Abstract
Prostate cancer is the most common cancer and second leading cause of cancer deaths among men in the United States. Most men have localized disease diagnosed following an elevated serum prostate specific antigen test for cancer screening purposes. Standard treatment options consist of surgery or definitive radiation therapy directed by clinical factors that are organized into risk stratification groups. Current clinical risk stratification systems are still insufficient to differentiate lethal from indolent disease. Similarly, a subset of men in poor risk groups need to be identified for more aggressive treatment and enrollment into clinical trials. Furthermore, these clinical tools are very limited in revealing information about the biologic pathways driving these different disease phenotypes and do not offer insights for novel treatments which are needed in men with poor-risk disease. We believe molecular biomarkers may serve to bridge these inadequacies of traditional clinical factors opening the door for personalized treatment approaches that would allow tailoring of treatment options to maximize therapeutic outcome. We review the current state of prognostic and predictive tissue-based molecular biomarkers which can be used to direct localized prostate cancer treatment decisions, specifically those implicated with definitive and salvage radiation therapy.
Keywords: Biomarkers, prostate cancer, radiation therapy, RTOG, salvage radiation therapy.
INTRODUCTION
Prostate cancer is the most common cancer diagnosed in men in the United States. An estimated 217,000 new cases of prostate cancer were diagnosed in the United States in 2010 of which an approximate 85-90 percent were clinically localized at diagnosis [1]. Despite intense efforts to screen using clinical-pathologic factors, such as serum prostate specific antigen (PSA), and aggressively treat localized disease with surgery or radiation therapy, an unacceptable number of even early stage prostate cancer patients will ultimately succumb to their disease [2]. Prostate cancer as a whole still remains the second leading cause of cancer related death among men [1, 3]. Thus, key issues in the management of localized prostate cancer include not only the stratification of patients with aggressive disease from those with indolent cancer but also the development and improvement of therapies to treat localized prostate disease that is ultimately lethal. Taken a step further, a personalized approach would allow different treatment options to be tailored to specific traits of the patient and their prostate cancer to maximize clinical outcome. To address these issues, it is imperative to identify and validate new prognostic and predictive molecular biomarkers which can be used to direct localized prostate cancer treatment decisions. In this review, we catalog some of the work on tissue-based molecular biomarkers for prostate cancer, specifically those implicated with definitive and salvage radiation therapy treatment. We direct the reader to excellent reviews on prostate cancer biomarkers from other biologic specimens such as urine and blood [4, 5].
CLINICAL FEATURES AND DEFINITIVE RADIATION TREATMENT FOR LOCALIZED PROSTATE CANCER
Presentation
Clinically localized prostate cancer in the modern era usually presents initially as symptoms resulting in an abnormal finding on digital rectal examination (DRE) of the prostate and/or subsequent serum elevation of the biomarker PSA secondary to abnormal prostate exam or for screening purposes [6]. Serum PSA is currently the best molecular biomarker for prostate cancer when used for diagnostic and prognostic purposes [7]. Either of these clinical findings, abnormal DRE and elevated PSA, eventually prompt a prostate biopsy and can lead to the histological diagnosis of prostate cancer.
Standard Clinical Staging Systems for Localized Prostate Cancer
Current standard of care paradigms use pretreatment patient and prostate cancer clinical factors for prognosis and to dictate treatment options [8]. Clinical factors that help risk stratify localized prostate cancer patients are the clinical cancer stage groupings which are determined by the anatomic Tumor-Node-Metastasis (TNM) staging system, the Gleason score or summed tumor grade and pretreatment serum PSA level [9-11]. Using this staging system, clinically localized prostate cancer patients can be assigned to four risk groupings: low-risk (Group I), intermediate-risk (Group IIA), high-risk (Group IIB) and locally advanced (Group III) [8, 9], each connoting progressively increasing chances of recurrence following definitive local therapy [12, 13]. In addition, patient clinical factors such as age and comorbid conditions are integrated with the risk groupings to determine the optimal therapy [8]. With the recent realization that within the low-risk group there is a sub-group of extraordinarily favorable patients as defined by the so-called “Epstein criteria” [14], the National Comprehensive Cancer Network (NCCN) have created a another localized prostate cancer risk group, termed very low-risk. Very low-risk patients are advised by the NCCN to undergo active surveillance and not pursue definitive local therapy if their life expectancy, disregarding their prostate cancer diagnosis, is less than twenty years [8].
Radiation Therapy for Localized Prostate Cancer
Definitive treatment options for localized prostate cancer in general involve the use of surgery or radiation therapy [2, 8]. As mentioned above, active surveillance can also be a viable option for many localized prostate cancer patients in the very low-risk group [8, 14] and even for select patients of low [8, 15, 16] and intermediate-risk [8, 17]. Given the focus of this review is on radiation therapy biomarkers, we will only detail the use of radiation therapy further. Definitive radiation therapy typically involves the delivery of tumoricidal doses of high energy ionizing radiation to the whole prostate gland and sometimes to include the periprostatic tissues. Radiation is produced external to the body usually by a linear accelerator and directed via a number of different, but similar techniques, to pass through the patient with the highest dose deposited to the entire prostate [2, 8, 18]. Focal therapy of prostate cancer or treatments directed at less than the entire prostate is generally not advocated in the upfront setting unless as part of a clinical trial [16]. Another common form of radiation therapy used is the physical implantation of radioactive sources or brachytherapy, into the prostate which then decay and emit high energy radiation to the prostate gland [18]. The underlying mechanism for prostate cancer cell death from radiation therapy is radiation induced DNA damage. Radiation therapy damages prostate cancer DNA by rare direct ionization events or by the much more common indirect DNA damage from free radicals that occur as a by-product of the hydrolysis of water [19, 20].
High dose radiation therapy for localized prostate cancer can be very effective resulting in freedom from relapse of disease (or cure as determined by PSA) as high as 93% at 10 years in the most favorable or low-risk cases [21]. However, clinical experience with the use of radiation therapy alone in higher risk localized prostate cancer (intermediate [22, 23], high-risk [22, 24-27] and locally advanced groups [25-29]) results in relatively poor outcomes. This has lead to the standard approach of combination treatment with radiation therapy and hormone deprivation therapy (ADT) for variable duration, resulting in moderate improvements in clinical outcomes [30]. ADT is the surgical or pharmacologic depletion of testosterone to very low or castrate levels in prostate cancer patients [30]. The mechanism of action by which ADT increases radiation therapy effectiveness is complicated and not fully appreciated yet, but is thought to involve increased radiation-induced apoptosis, decreased prostate cell proliferation and perhaps suppression of systemic micrometastases [31-34]. Taken altogether, the standard radiation treatment recommendations for the localized prostate cancer risk groups that require definitive local therapy are as follows: (1) low-risk patients should receive definitive high dose radiation therapy alone; (2) intermediate-risk patients can receive high dose definitive radiation therapy alone, but more commonly a combination of definitive radiation therapy and short-term duration ADT are recommended; and (3) high-risk and locally advanced patients should receive a combination of definitive radiation therapy and long-term duration ADT [8]. It should be noted that intermediate-risk and high-risk patients are often treated with high-dose radiation therapy in combination with ADT because of the ability to safely deliver high dose radiation even though high level data in support of this approach is lacking. The target volumes treated with high-risk patients, sometimes referred to as prostate alone versus the inclusion of larger treatment volumes that include draining lymph nodes also known as pelvic fields, is controversial and will not be discussed further, but the reader is directed towards references on this subject [35-37].
CLINICAL PREDICTIVE MODELS OR NOMOGRAMS FOR PROSTATE CANCER RADIATION THERAPY
To improve upon these more simplistic risk stratification tools in an effort to better guide treatment decisions, multiple groups have produced more elaborate predictive prostate cancer treatment models also sometimes referred to as prostate cancer nomograms [38-40]. These models incorporate the pretreatment patient and tumor clinical factors detailed above, but also can predict the effects of treatment related factors such as radiation therapy dose and the use of combined radiation therapy and ADT on clinical outcomes. Although, these prostate cancer nomograms can be very useful in the clinical setting, as is the case for any model, there are still questions regarding their general validity for all localized prostate cancer patients. An excellent review detailing the clinical experience, utility and shortcomings of these clinical predictive models for prostate cancer radiation therapy by Roach and colleagues is referenced [41].
TISSUE BIOMARKERS FOR PROSTATE CANCER RADIATION THERAPY
While the clinical staging systems and predictive tools described above have helped tremendously to guide current standards in management of localized prostate cancer, they still do not uniformly distinguish indolent from lethal disease and are limited in revealing information about the genetic and biologic pathways driving these different disease phenotypes. In addition, they do not offer clear targets for the development of therapies to treat localized prostate cancer which are needed in patients with poor-risk disease. We believe molecular biomarkers may serve to bridge these inadequacies of traditional clinical factors.
A biomarker is as a factor, clinical or molecular, that can be correlated with a physiologic pathway, pathologic natural history or treatment response to a particular therapy [42]. Biomarkers can thus be very important tools to distinguish presymptomatic patients from unaffected healthy individuals such as with serum PSA screening, to monitor disease progression, to recognize those who are susceptible to adverse effects and to determine the efficacy of specific therapies. Biomarkers come in every imaginable flavor: clinical exam findings; physiologic parameters; results from imaging studies; and the focus of this review, molecules from biologic material such as body fluids or tissue specimens, are all biomarkers. The commonality between these diverse types of biomarkers is at the minimum they serve as surrogate measures for a pathophysiologic endpoint [43] and in the case of molecular biomarkers can also be a critical causative effector target for the desired process under scrutiny. Examples of the later, where predictive molecular biomarkers are also critical nodal targets of a tumor maintenance pathway are c-KIT mutations in gastrointestinal stromal tumors (GIST) [44, 45] and Epidermal Growth Factor Receptor (EGFR) mutations in non-small cell lung cancer (NSCLC) [46-49] that predict response to and are both targets of tyrosine kinase inhibitors (TKIs). For the remainder of the review we will restrict our use of tissue-based molecular biomarkers to surrogates of important prostate cancer clinical outcomes following radiation therapy.
