Daiber A, Brandes RP, Hortman M, Wenzel P, Hink U, Schultz E, Mollnau H, von Sandersleben A, Kleschyov AL, Mulsch A, LI H, Forstermann U, Munzel T. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II treated rats. Hypertension. 2006;48:677–684. doi: 10.1161/01.HYP.0000239207.82326.29. [DOI] [PubMed] [Google Scholar]
- Oğuz A, Uzunlulu M, Yorulmaz E, Yalçin Y, Hekim N, Fici F. Effect of nebivolol and metoprolol treatments on serum asymmetric dimethylarginine levels in hypertensive patients with type 2 diabetes mellitus. Anadolu Kardiyol Derg. 2007;7:383–387. [PubMed] [Google Scholar]
- Pasini AF, Garbin U, Stranieri C, Boccioletti V, Mozzini C, Manfro S, Pasini A, Cominacini M, Cominacini L. Nebivolol treatment reduces serum levels of asymmetric dimethylarginine and improves endothelial dysfunction in essential hypertensive patients. Am J Hypertens. 2008;21:1251–1257. doi: 10.1038/ajh.2008.260. [DOI] [PubMed] [Google Scholar]
- Pires MJ, Rodríguez-Peña AB, Arévalo M, Cenador B, Evangelista S, Esteller A, Sánchez-Rodríguez A, Colaço A, López-Novoa JM. Long-term nebivolol administration reduces renal fibrosis and prevents endothelial dysfunction in rats with hypertension induced by renal mass reduction. J Hypertens. 2007;25:2486–2496. doi: 10.1097/HJH.0b013e3282efeecb. [DOI] [PubMed] [Google Scholar]
- Reidenbach C, Schwinger RH, Steinritz D, Kehe K, Thiermann H, Klotz T, Sommer F, Bloch W, Brixius K. Nebivolol induces eNOS activation and NO-liberation in murine corpus cavernosum. Life Sci. 2007;80:2421–2427. doi: 10.1016/j.lfs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Rozec B, Erfanian M, Laurent K, Trochu JN, Gauthier C. Nebivolol, a vasodilating selective beta(1)-blocker, is a beta(3)-adrenoceptor agonist in the nonfailing transplanted human heart. J Am Coll Cardiol. 2009;53:1532–1538. doi: 10.1016/j.jacc.2008.11.057. [DOI] [PubMed] [Google Scholar]
- Sasser JM, Moningka NC, Cunningham MW, Jr., Croker BP, Baylis C. Asymmetric Dimethylarginine in Angiotensin II Induced Hypertension. Am J Physiol. 2010;298:R740–R746. doi: 10.1152/ajpregu.90875.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser JM, Molnar M, Baylis C. Relaxin ameliorates hypertension and increases nitric oxide metabolite excretion in angiotensin II but not N(ω)-nitro-L-arginine methyl ester hypertensive rats. Hypertension. 2011;58:197–204. doi: 10.1161/HYPERTENSIONAHA.110.164392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N, Tavil Y, Erdamar H, Yazici HU, Cakir E, Akgül EO, Bilgi C, Erbil MK, Poyraz F, Okyay K, Turfan M, Cemri M. Nebivolol therapy improves endothelial function and increases exercise tolerance in patients with cardiac syndrome X. Anadolu Kardiyol Derg. 2009;9:371–379. [PubMed] [Google Scholar]
- Smith C, Merchant M, Fekete A, Nyugen HL, Oh P, Tain YL, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrol Dial Transplant. 2009;24:1422–1428. doi: 10.1093/ndt/gfn676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto T, Losonczy G, Qiu C, Hill C, Samsell L, Ruby J, Charon N, Venuto R, Baylis C. Acute Changes in Urinary Excretion of Nitrite+Nitrate (UNOXV) do not Predict Renal Vascular NO Production. Kidney Int. 1995;48:1272–1277. doi: 10.1038/ki.1995.411. [DOI] [PubMed] [Google Scholar]
- Szabo A, Wagner L, Erdely A, Lau K, Baylis C. Renal neuronal nitric oxide synthase protein expression as a marker of renal function. Kidney Int. 2003;64:1765–1771. doi: 10.1046/j.1523-1755.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- Tain Y-L, Freshour G, Dikalova A, Griendling K, Baylis C. Vitamin E reduces glomerulosclerosis, restores renal nNOS and suppresses oxidative stress in the 5/6 nephrectomized rat. Am J Physiol. 2007;292:F1404–F1410. doi: 10.1152/ajprenal.00260.2006. [DOI] [PubMed] [Google Scholar]
- Tain YL, Muller V, Szabo AJ, Erdely A, Smith C, Baylis C. Sex differences in response to rapamycin in kidney transplantation: Impact on constitutive nitric oxide synthase. Nitric Oxide. Biology and Chemistry. 2008;18:80–86. doi: 10.1016/j.niox.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain YL, Ghosh S, Krieg RJ, Baylis C. Reciprocal changes of renal neuronal nitric oxide synthase-α and –β associated with renal progression in a neonatal 5/6 nephrectomized rat model. Pediatr Neonatol. 2011;52:66–72. doi: 10.1016/j.pedneo.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toblli JE, Cao G, Giani JF, Muñoz MC, Angerosa M, Dominici FP. Long-term treatment with nebivolol attenuates renal damage in Zucker diabetic fatty rats. J Hypertens. 2011;29:1613–1623. doi: 10.1097/HJH.0b013e328349064c. [DOI] [PubMed] [Google Scholar]
- Varagic J, Ahmad S, Brosnihan KB, Habibi J, Tilmon RD, Sowers JR, Ferrario CM. Salt-induced renal injury in spontaneously hypertensive rats: effects of nebivolol. Am J Nephrol. 2010;32:557–566. doi: 10.1159/000321471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang M, Liu Y, Liu Y, Chen M. The effect of nebivolol on asymmetric dimethylarginine system in spontaneously hypertension rats. Vascul Pharmacol. 2011;54:36–43. doi: 10.1016/j.vph.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Whaley-Connell A, Habibi J, Johnson M, Tilmon R, Rehmer N, Rehmer J, Wiedmeyer C, Ferrario CM, Sowers JR. Nebivolol reduces proteinuria and renal NADPH oxidase-generated reactive oxygen species in the transgenic Ren2 rat. Am J Nephrol. 2009;30:354–360. doi: 10.1159/000229305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol. 2001;280:F996–F1000. doi: 10.1152/ajprenal.2001.280.6.F996. [DOI] [PubMed] [Google Scholar]