Prognostic versus Predictive Biomarkers
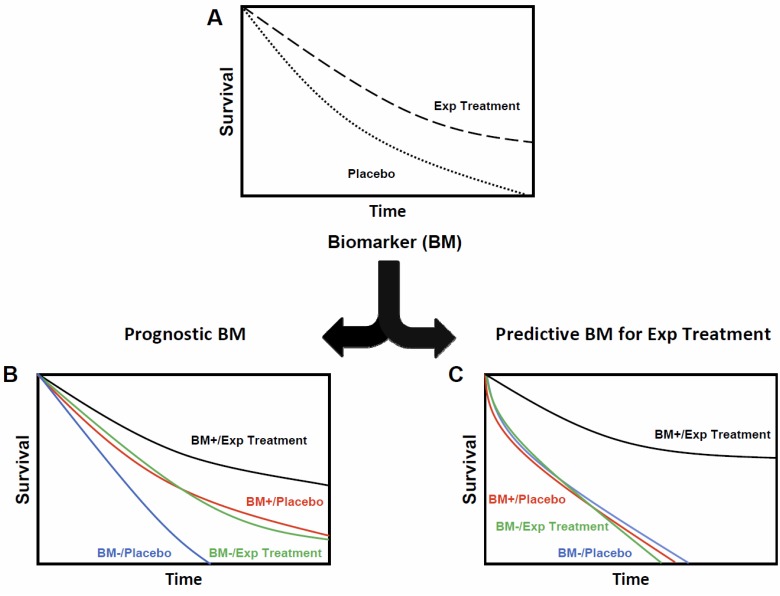
Distinguishing biomarkers as prognostic versus predictive is not simply semantics, but denotes specific properties of each biomarker type for treatment response and clinical outcomes (Fig. 1) [50]. A prognostic biomarker is correlated with an endpoint irrespective of therapy. In practice, prognostic biomarkers help guide treatment decisions made by clinicians. Using clinical risk based staging systems as an example, in general, low risk prostate cancer patients are treated with one modality, i.e. radiation therapy alone, while higher risk patients are directed towards combined modality treatments, i.e. radiation therapy and ADT [8]. However, purely prognostic biomarkers do not correlate with clinical outcomes to a specific treatment. In contrast, a predictive biomarker is correlated with an improvement or lack of improvement in clinical outcomes to a specific treatment. This prediction of outcome to a specific treatment is dependent on the status of the predictive biomarker. As cited above, predictive biomarkers have also been shown to be targets for therapy [44-49]. The determination of the prognostic versus predictive properties of a biomarker can be complex and is beyond the scope of this review (for an excellent reference on this topic [50]), however, one point is worth explicitly stating. The determination of the prognostic and predictive properties of a biomarker is best evaluated in a randomized clinical trial with a control group, because of the confounding effects present in most single-arm treatment trials (see Fig. 1; this type of analysis is not possible without a placebo or control group). The need for a comparator or control group to determine predictive properties of a biomarker will restrict our review to the high-level prostate cancer radiation therapy trials performed by the Radiation Therapy Oncology Group (RTOG).
Fig. (1).
Prognostic versus predictive biomarkers. An idealized example of the interrogation of the prognostic versus predictive properties of a biomarker (BM) are shown. (A) An experimental treatment (Exp Treatment) is tested in a randomized controlled fashion against a placebo or control arm and shown to confer a survival advantage. A biomarker (BM) has been shown to correlate with improved benefit with the Exp Treatment in prior uncontrolled studies without a control arm. By segregating the groups based on their treatment arms and BM status (BM+/Placebo; BM+/Exp Treatment; BM-/Placebo; and BM-/Exp Treatment) it is possible to distinguish whether the BM is purely prognostic versus predictive. (B) The BM is purely prognostic and therefore independent of the treatment effect. The relative magnitude of the benefit from the Exp Treatment is similar for each BM group. (C) The BM is purely predictive for the Exp Treatment and all the benefit from the Exp Treatment is exhibited only for patients in the BM+ group.
Radiation Therapy Oncology Group (RTOG) Prostate Cancer Biomarker Studies
The RTOG is a long standing cooperative research groups which has conducted numerous prospective trials in the field of radiation oncology, including those pertaining to prostate cancer. Two seminal phase III randomized trials, RTOG 86-10 [25] and 92-02 [27], were conducted in high-risk localized and locally advanced prostate cancer patients to address the role of combination treatment of radiation therapy and ADT and duration of ADT (short-term versus long-term), respectively. These two trials have also been the source of biologic material, in the form of prostate specimens, which has allowed RTOG investigators to explore the prognostic and in some cases predictive power of a number of tissue-based molecular biomarkers (Table 1). In order to understand the implications that these biomarkers may have on prognosis and predictive ability following radiation therapy with or without the addition of ADT, we will review the specifics of each trial below. Given the use of ADT in the vast majority of patients on these two trials, it should be noted that these tissue biomarkers are probably best described as surrogates of prostate cancer treatment with radiation and androgen deprivation therapy.
Table 1.
Selected Prostate Cancer Radiation Therapy Biomarker Studies
| DEFINITIVE RADIATION THERAPY | BIOMARKER | PROGNOSTIC | PREDICTIVE | REFERENCE |
| p53 DNA | DM, PFS1, CSM2 & OS1 | CSM2 | [62-64] | |
| DNA Ploidy | OS1 | OS1 | [86] | |
| COX-2 | BF2, DM2 | BF2 | [96] | |
| Protein Kinase A | BF, LF & DM | BF2, LF2, CSM2 & DM2 | [102, 103] | |
| BCL-2/BAX | PFS2 & BF | BF | [113, 114, 159] | |
| p16 | LF1, DM & CSM1 | [118, 119] | ||
| Ki-67 | BF2, DM, CSM & OS2 | [64, 129, 130] | ||
| VEGF/HIF-1 alpha | BF3 | [160] | ||
| MDM2 | DM2 | [64, 138] | ||
| Survivin | CSM1 & OS1 | [153] | ||
| SALVAGE RADIATION THERAPY | p21 | BF | [173] | |
| Ki-67 | BF | [187] | ||
| E-cadherin | BF | [186] | ||
| B7-H3 | BF | [197] |
Found only in RTOG 86-10
Found only in RTOG 92-02
Found only in MRC RT01
BF – biochemical or PSA failure; LF – local failure; DM – distant metastases; PFS – progression free survival; CSM – cause specific mortality; OS – overall survival.
RTOG 86-10 was a randomized trial conducted in the pre-PSA era of radiation therapy (RT) alone versus radiation therapy and ADT for patients (n=471 randomized) with bulky tumors (≥25 cm2 by DRE) and T stage T2b–4. One of the original hypotheses for this trial was to determine whether ADT before and during radiation therapy may reduce tumor bulk and enhance tumor cell kill and thus improve tumor control and survival [51]. Thus, patients in the ADT arm were pharmacologically castrated using goserelin (luteinizing hormone-releasing hormone agonist) and flutamide (anti-androgen) for 2 months before and 2 months during radiation therapy. The most updated results of this trial [25], in keeping with an earlier report [51], demonstrated statistically significant improvements in 10-year disease-specific mortality or death from prostate cancer (23% vs. 36%; p = .01), distant metastasis (35% vs. 47%; p = .006), disease-free survival (11% vs. 3%; p < .0001), and PSA failure (65% vs. 80%; p < .0001), all favoring the combination arm.
RTOG 92-02 is the largest randomized trial of prostate cancer that tested the optimal duration of ADT when combined with radiation therapy for prostate cancer patients (n=1554 randomized) with T2c–4 primary tumors and PSA <150 ng/ml. The control arm was based on RTOG 86-10, ADT using goserelin and flutamide for 2 months before and 2 months (or short-term ADT = STAD) during radiation therapy (RT + STAD). The experimental arm added 24 months of additional goserelin alone also known as long-term ADT (LTAD). The mature results (median 11 years of follow-up) [27] from this trial demonstrate the superiority of RT + LTAD over RT + STAD for patients with high-risk/locally advanced prostate cancer for 10-year disease-specific survival (83.9% vs. 88.7%; p = .0042), local progression (22.2% vs. 12.3%; p < .0001), distant metastasis (22.8% vs. 14.8%; p < .0001), disease-free survival (13.2% vs. 22.5%; p < .0001) and PSA failure (68.1% vs. 51.9%; p ≤ .0001). Neither RTOG trial demonstrated an overall survival benefit. Multiple reasons have been postulated to explain this lack of overall survival benefit for RTOG 92-02 including the advanced age of the patients and the large enrollment of lower Gleason score (GS ≤7) patients. Regardless, hypothesis generating subset analysis has demonstrated an overall survival benefit for patients with GS 8-10 (31.9% vs. 45.1%; p = .0061). These RTOG trials helped form the rationale for the use of combination radiation therapy and LTAD for high-risk and locally advanced prostate cancer patients. In addition, they have been an incredible resource to study candidate prognostic and predictive biomarkers for prostate cancer radiation therapy and androgen deprivation therapy.
p53 – Prognostic and Predictive
The p53 protein encoded by the TP53 gene is the most commonly mutated gene in human cancer. The p53 tumor suppressor protein has multifaceted roles as a stress-responsive transcription factor to a variety of intracellular and extracellular insults, notably including those that are produced by radiation therapy [52]. Preclinical studies in prostate cancer cells suggest that p53 loss of function results in radiation resistance [53, 54]. As a biomarker, p53 accumulation or TP53 mutation has been associated with poor prognosis in multiple different cancer types. Other groups have also looked at the role of p53 as a biomarker for prostate cancer patients treated with radiation therapy, often as part of a heterogeneously treated group, producing as expected varied results [55-61]. Many of these groups did not have the benefit of tissue collection from a prospective randomized clinical trial such as with the RTOG with a control group of patients, thus as explained above negating the ability to look at predictive power.
Give these circumstances, p53 was an ideal biomarker candidate for the RTOG to test for prognostic and predictive power using the biospecimens from RTOG 86-10 [62] and 92-02 [63]. From RTOG 86-10, abnormal accumulation of p53, defined as ≥20% positive nuclear staining using immunohistochemistry (IHC), was present in 23/129 (18%) patient tumor samples available from the original 471 enrolled on the trial (or only 27%). The p53 biomarker was independently prognostic for increased distant metastasis (RR = 2.15, p = 0.04), poor progression-free (RR = 2.45, p = 0.003) and overall survival (RR = 2.34, p = 0.02) for the entire group (RT and RT + STAD arms). There was also a curious finding that the p53 biomarker seemed to predict increased metastasis in the RT+STAD suggesting that patients with abnormal p53 biomarker status do not react favorably to RT + STAD. This is likely an artifact from confounding due to the small numbers of cases with abnormal p53 biomarker status (12 with RT and 11 with RT + STAD) from a subset (only 27% of the original trial enrollment) that was not representative of the original trial cohort. Nonetheless, this was arguably the first study to demonstrate a clear prognostic role for the p53 as a molecular biomarker for prostate cancer patients treated with radiation therapy.
For RTOG 92-02 [63], tissue was available from 379 STAD + RT cases and 398 LTAD + RT cases for a total of 777 cases. Abnormal p53 was present in 168/777 (22%) patient samples and was an independent prognostic factor correlated with increased distant metastasis (HR = 1.72, p = 0.013) and cause-specific mortality (HR = 1.89, p = 0.014). When checking for the predictive power of p53 expression between RT + STAD and RT + LTAD treatment groups, the RTOG demonstrated that for patients treated with RT + STAD, p53 was a predictive biomarker associated with increased cause-specific mortality (HR = 3.81, p = 0.009). This suggest that p53 is a both a prognostic and predictive biomarker for high-risk/locally advanced prostate patients receiving radiation therapy and those with abnormal p53 status should be treated with RT + LTAD. Some caveats regarding these p53 biomarker studies and IHC data driven biomarker studies in general need to be introduced before proceeding further. The methods by which samples are scored: (1) manual methods versus quantitative image analysis; and (2) choice of cut-points, can dramatically influence the prognostic and predictive associations determined. Using the p53 biomarker as an example, a second analysis using RTOG 92-02 specimens and using quantitative image analysis systems with different cut-points was performed for the p53 biomarker [64]. The results of this second analysis resulted in weaker prognostic association with overall survival (RR ~ 1.3 p =0.02) and was no longer prognostic for distant metastasis or prostate cancer death. Hence great detail should be paid to the methods and even greater caution should be emphasized in the interpretation of biomarker data. Ultimately, these data are hypothesis generating at best and need themselves to be prospectively answered in a clinical trial. After acknowledging these caveats, these RTOG data in two different but complimentary patient data sets, reanalyzed twice in the case of RTOG 92-02, suggest that p53 appears to be a prognostic molecular biomarker. Furthermore, abnormal p53 biomarker status appears to predict patients that may benefit from RT + LTAD.
DNA Ploidy - Prognostic and Predictive
DNA ploidy or chromosome complement is a crude measure of genomic instability, a hallmark of tumorigenesis [65], and in most cases has been correlated as a biomarker portending worse prognosis for prostate cancer [66-79]. An even more limited number of studies have produced mixed results after examining DNA ploidy as a biomarker for prostate cancer patients treated with radiation therapy [80-85].
The RTOG using 149 specimens from trial 86-10 examined DNA ploidy and found 55/149 (37%) patients to be non-diploid [86]. This non-diploid biomarker status was independently prognostic for worse 5-year overall survival (70% versus 42%, p = 0.031), but not any other clinical endpoints. The reduced overall survival in the absence of an increase in other endpoints was explained by what appeared to be increased resistance to salvage ADT by patients with non-diploid tumors who had been treated with RT+STAD. The DNA ploidy biomarker showed a predictive interaction with the patients on the RT + STAD arm of RTOG 86-10 demonstrating a worse overall survival (p = 0.02) and worse survival following salvage ADT with non-diploid biomarker status (p = 0.01), which was not present in the control RT alone arm (p = 0.56 and p = 0.37, respectively). These results were have not been duplicated in published form using the RTOG 92-02 dataset.
COX-2 – Prognostic and Predictive
Inflammation as a driving event in prostate tumorigenesis and tumor progression is acknowledged [87]. Mediators and modifiers of inflammation such as cyclooxygenase-2 (COX-2) have been studied with great interest. Potential roles of COX-2 in tumor related processes such as tumorigenesis, angiogenesis [88-90] and radiation treatment resistance makes this an attractive biomarker candidate and potential therapeutic target [91-95].
From a cohort of 1554 patients from RTOG 92-02, 586 patient samples (270 STAD + RT cases and 316 LTAD + RT) were analyzed by COX-2 IHC [96]. By multivariate analysis COX-2 staining was an independent prognostic factor of distant metastases (p < 0·02) as a continuous factor or by using a cut-point. COX-2 was also prognostic for PSA failure [using two separate definitions (ASTRO: HR = 1·073, p=0·008; Phoenix: HR = 1·073, p=0·014) when COX-2 staining was considered as a continuous variable. Interestingly, COX-2 overexpression was associated with worse PSA failure (by ASTRO definition) for those on the control arm of RT + STAD but not those on the RT + LTAD (p = 0.002), suggesting that COX-2 may serve as a predictive biomarker for length of ADT therapy in high-risk and locally advanced prostate cancer patients.
Protein Kinase A – Prognostic and Predictive
The protein kinase A (PKA) is a holoenzyme of two regulatory and two catalytic subunits dependent on cyclic AMP [97] that is overexpressed in a variety of cancer types and associated with poor prognosis [98-100]. Knockdown of the gene encoding PKA enhanced the response of androgen-sensitive prostate cancer cells to ADT with or without RT and androgen-insensitive cells to RT [101].
PKA overexpression was determined for 80/456 (17.5%) samples from RTOG 86-10 [102]: 36 men from RT alone control arm and 44 from the RT + STAD arm. The PKA IHC was analyzed using both manual and image analysis methods. PKA expression was found to be very weakly prognostic for PSA failure (using image analysis as a continuous variable, HR = 1.01, p = 0.03), but stronger for local recurrence (using image analysis with a cut-point of >2 using a scale of 0-3, HR = 3.66, p = 0.002), and distant metastases (manual analysis with a cut-point of >2 using a scale of 0-3, HR = 2.27, p = 0.018). No interaction between PKA biomarker expression and the treatment arms of RTOG 86-10 was found.
Examining the RTOG 92-02 biorepository for PKA overexpression resulted in IHC analysis on 161 patients from the STAD + RT control arm and 152 patient samples from the LTAD + RT arm (313 total cases) [103]. In contrast to RTOG 86-10, the IHC was analyzed using both manual and image analysis methods. PKA overexpression by IHC was independently prognostic for PSA failure (p ≤ 0.01), local recurrence (p < 0.05), and distant metastases (p < 0.01) confirming the results from RTOG 86-10. In contrast to RTOG 96-10, when the investigators examined for potential interactions between PKA biomarker expression and the treatment arms of RTOG 92-02 they found significant prediction of the outcome for patients treated on the RT + LTAD arm. Low PKA expression was predictive of decreased PSA failure (HR = 0.54, p = 0.0003), local recurrence (HR = 0.31, p = 0.007), distant metastases (HR = 0.23, p = 0.003) and prostate specific death (HR = 0.25, p = 0.005). These results suggest that the benefits from RT + LTAD are the most pronounced in groups of patients with low PKA expression and consequently, novel strategies may be needed for patients whose tumors have high PKA levels.
BCL-2/BAX – Prognostic and Predictive
The BCL-2 family are dimeric proteins that are defined by containing Bcl-2 homology (BH) domains and act as anti- or pro-apoptotic regulators [104, 105]. BAX is a pro-apoptotic cytosolic family member that following initiation of apoptotic signaling inserts itself into the outer mitochondrial membrane contributing to the release of cytochrome c and other pro-apoptotic factors. In contrast, BCL-2 is the prototypical anti-apoptotic protein that exerts its effects by titrating out pro-apoptotic proteins such as BAX. The relative amounts of BCL-2 and/or BAX has been shown to correlate with tumor aggressiveness and radiation resistance in prostate cancer [58, 106-112].
The RTOG examined the relative levels of BCL-2 and BAX by IHC in both 86-10 [113] and 92-02 [114] trials and correlated with prognostic and predictive ability for clinical outcomes. The findings from RTOG 86-10 were negative and we will instead focus on those from RTOG 92-02. BCL-2 was positive in 45.6% (229/502) of cases and BAX expression altered in 53.9% (185/343) of cases. BCL-2 was not found to correlate to any of the clinical end points. However, altered BAX expression was independently prognostic for any type of disease failure (RR = 1.43, p = 0.0226). Next the investigators analyzed the BCL-2 and BAX staining as composite groups given their biologic interaction for apoptosis regulation. When examining the composite group of positive BCL-2 and/or altered BAX, this biomarker group was independently prognostic for any failure (RR = 1.45; p = 0.046) and PSA failure (ASTRO definition, RR = 1.60; p = 0.036). Lastly, this same biomarker group was predictive for worse 5-year PSA failure when comparing RT + STAD versus RT + LTAD (61% versus 24%, p < 0.0001).
p16 – Prognostic
The CDKN2A tumor suppressor gene is commonly homozygously deleted in a wide variety of primary tumors cancer and cell lines which helped lead to its positional cloning [115, 116]. CDKN2A encodes for p16 which is a cyclin-dependent kinase inhibitor (CDKI) and a critical component of the pRB/p16 tumor suppressor axis. This axis forms a critical regulatory system for the G1-S-phase transition and is found to be dysregulated in a majority of human cancers [117].
A subset of tissue blocks (67/471) from RTOG 86-10 were examined by IHC for decreased p16 and pRB levels [118]. Using cut-off values of ≤25% for p16 and ≤20% for pRB staining cells, RTOG investigators found that 18/67 (27%) and 54/67 (81%) samples demonstrated decreased p16 and pRB levels, respectively. Decreased p16 expression was prognostic for higher risk of local progression (p = 0.0035), distant metastasis (p = 0.026) and prostate cancer death (p = 0.01). Decreased pRB levels were oddly prognostic for decreased risk of death from prostate cancer (p = 0.03). The predictive power of the p16 and pRB biomarkers were not tested for interaction with the treatment arms of RTOG 86-10 likely because of the small subset sample sizes.
A follow-up study using samples from RTOG 92-02 was performed examining the p16 biomarker only and by utilizing a quantitative image analysis system [119]. Tissue was available from 285 STAD + RT and 327 LTAD + RT for 612 total cases. The p16 biomarker was prognostic for distant metastases (p = 0.0332) similar to RTOG 86-10. Tests for interaction between p16 biomarker levels and treatment effect revealed no significant interactions. However, this study represented a sub-group that may not have represented the original cohort of patients of RTOG 92-02 and investigators used arbitrary cut-points with their quantitative analysis perhaps limiting the power to detect predictive interactions with p16 and the treatment arms. In summary, the data from RTOG 86-10 and 92-02 suggest that p16 is a prognostic marker for distant metastasis and perhaps local progression and prostate cancer death. Confirmatory studies are warranted before p16 can be used as prognostic biomarker for prostate cancer patients and no data is available to suggest that p16 is predictive for who should receive RT alone, RT + STAD or RT + LTAD.
Ki-67 – Prognostic
The Ki-67 antigen is a biomarker used to determine proliferative rate for many other types of tumors [120], as well as been shown to correlate with clinical outcomes of prostate cancer patients treated with surgery or radiation therapy [80, 108, 109, 121-128]. One previous study had suggested a Ki-67 index of ≥3.5% was prognostic for PSA failure following radiation therapy [126].
RTOG 86-10 samples were used to validate this cut-point for Ki-67 staining [129]. Diagnostic samples from 108 patients were available for Ki-67 analysis, 60 cases from the RT alone arm and 48 patient samples from RT + STAD arm with a median follow-up of 9 years. Using the 3.5% cut-point Ki-67 was independently prognostic for 5-year risk of distant metastases (13.5% versus 50.8%; p = 0.0005) and prostate specific survival (97.3% versus 67.7%; p = 0.0039). In a separate analysis from the same study, the investigators also found the 7.1% cut-point to be prognostic on univariate testing for distant metastases and prostate specific survival. No tests for Ki-67 interaction with the treatment arms of RTOG 86-10 were performed to allow predictive assessment.
Ki-67 staining was studied in the RTOG 92-02 data set using 537 patient specimens, 257 from the RT + STAD arm and 280 from RT + LTAD arm [130]. The investigators looked at the Ki-67 staining as both a continuous variable and also using their 3.5% and 7.1% cut-points from above. As a continuous variable Ki-67 staining was independently prognostic for PSA failure (p = 0.0445), distant metastases (p < 0.0001), prostate specific survival (p < 0.0001) and overall survival (p = 0.0094). The 7.1% cut-point was also independently prognostic for distant metastases (p = 0.0008) and prostate specific survival (p = 0.017). No definitive predictive correlations were made with Ki-67 staining and the treatment arms of RTOG 92-02. The investigators did identify some possible groups of prostate cancer patients that may not require LTAD by looking at various permutations of the 7.1% Ki-67 cut-point with other traditional clinical risk factors. In a separate study this same IHC staining was duplicated, but the RTOG investigators used quantitative image analysis and different cut-points [64]. This duplicate analysis found Ki-67 to again be independently prognostic for distant metastases (p < 0.0001), prostate specific survival (p = 0.0007) and overall survival (p = 0.01). Taken altogether, the Ki-67 biomarker was found to be strongly prognostic in two separate validation sets for distant metastases and prostate specific survival.
MDM2 – Prognostic
The human orthologue of the murine double minute (MDM2) oncoprotein is a negative regulator of p53 that regulates p53 degradation and represses p53 transcriptional targets [131, 132]. MDM2 also appears to confer androgen independent survival and radioresistance to prostate cancer cells via p53-dependent and independent pathways [133-135]. As described above, p53 as a biomarker has been shown to have promising prognostic and predictive potential for patients treated on RTOG 86-10 and 92-02. Only a limited number of studies had examined MDM2 as a biomarker in prostate cancer specimens with mostly uncontrolled cohorts [136, 137].
When RTOG investigators looked at MDM2 by IHC as a biomarker in the 86-10 cohort, they were able to examine 108 patients (62 from the RT arm and 46 from the RT + STAD arm) and found at least 47 specimens (44%) that had MDM2 overexpression. The investigators analyzed their data using a cut-point of >5% nuclear staining by manual analysis and with at 3% cut-point using a quantitative method, but did not find that MDM2 was independently prognostic or predictive for any clinical outcome [138].
A larger study using RTOG 92-02 specimens (589 total) of MDM2 IHC and quantitative image analysis was performed (64). MDM2 was found to be independently prognostic for distant metastases (p = 0.02) and overall survival (p = 0.003). In addition, the group performed a concurrent analysis of the individual prognostic power of the proliferative index by Ki-67 IHC and p53 overexpression which we described above separately. Combining MDM2 overexpression and high Ki-67 resulted in increased prognostic significance for distant metastasis (p < 0.0001), prostate cancer death (p < 0.0001) and overall survival (p = 0.0002). However, no predictive correlations could be made between MDM2 and Ki-67 and any of the clinical outcomes depending on treatment arm. MDM2 individually and particularly in combination with Ki-67 appears to be a robust molecular biomarker for prostate cancer patients treated with radiation therapy.
Survivin – Prognostic
Survivin is a member of the inhibitor of apoptosis (IAP) family and inhibits caspase activation preventing apoptosis. Survivin is almost exclusively expressed during embryogenesis [139], but is found to be overexpressed in a wide variety of tumors types including prostate cancer, where it is associated with prognosis [140, 151]. Survivin also mediates paclitaxel and androgen deprivation resistance in prostate cancer cells [140, 152].
The RTOG examined nuclear survivin as a biomarker using the 86-10 cohort from 68 patient samples [153]. The samples analyzed were not representative for prostate cancer survival and distant metastases compared to the original 86-10 larger cohort of 456 patients and thus the results below may not generalize. With these caveats, nuclear survivin was independently prognostic for improved prostate cancer survival (HR = 0.36, p = 0.0173) and overall survival (HR = 0.46, p = 0.0156). Survivin exists in cytoplasmic and nuclear pools [154], with cytoplasmic survivin associated with poor outcomes [150]. Therefore, another analysis scored for cytoplasmic staining on 65 patient samples from trial 86-10. An association for increased local recurrence and cytoplasmic surviving was established, but was not upheld on multivariate analysis. Further analyses of survivin as a biomarker are warranted with a larger data such as with RTOG 92-02.
Other Biomarkers Tested on RTOG 86-10 and 92-02 Trials
Five additional molecular biomarkers where tested using the patient samples from RTOG 86-10 or 92-02. The nuclear factor-κB [155], chemokine receptor CXCR4 [155], STAT3 [156] and CAG triplet repeat number located within the gene for the androgen receptor (AR) [157] were examined using materials from ROG 86-10. A very small subset of patients from RTOG 92-02 were examined for racial polymorphisms of the cytochrome P450 3A4 (CYP3A4) gene responsible for androgen metabolism [158]. The hypotheses for studying these molecules were all well conceived and experiments performed in keeping with the high standards of the studies as detailed above. However, these studies were either under-powered, demonstrated no/weak or paradoxical associations with clinical outcomes and so will not be discussed. The reader is directed to these original publications for further descriptions of these works.
SELECT NON-RTOG PROSTATE CANCER TISSUE BIOMARKER STUDIES
Two additional non-RTOG tissue biomarker studies using patient samples from randomized dose escalation studies conducted by the Medical Research Council (MRC) from the United Kingdom will also be reviewed [159, 160]. The MRC RT01 trial (n=843) and the phase II pilot predecessor trial (n=127) were almost identical in design. Patients received 3–6 months of ADT followed by prostate radiation therapy that was randomly assigned to 64 Gy versus 74 Gy. In their initial study, the MRC investigators examined hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF) and osteopontin. These three markers have been implicated in intratumoral hypoxia which has been shown to have a role in modulating radiation response in multiple tumor types [161]. HIF-1 alpha is a transcription factor that is stabilized under hypoxic conditions and induces a number of genes, such as VEGF resulting in tumor progression, angiogenesis, and metabolic changes, making it an ideal biomarker and potential therapeutic target [162]. The MRC were able to obtain 201 patient samples from the randomized studies (n=103 from the 64 Gy arm and n=98 from the 74 Gy arm) and perform IHC from tissue microarrays. The predictive properties of these hypoxia tissue biomarkers were not analyzed. However, HIF-1 alpha (HR = 1.46, p = 0.02) and VEGF (HR = 1.45, p = 0.008) were both found to be independently prognostic for PSA failure (nadir + 2 ng/mL).
The MRC investigators also examined some of the same tissue biomarkers as the RTOG: Bcl-2, p53 and MDM2, and where able to replicate that Bcl-2 was both a prognostic (HR = 3.57, p = 0.001) and interestingly, a predictive biomarker for radiation therapy with ADT. Using the same tissues as above, they specifically showed that patients with Bcl-2 positive tumors demonstrated improved 7-year biochemical control (41% versus 61%, p = 0.0122) with escalated doses of radiation therapy (64 Gy versus 74 Gy) and ADT. Whereas patients with Bcl-2 negative tumors did not demonstrate any improvement with dose escalated radiation therapy (p = 0.423), suggesting that Bcl-2 status could be used to predict patients requiring more aggressive treatment in the form of radiation dose escalation or longer duration of ADT.
MOLECULAR BIOMARKERS FOR ADJUVANT AND SALVAGE RADIATION THERAPY
As mentioned above, localized prostate cancer can be treated successfully with definitive local therapies such as surgery or radiation therapy. Surgery in the form of a radical prostatectomy is the gold standard by which other local therapies are measured [2]. Unfortunately, in the United States approximately 30,000 men per year will still experience PSA failure following their surgery with many of these men ultimately developing metastases [163, 164]. Radiation therapy to the prostatic fossa or surgical bed is the only potentially curable remaining option for these men. Radiation therapy to the prostatic fossa shortly after surgery in the absence of known disease, i.e. undetectable PSA, based on known poor pathologic prognostic factors such as extra-prostatic disease and positive surgical margins is known as adjuvant radiation therapy (ART). Three phase III randomized trials have demonstrated improvement in clinical outcome for patients undergoing ART who display these poor pathologic risk factors [165-167] (and we refer readers to an excellent review of these seminal studies [168]). However, the number of men needed to treat for a survival benefit is still too high (12 men treated to save 1 life) [165]. No published studies on these phase III randomized trials or any single-institution cohort experiences are available examining molecular biomarkers for ART. The phase III trials [165-167] in particular provide an optimal opportunity to assess the prognostic and more importantly the predictive power of molecular biomarkers for ART. We expect these types of molecular biomarker studies to provide important tools to personalize ART for patients in the future.
The use of radiation therapy following surgery for clinically persistent or recurrent disease, typically a detectable PSA, is known as salvage radiation therapy (SRT). The success of SRT is highly variable [169, 170]. Similar to initial diagnosis, additional clinical tests including imaging to distinguish the extent of disease are sometimes warranted [8, 168, 171]. However, in general these additional clinical tests are not helpful at determining whether men with persistent prostate cancer will benefit from SRT. Clinical and pathologic factors have been identified to help refine the group of men likely to benefit [169, 172], but these risk stratification groups are still crude. Similar to definitive prostate cancer treatment, SRT prostate cancer nomograms have been created that also incorporate treatment related factors such as SRT dose and the use of ADT [170]. Again, the use of molecular biomarkers for the salvage setting should similarly improve treatment decisions and identify potential targets for therapy. However, prognostic molecular biomarkers for SRT, although further along than for ART, are still in their infancy and consist of only a few single-institution, retrospectively gathered cohort studies. A subtle but important implication of ART-SRT biomarkers; they may be surrogates for presence or absence of metastatic disease at the time of ART-SRT and/or they may be surrogates for the intrinsic response of disease to radiation therapy. Lastly, since these are all single-arm biomarker studies, no appreciation of predictive power can be made. We will review these few studies in detail below.
p21
The CDKN1A gene encodes the p21 cyclin-dependent kinase inhibitor 1 (CDKI1) protein. The p21 protein is a critical regulator of cell cycle progression through G1 and forms a potent G1 checkpoint with the p53 tumor suppressor following various cellular stressors including radiation. Previous studies on prostatectomy specimens have indicated that p21 overexpression correlated with worse prognosis possibly mediated by the ability of p21 to inhibit genotoxic cell death from apoptosis. Riguad et al. [173] examined p21 as a biomarker for prognosis in 74 patients with a median age of 65 years (range 48-74) who received an average of 60 Gy SRT (range 50–70) 34.5 months (mean 42, range 4–112) following their surgery. Most had a pGleason score ≤ 7 (63/74), extraprostatic disease ≥pT3 (50/74) and positive surgical margins (48/74). The median pre-SRT PSA was 0.8 ng/ml (range 0.3–36.8). The p21 levels were assessed by IHC and scored manually demonstrating that 15/74 (20%) of the specimens overexpressed p21. The p21 biomarker was not correlated in this group with PSA failure following surgery before SRT. However, p21 was found to be independently prognostic for PSA failure (defined as > 0.3 ng/ml) following SRT when controlling for other variables such as pre-SRT PSA and pGleason score (p = 0.004). Risk stratification using p21 negative status and pre-SRT <1 ng/ml levels resulted in a subset of patients with a 5-yr PSA failure free survival of 83%. This group also conducted a concurrent IHC biomarker analysis of p53 and another CDKI, p27, but found no prognostic associations with their cohort of SRT patients.
E-Cadherin
E-cadherin is associated with tumor invasiveness, metastatic dissemination, and poor patient prognosis [174, 175] including in prostate cancer [176-181]. E-Cadherin is a single-span transmembrane glycoprotein that establishes homophilic interactions with adjacent E-cadherin molecules expressed by neighboring cells assisting in formation of adherens junctions [182, 183]. Preclinical data suggests that knock-down of E-cadherin is sufficient to confer a phenotype of epithelial-mesenchymal transition (EMT) to normal and malignant cells and these cells that have undergone EMT are subsequently chemoresistant [184]. Some preclinical and clinical data suggest an EMT phenotype may confer a worse prognosis for prostate cancer patients [185]. Ray et al. [186] examined a small cohort of 37 patient samples for abnormal localization of E-cadherin by IHC who had been treated with a median of 68.4 Gy (range 64.8-70.2) SRT. Clinical characteristics of this cohort were most men had a pGleason score ≤ 7 (29/37), extraprostatic disease ≥pT3 (25/37) and positive surgical margins (24/37). Most patients had pre-SRT PSA that was <1 ng/ml (29/37). They found 68% (25/37) of their cohort had abnormal localization of E-cadherin following manual analysis of their IHC. Aberrant E-cadherin was an independent prognostic biomarker in this SRT population and conferred a worse PSA failure rate [HR = 3.8, p = 0.03; (defined as > 0.2 ng/ml greater than the nadir)].
Ki-67
Ki-67 staining index as a marker of proliferation and use as a biomarker has demonstrated impressive independent prognostic ability in definitive cases of prostate cancer as described above and is a logical molecular biomarker candidate in the salvage setting. A group from the Mayo Clinic [187] assayed their a cohort of 147 patients treated with SRT (average 66.6 Gy) using Ki-67 IHC with techniques similar to those used above in the RTOG 92-02 trial. Other germane clinical characteristics of this cohort were most men had a pGleason score ≤ 7 (115/147), extraprostatic disease ≥pT3 (97/147), positive surgical margins (87/147) and follow-up was 6.2 years on average (range 0.6-14.7 years). The average pre-SRT PSA was 0.05 ng/ml (range 0.1-15.3). The average Ki-67 staining index was 2.5 in their cohort (range 0-20.9). High Ki-67 (as defined as >4) was an independent prognostic biomarker in this SRT population and conferred a worse PSA failure rate [RR = 2.02, p = 0.005; (defined as > 0.4 ng/ml or greater than the nadir)]. These SRT data and the RTOG studies in combination suggest that Ki-67 staining may be a general prognostic biomarker for prostate cancer.
B7-H3
The B7 ligand (encode by CD276) is a member of the B7 family of immuno-moldulation molecules [188-191]. B7-H3 functions are likely context dependent and can involve inhibition or stimulation of the immune response [192-195], but overexpression of B7-H3 has been correlated to worse clinical outcomes in prostate cancer [196]. The same group of investigators from the Mayo Clinic analyzed their SRT cohort of patients above for B7-H3 overexpression using IHC [197]. Manual scoring was performed and a majority of the tumors expressed B7-H3, but the intensity varied with 49 (33%) patient tumors showing weak B7-H3 staining, 70 (47%) with moderate B7-H3 intensity and 29 (20%) with strong B7-H3 staining. Interestingly, strong B7-H3 staining was independently prognostic for more likely PSA failure following SRT (RR = 2.87, p = 0.003).
FUTURE PERSPECTIVE
The immediate future for the existing tissue-based molecular biomarkers as we have detailed above, is their prospective validation in clinical trials. The creation of integrative clinical-biomarker nomograms is on the near horizon for prostate cancer radiation therapy [41]. Combining the prognostic and predictive abilities of molecular biomarkers with other clinical factors that are independently prognostic and/or predictive is the next straightforward extension of current nomograms. We have focused our review on tissue-based molecular biomarkers, but an explosion of data is emerging from biomarker studies in other specimen types [4, 5]. In addition, imaging-based biomarker research is provocative and has the potential to further augment the tools for directing prostate cancer therapy [198]. Ultimately, the future of prostate cancer biomarkers, and medical biomarkers in general, will be a multiscale [199] and personalized approach that integrates genomic [200], transcriptomic [201], proteomic [202] and metabolomic [203] data to provide direction for personalized prostate cancer management.
ACKNOWLEDGEMENTS
This work was supported by grants from the Department of Defense, Patrick C. Walsh Fund, Francis Family Foundation and the American Society of Radiation Oncology.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N Engl J Med. 2007;357(26):2696–705. doi: 10.1056/NEJMcp0706784. [DOI] [PubMed] [Google Scholar]
- 3.Kamidono S, Ohshima S, Hirao Y, et al. Evidence-based clinical practice Guidelines for Prostate Cancer (Summary - JUA 2006 Edition) Int J Urol. 2008;15(1):1–18. doi: 10.1111/j.1442-2042.2007.01959.x. [DOI] [PubMed] [Google Scholar]
- 4.Sardana G, Dowell B, Diamandis EP. Emerging biomarkers for the diagnosis and prognosis of prostate cancer. Clin Chem. 2008;54(12):1951–60. doi: 10.1373/clinchem.2008.110668. [DOI] [PubMed] [Google Scholar]
- 5.Tosoian J, Loeb S. PSA and beyond: the past, present, and future of investigative biomarkers for prostate cancer. ScientificWorldJournal. 2010;10:1919–31. doi: 10.1100/tsw.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23(32):8146–51. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 7.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8(4):268–78. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 8.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 9.AJCC Cancer Staging Manual. New York: Springer; 2010. Prostate; p. 457. [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 11.Abuzallouf S, Dayes I, Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol. 2004;171(6 Pt 1):2122–7. doi: 10.1097/01.ju.0000123981.03084.06. [DOI] [PubMed] [Google Scholar]
- 12.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 13.D'Amico AV, Whittington R, Malkowicz SB, et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol. 1999;17(1):168–72. doi: 10.1200/JCO.1999.17.1.168. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271(5):368–74. [PubMed] [Google Scholar]
- 15.Bastian PJ, Carter BH, Bjartell A, et al. Insignificant prostate cancer and active surveillance: from definition to clinical implications. Eur Urol. 2009;55(6):1321–30. doi: 10.1016/j.eururo.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Lawrentschuk N, Klotz L. Active surveillance for low-risk prostate cancer: an update. Nat Rev Urol. 2011;8:312–20. doi: 10.1038/nrurol.2011.50. [DOI] [PubMed] [Google Scholar]
- 17.Johansson JE, Holmberg L, Johansson S, Bergstrom R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA. 1997;277(6):467–71. [PubMed] [Google Scholar]
- 18.Khoo VS. Radiotherapeutic techniques for prostate cancer, dose escalation and brachytherapy. Clin Oncol (R Coll Radiol) 2005;17(7):560–71. doi: 10.1016/j.clon.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4(9):737–47. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 20.Hall EJ, Giaccia AJ, editors. Radiobiology for the radiologist. 6th. USA: Lippincott Wilkins & Williams; 2007. [Google Scholar]
- 21.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28(7):1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289–95. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 23.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107–18. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 24.Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6(11):841–50. doi: 10.1016/S1470-2045(05)70348-X. [DOI] [PubMed] [Google Scholar]
- 25.Roach M, 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26(4):585–91. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 26.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26(15):2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 28.Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–8. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 29.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61(5): 285–90. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 30.Gottschalk AR, Roach M., 3rd The use of hormonal therapy with radiotherapy for prostate cancer: analysis of prospective randomised trials. Br J Cancer. 2004;90(5):950–4. doi: 10.1038/sj.bjc.6601625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zietman AL, Prince EA, Nakfoor BM, Shipley WU. Neoadjuvant androgen suppression with radiation in the management of locally advanced adenocarcinoma of the prostate: experimental and clinical results. Urology. 1997;49(3A Suppl):74–83. doi: 10.1016/s0090-4295(97)00173-8. [DOI] [PubMed] [Google Scholar]
- 32.Zietman AL, Nakfoor BM, Prince EA, Gerweck LE. The effect of androgen deprivation and radiation therapy on an androgen-sensitive murine tumor: an in vitro and in vivo study. Cancer J Sci Am. 1997;3(1):31–6. [PubMed] [Google Scholar]
- 33.Joon DL, Hasegawa M, Sikes C, et al. Supraadditive apoptotic response of R3327-G rat prostate tumors to androgen ablation and radiation. Int J Radiat Oncol Biol Phys. 1997;38(5):1071–7. doi: 10.1016/s0360-3016(97)00303-9. [DOI] [PubMed] [Google Scholar]
- 34.Pollack A, Salem N, Ashoori F, et al. Lack of prostate cancer radiosensitization by androgen deprivation. Int J Radiat Oncol Biol Phys. 2001;51(4):1002–7. doi: 10.1016/s0360-3016(01)01750-3. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen PL, D'Amico AV. Targeting Pelvic Lymph Nodes in Men With Intermediate- and High-Risk Prostate Cancer Despite Two Negative Randomized Trials. J Clin Oncol. 2008;26(12):2055–2056. doi: 10.1200/JCO.2007.15.9939. [DOI] [PubMed] [Google Scholar]
- 36.Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25(34):5366–73. doi: 10.1200/JCO.2006.10.5171. [DOI] [PubMed] [Google Scholar]
- 37.Lawton CA, DeSilvio M, Roach M, 3rd, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69(3):646–55. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;8(10):715–7. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27(26):4300–5. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zelefsky MJ, Kattan MW, Fearn P, et al. Pretreatment nomogram predicting ten-year biochemical outcome of three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for prostate cancer. Urology. 2007;70(2):283–7. doi: 10.1016/j.urology.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 41.Roach M, 3rd, Waldman F, Pollack A. Predictive models in external beam radiotherapy for clinically localized prostate cancer. Cancer. 2009;115(13 Suppl):3112–20. doi: 10.1002/cncr.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 43.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125(7):605–13. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 44.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002;20(6):1692–703. doi: 10.1200/JCO.2002.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 45.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342–9. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 46.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 47.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 48.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101(36):13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305(5687):1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 50.Clark GM. Prognostic factors versus predictive factors: Examples from a clinical trial of erlotinib. Mol Oncol. 2008;1(4):406–12. doi: 10.1016/j.molonc.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50(5):1243–52. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 52.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137(3):413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 53.Cowen D, Salem N, Ashoori F, et al. Prostate cancer radiosensitization in vivo with adenovirus-mediated p53 gene therapy. Clin Cancer Res. 2000;6(11):4402–8. [PubMed] [Google Scholar]
- 54.Colletier PJ, Ashoori F, Cowen D, et al. Adenoviral-mediated p53 transgene expression sensitizes both wild-type and null p53 prostate cancer cells in vitro to radiation. Int J Radiat Oncol Biol Phys. 2000;48(5):1507–12. doi: 10.1016/s0360-3016(00)01409-7. [DOI] [PubMed] [Google Scholar]
- 55.Vesalainen SL, Lipponen PK, Talja MT, Alhava EM, Syrjanen KJ. Proliferating cell nuclear antigen and p53 expression as prognostic factors in T1-2M0 prostatic adenocarcinoma. Int J Cancer. 1994;58(2):303–8. doi: 10.1002/ijc.2910580226. [DOI] [PubMed] [Google Scholar]
- 56.Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of p53 protein as a prognostic indicator in prostate cancer. Hum Pathol. 1995;26(1):106–9. doi: 10.1016/0046-8177(95)90122-1. [DOI] [PubMed] [Google Scholar]
- 57.Visakorpi T, Kallioniemi OP, Heikkinen A, Koivula T, Isola J. Small subgroup of aggressive, highly proliferative prostatic carcinomas defined by p53 accumulation. J Natl Cancer Inst. 1992;84(11):883–7. doi: 10.1093/jnci/84.11.883. [DOI] [PubMed] [Google Scholar]
- 58.Scherr DS, Vaughan ED, Jr, Wei J, et al. BCL-2 and p53 expression in clinically localized prostate cancer predicts response to external beam radiotherapy. J Urol. 1999;162(1):12–6. doi: 10.1097/00005392-199907000-00003. discussion 16-7. [DOI] [PubMed] [Google Scholar]
- 59.Ritter MA, Gilchrist KW, Voytovich M, Chappell RJ, Verhoven BM. The role of p53 in radiation therapy outcomes for favorable-to-intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53(3):574–80. doi: 10.1016/s0360-3016(02)02781-5. [DOI] [PubMed] [Google Scholar]
- 60.Huang A, Gandour-Edwards R, Rosenthal SA, Siders DB, Deitch AD, White RW. p53 and bcl-2 immunohistochemical alterations in prostate cancer treated with radiation therapy. Urology. 1998;51(2):346–51. doi: 10.1016/s0090-4295(97)00636-5. [DOI] [PubMed] [Google Scholar]
- 61.Stattin P, Damber JE, Modig H, Bergh A. Pretreatment p53 immunoreactivity does not infer radioresistance in prostate cancer patients. Int J Radiat Oncol Biol Phys. 1996;35(5):885–9. doi: 10.1016/0360-3016(96)00134-4. [DOI] [PubMed] [Google Scholar]
- 62.Grignon DJ, Caplan R, Sarkar FH, et al. p53 status and prognosis of locally advanced prostatic adenocarcinoma: a study based on RTOG 8610. J Natl Cancer Inst. 1997;89(2):158–65. doi: 10.1093/jnci/89.2.158. [DOI] [PubMed] [Google Scholar]
- 63.Che M, DeSilvio M, Pollack A, et al. Prognostic value of abnormal p53 expression in locally advanced prostate cancer treated with androgen deprivation and radiotherapy: a study based on RTOG 9202. Int J Radiat Oncol Biol Phys. 2007;69(4):1117–23. doi: 10.1016/j.ijrobp.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khor LY, Bae K, Paulus R, et al. MDM2 and Ki-67 predict for distant metastasis and mortality in men treated with radiotherapy and androgen deprivation for prostate cancer: RTOG 92-02. J Clin Oncol. 2009;27(19):3177–84. doi: 10.1200/JCO.2008.19.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Wirth MP, Muller HA, Manseck A, Muller J, Frohmuller HG. Value of nuclear DNA ploidy patterns in patients with prostate cancer after radical prostatectomy. Eur Urol. 1991;20(3):248–52. doi: 10.1159/000471708. [DOI] [PubMed] [Google Scholar]
- 67.Winkler HZ, Rainwater LM, Myers RP, et al. Stage D1 prostatic adenocarcinoma: significance of nuclear DNA ploidy patterns studied by flow cytometry. Mayo Clin Proc. 1988;63(2):103–12. doi: 10.1016/s0025-6196(12)64942-8. [DOI] [PubMed] [Google Scholar]
- 68.Visakorpi T, Kallioniemi OP, Paronen IY, Isola JJ, Heikkinen AI, Koivula TA. Flow cytometric analysis of DNA ploidy and S-phase fraction from prostatic carcinomas: implications for prognosis and response to endocrine therapy. Br J Cancer. 1991;64(3):578–82. doi: 10.1038/bjc.1991.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nativ O, Winkler HZ, Raz Y, et al. Stage C prostatic adenocarcinoma: flow cytometric nuclear DNA ploidy analysis. Mayo Clin Proc. 1989;64(8):911–9. doi: 10.1016/s0025-6196(12)61218-x. [DOI] [PubMed] [Google Scholar]
- 70.Montgomery BT, Nativ O, Blute ML, et al. Stage B prostate adenocarcinoma. Flow cytometric nuclear DNA ploidy analysis. Arch Surg. 1990;125(3):327–31. doi: 10.1001/archsurg.1990.01410150049010. [DOI] [PubMed] [Google Scholar]
- 71.Lee SE, Currin SM, Paulson DF, Walther PJ. Flow cytometric determination of ploidy in prostatic adenocarcinoma: a comparison with seminal vesicle involvement and histopathological grading as a predictor of clinical recurrence. J Urol. 1988;140(4):769–74. doi: 10.1016/s0022-5347(17)41808-8. [DOI] [PubMed] [Google Scholar]
- 72.Haugen OA, Mjolnerod O. DNA-ploidy as prognostic factor in prostatic carcinoma. Int J Cancer. 1990;45(2):224–8. doi: 10.1002/ijc.2910450203. [DOI] [PubMed] [Google Scholar]
- 73.Fordham MV, Burdge AH, Matthews J, Williams G, Cooke T. Prostatic carcinoma cell DNA content measured by flow cytometry and its relation to clinical outcome. Br J Surg. 1986;3(5):400–3. doi: 10.1002/bjs.1800730530. [DOI] [PubMed] [Google Scholar]
- 74.Adolfsson J, Ronstrom L, Hedlund PO, Lowhagen T, Carstensen J, Tribukait B. The prognostic value of modal deoxyribonucleic acid in low grade, low stage untreated prostate cancer. J Urol. 1990;144(6):1404–6. doi: 10.1016/s0022-5347(17)39754-9. discussion 1406-7. [DOI] [PubMed] [Google Scholar]
- 75.Di Silverio F, D'Eramo G, Buscarini M, et al. DNA ploidy, Gleason score, pathological stage and serum PSA levels as predictors of disease-free survival in C-D1 prostatic cancer patients submitted to radical retropubic prostatectomy. Eur Urol. 1996;30(3):316–21. doi: 10.1159/000474189. [DOI] [PubMed] [Google Scholar]
- 76.Ross JS, Figge H, Bui HX, et al. Prediction of pathologic stage and postprostatectomy disease recurrence by DNA ploidy analysis of initial needle biopsy specimens of prostate cancer. Cancer. 1994;74(10):2811–8. doi: 10.1002/1097-0142(19941115)74:10<2811::aid-cncr2820741012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 77.Carmichael MJ, Veltri RW, Partin AW, Miller MC, Walsh PC, Epstein JI. Deoxyribonucleic acid ploidy analysis as a predictor of recurrence following radical prostatectomy for stage T2 disease. J Urol. 1995;153(3 Pt 2):1015–9. [PubMed] [Google Scholar]
- 78.Amling CL, Lerner SE, Martin SK, Slezak JM, Blute ML, Zincke H. Deoxyribonucleic acid ploidy and serum prostate specific antigen predict outcome following salvage prostatectomy for radiation refractory prostate cancer. J Urol. 1999;161(3):857–62. [PubMed] [Google Scholar]
- 79.Ahlgren G, Lindholm K, Falkmer U, Abrahamsson PA. A DNA cytometric proliferation index improves the value of the DNA ploidy pattern as a prognosticating tool in patients with carcinoma of the prostate. Urology. 1997;50(3):379–84. doi: 10.1016/S0090-4295(97)00223-9. [DOI] [PubMed] [Google Scholar]
- 80.Khoo VS, Pollack A, Cowen D, et al. Relationship of Ki-67 labeling index to DNA-ploidy, S-phase fraction, and outcome in prostate cancer treated with radiotherapy. Prostate. 1999;41(3):166–72. doi: 10.1002/(sici)1097-0045(19991101)41:3<166::aid-pros3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 81.Song J, Cheng WS, Cupps RE, Earle JD, Farrow GM, Lieber MM. Nuclear deoxyribonucleic acid content measured by static cytometry: important prognostic association for patients with clinically localized prostate carcinoma treated by external beam radiotherapy. J Urol. 1992;147(3 Pt 2):794–7. doi: 10.1016/s0022-5347(17)37388-3. [DOI] [PubMed] [Google Scholar]
- 82.Pollack A, Zagars GK, el-Naggar AK, Terry NH. Relationship of tumor DNA-ploidy to serum prostate-specific antigen doubling time after radiotherapy for prostate cancer. Urology. 1994;44(5):711–8. doi: 10.1016/s0090-4295(94)80213-0. [DOI] [PubMed] [Google Scholar]
- 83.Gauwitz MD, Pollack A, el-Naggar AK, Terry NH, von Eschenbach AC, Zagars GK. The prognostic significance of DNA ploidy in clinically localized prostate cancer treated with radiation therapy. Int J Radiat Oncol Biol Phys. 1994;28(4):821–8. doi: 10.1016/0360-3016(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 84.Pollack A, Zagars GK, el-Naggar AK, Gauwitz MD, Terry NH. Near-diploidy: a new prognostic factor for clinically localized prostate cancer treated with external beam radiation therapy. Cancer. 1994;73(7):1895–903. doi: 10.1002/1097-0142(19940401)73:7<1895::aid-cncr2820730721>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 85.Centeno BA, Zietman AL, Shipley WU, et al. Flow cytometric analysis of DNA ploidy, percent S-phase fraction, and total proliferative fraction as prognostic indicators of local control and survival following radiation therapy for prostate carcinoma. Int J Radiat Oncol Biol Phys. 1994;30(2):309–15. doi: 10.1016/0360-3016(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 86.Pollack A, Grignon DJ, Heydon KH, et al. Prostate cancer DNA ploidy and response to salvage hormone therapy after radiotherapy with or without short-term total androgen blockade: an analysis of RTOG 8610. J Clin Oncol. 2003;21(7):1238–48. doi: 10.1200/JCO.2003.02.025. [DOI] [PubMed] [Google Scholar]
- 87.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60(5):1306–11. [PubMed] [Google Scholar]
- 89.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276(21):18563–9. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 90.Kirschenbaum A, Liu X, Yao S, Levine AC. The role of cyclooxygenase-2 in prostate cancer. Urology. 2001;58(2 Suppl 1):127–31. doi: 10.1016/s0090-4295(01)01255-9. [DOI] [PubMed] [Google Scholar]
- 91.Pruthi RS, Derksen JE, Moore D, et al. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clin Cancer Res. 2006;12(7 Pt 1):2172–7. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 92.Raju U, Ariga H, Dittmann K, Nakata E, Ang KK, Milas L. Inhibition of DNA repair as a mechanism of enhanced radioresponse of head and neck carcinoma cells by a selective cyclooxygenase-2 inhibitor, celecoxib. Int J Radiat Oncol Biol Phys. 2005;63(2):520–8. doi: 10.1016/j.ijrobp.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 93.Wen B, Deutsch E, Eschwege P, et al. Cyclooxygenase-2 inhibitor NS398 enhances antitumor effect of irradiation on hormone refractory human prostate carcinoma cells. J Urol. 2003;170(5):2036–9. doi: 10.1097/01.ju.0000092239.98832.52. [DOI] [PubMed] [Google Scholar]
- 94.Palayoor ST, Arayankalayil MJ, Shoaibi A, Coleman CN. Radiation sensitivity of human carcinoma cells transfected with small interfering RNA targeted against cyclooxygenase-2. Clin Cancer Res. 2005;11(19 Pt 1):6980–6. doi: 10.1158/1078-0432.CCR-05-0326. [DOI] [PubMed] [Google Scholar]
- 95.Nakata E, Mason KA, Hunter N, et al. Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int J Radiat Oncol Biol Phys. 2004;58(2):369–75. doi: 10.1016/j.ijrobp.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 96.Khor LY, Bae K, Pollack A, et al. COX-2 expression predicts prostate-cancer outcome: analysis of data from the RTOG 92-02 trial. Lancet Oncol. 2007;8(10):912–20. doi: 10.1016/S1470-2045(07)70280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mellon PL, Clegg CH, Correll LA, McKnight GS. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci USA. 1989;86(13):4887–91. doi: 10.1073/pnas.86.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi SS, He ZG, Shao K, et al. Relationship between overexpression of the RIalpha subunit of the cAMP-dependent protein kinase and clinicopathological features of lung cancer. Zhonghua Zhong Liu Za Zhi. 2004;26(9):547–50. [PubMed] [Google Scholar]
- 99.Miller WR, Hulme MJ, Cho-Chung YS, Elton RA. Types of cyclic AMP binding proteins in human breast cancers. Eur J Cancer. 1993;29A(7):989–91. doi: 10.1016/s0959-8049(05)80207-2. [DOI] [PubMed] [Google Scholar]
- 100.Bradbury AW, Carter DC, Miller WR, Cho-Chung YS, Clair T. Protein kinase A (PK-A) regulatory subunit expression in colorectal cancer and related mucosa. Br J Cancer. 1994;69(4):738–42. doi: 10.1038/bjc.1994.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hensley HH, Hannoun-Levi JM, Hachem P, et al. PKA knockdown enhances cell killing in response to radiation and androgen deprivation. Int J Cancer. 2011;128(4):962–73. doi: 10.1002/ijc.25634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khor LY, Bae K, Al-Saleem T, et al. Protein kinase A RI-alpha predicts for prostate cancer outcome: analysis of radiation therapy oncology group trial 86-10. Int J Radiat Oncol Biol Phys. 2008;71(5):1309–15. doi: 10.1016/j.ijrobp.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pollack A, Bae K, Khor LY, et al. The importance of protein kinase A in prostate cancer: relationship to patient outcome in Radiation Therapy Oncology Group trial 92-02. Clin Cancer Res. 2009;15(17):5478–84. doi: 10.1158/1078-0432.CCR-08-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813(4):521–31. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 105.Karnak D, Xu L. Chemosensitization of prostate cancer by modulating Bcl-2 family proteins. Curr Drug Targets. 2010;11(6):699–707. doi: 10.2174/138945010791170888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsuji M, Murakami Y, Kanayama H, Sano T, Kagawa S. Immunohistochemical analysis of Ki-67 antigen and Bcl-2 protein expression in prostate cancer: effect of neoadjuvant hormonal therapy. Br J Urol. 1998;81(1):116–21. doi: 10.1046/j.1464-410x.1998.00492.x. [DOI] [PubMed] [Google Scholar]
- 107.Szostak MJ, Kaur P, Amin P, Jacobs SC, Kyprianou N. Apoptosis and bcl-2 expression in prostate cancer: significance in clinical outcome after brachytherapy. J Urol. 2001;165(6 Pt 1):2126–30. doi: 10.1097/00005392-200106000-00082. [DOI] [PubMed] [Google Scholar]
- 108.Rosser CJ, Reyes AO, Vakar-Lopez F, et al. Bcl-2 is significantly overexpressed in localized radio-recurrent prostate carcinoma, compared with localized radio-naive prostate carcinoma. Int J Radiat Oncol Biol Phys. 2003;56(1):1–6. doi: 10.1016/s0360-3016(02)04468-1. [DOI] [PubMed] [Google Scholar]
- 109.Pollack A, Cowen D, Troncoso P, et al. Molecular markers of outcome after radiotherapy in patients with prostate carcinoma: Ki-67, bcl-2, bax, and bcl-x. Cancer. 2003;97(7):630–8. doi: 10.1002/cncr.11230. [DOI] [PubMed] [Google Scholar]
- 110.McDonnell TJ, Troncoso P, Brisbay SM, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992;52(24):6940–4. [PubMed] [Google Scholar]
- 111.Mackey TJ, Borkowski A, Amin P, Jacobs SC, Kyprianou N. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology. 1998;52(6):1085–90. doi: 10.1016/s0090-4295(98)00360-4. [DOI] [PubMed] [Google Scholar]
- 112.Bylund A, Stattin P, Widmark A, Bergh A. Predictive value of bcl-2 immunoreactivity in prostate cancer patients treated with radiotherapy. Radiother Oncol. 1998;49(2):143–8. doi: 10.1016/s0167-8140(98)00111-x. [DOI] [PubMed] [Google Scholar]
- 113.Khor LY, Desilvio M, Li R, et al. Bcl-2 and bax expression and prostate cancer outcome in men treated with radiotherapy in Radiation Therapy Oncology Group protocol 86-10. Int J Radiat Oncol Biol Phys. 2006;66(1):25–30. doi: 10.1016/j.ijrobp.2006.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khor LY, Moughan J, Al-Saleem T, et al. Bcl-2 and Bax expression predict prostate cancer outcome in men treated with androgen deprivation and radiotherapy on radiation therapy oncology group protocol 92-02. Clin Cancer Res. 2007;13(12):3585–90. doi: 10.1158/1078-0432.CCR-06-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kamb A, Gruis NA, Weaver-Feldhaus J, et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264(5157):436–40. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- 116.Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368(6473):753–6. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- 117.Matheu A, Maraver A, Serrano M. The Arf/p53 pathway in cancer and aging. Cancer Res. 2008;68(15):6031–4. doi: 10.1158/0008-5472.CAN-07-6851. [DOI] [PubMed] [Google Scholar]
- 118.Chakravarti A, Heydon K, Wu CL, et al. Loss of p16 expression is of prognostic significance in locally advanced prostate cancer: an analysis from the Radiation Therapy Oncology Group protocol 86-10. J Clin Oncol. 2003;21(17):3328–34. doi: 10.1200/JCO.2003.12.151. [DOI] [PubMed] [Google Scholar]
- 119.Chakravarti A, DeSilvio M, Zhang M, et al. Prognostic value of p16 in locally advanced prostate cancer: a study based on Radiation Therapy Oncology Group Protocol 9202. J Clin Oncol. 2007;25(21):3082–9. doi: 10.1200/JCO.2006.08.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson GD, Saunders MI, Dische S, et al. Direct comparison of bromodeoxyuridine and Ki-67 labelling indices in human tumours. Cell Prolif. 1996;29(3):141–52. [PubMed] [Google Scholar]
- 121.Scalzo DA, Kallakury BV, Gaddipati RV, et al. Cell proliferation rate by MIB-1 immunohistochemistry predicts postradiation recurrence in prostatic adenocarcinomas. Am J Clin Pathol. 1998;109(2):163–8. doi: 10.1093/ajcp/109.2.163. [DOI] [PubMed] [Google Scholar]
- 122.Halvorsen OJ, Haukaas S, Hoisaeter PA, Akslen LA. Maximum Ki-67 staining in prostate cancer provides independent prognostic information after radical prostatectomy. Anticancer Res. 2001;21(6A):4071–6. [PubMed] [Google Scholar]
- 123.Grossfeld GD, Olumi AF, Connolly JA, et al. Locally recurrent prostate tumors following either radiation therapy or radical prostatectomy have changes in Ki-67 labeling index, p53 and bcl-2 immunoreactivity. J Urol. 1998;159(5):1437–43. doi: 10.1097/00005392-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 124.Stattin P, Damber JE, Karlberg L, Bergh A. Cell proliferation assessed by Ki-67 immunoreactivity on formalin fixed tissues is a predictive factor for survival in prostate cancer. J Urol. 1997;157(1):219–22. [PubMed] [Google Scholar]
- 125.Stapleton AM, Zbell P, Kattan MW, et al. Assessment of the biologic markers p53, Ki-67, and apoptotic index as predictive indicators of prostate carcinoma recurrence after surgery. Cancer. 1998;82(1):168–75. doi: 10.1002/(sici)1097-0142(19980101)82:1<168::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 126.Cowen D, Troncoso P, Khoo VS, et al. Ki-67 staining is an independent correlate of biochemical failure in prostate cancer treated with radiotherapy. Clin Cancer Res. 2002;8(5):1148–54. [PubMed] [Google Scholar]
- 127.Bubendorf L, Sauter G, Moch H, et al. Ki67 labelling index: an independent predictor of progression in prostate cancer treated by radical prostatectomy. J Pathol. 1996;178(4):437–41. doi: 10.1002/(SICI)1096-9896(199604)178:4<437::AID-PATH484>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 128.Bettencourt MC, Bauer JJ, Sesterhenn IA, Mostofi FK, McLeod DG, Moul JW. Ki-67 expression is a prognostic marker of prostate cancer recurrence after radical prostatectomy. J Urol. 1996;156(3):1064–8. [PubMed] [Google Scholar]
- 129.Li R, Heydon K, Hammond ME, et al. Ki-67 staining index predicts distant metastasis and survival in locally advanced prostate cancer treated with radiotherapy: an analysis of patients in radiation therapy oncology group protocol 86-10. Clin Cancer Res. 2004;10(12 Pt 1):4118–24. doi: 10.1158/1078-0432.CCR-1052-03. [DOI] [PubMed] [Google Scholar]
- 130.Pollack A, DeSilvio M, Khor LY, et al. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004;22(11):2133–40. doi: 10.1200/JCO.2004.09.150. [DOI] [PubMed] [Google Scholar]
- 131.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69(7):1237–45. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 132.Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362(6423):857–60. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 133.Stoyanova R, Hachem P, Hensley H, et al. Antisense-MDM2 sensitizes LNCaP prostate cancer cells to androgen deprivation, radiation, and the combination in vivo. Int J Radiat Oncol Biol Phys. 2007;68(4):1151–60. doi: 10.1016/j.ijrobp.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mu Z, Hachem P, Agrawal S, Pollack A. Antisense MDM2 oligonucleotides restore the apoptotic response of prostate cancer cells to androgen deprivation. Prostate. 2004;60(3):87–96. doi: 10.1002/pros.20044. [DOI] [PubMed] [Google Scholar]
- 135.Mu Z, Hachem P, Agrawal S, Pollack A. Antisense MDM2 sensitizes prostate cancer cells to androgen deprivation, radiation, and the combination. Int J Radiat Oncol Biol Phys. 2004;58(2):336–43. doi: 10.1016/j.ijrobp.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 136.Leite KR, Franco MF, Srougi M, et al. Abnormal expression of MDM2 in prostate carcinoma. Mod Pathol. 2001;14(5):428–36. doi: 10.1038/modpathol.3880330. [DOI] [PubMed] [Google Scholar]
- 137.Osman I, Drobnjak M, Fazzari M, Ferrara J, Scher HI, Cordon-Cardo C. Inactivation of the p53 pathway in prostate cancer: impact on tumor progression. Clin Cancer Res. 1999;5(8):2082–8. [PubMed] [Google Scholar]
- 138.Khor LY, Desilvio M, Al-Saleem T, et al. MDM2 as a predictor of prostate carcinoma outcome: an analysis of Radiation Therapy Oncology Group Protocol 8610. Cancer. 2005;104(5):962–7. doi: 10.1002/cncr.21261. [DOI] [PubMed] [Google Scholar]
- 139.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3(6):401–10. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 140.Zhang M, Latham DE, Delaney MA, Chakravarti A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene. 2005;24(15):2474–82. doi: 10.1038/sj.onc.1208490. [DOI] [PubMed] [Google Scholar]
- 141.Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6(1):127–34. [PubMed] [Google Scholar]
- 142.Monzo M, Rosell R, Felip E, et al. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol. 1999;17(7):2100–4. doi: 10.1200/JCO.1999.17.7.2100. [DOI] [PubMed] [Google Scholar]
- 143.Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58(9):1808–12. [PubMed] [Google Scholar]
- 144.Liu JJ, Huang RW, Lin DJ, et al. Expression of survivin and bax/bcl-2 in peroxisome proliferator activated receptor-gamma ligands induces apoptosis on human myeloid leukemia cells in vitro. Ann Oncol. 2005;16(3):455–9. doi: 10.1093/annonc/mdi077. [DOI] [PubMed] [Google Scholar]
- 145.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58(22):5071–4. [PubMed] [Google Scholar]
- 146.Ikeguchi M, Ueta T, Yamane Y, Hirooka Y, Kaibara N. Inducible nitric oxide synthase and survivin messenger RNA expression in hepatocellular carcinoma. Clin Cancer Res. 2002;8(10):3131–6. [PubMed] [Google Scholar]
- 147.Grossman D, McNiff JM, Li F, Altieri DC. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest Dermatol. 1999;113(6):1076–81. doi: 10.1046/j.1523-1747.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 148.Chakravarti A, Zhai GG, Zhang M, et al. Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms. Oncogene. 2004;23(45):7494–506. doi: 10.1038/sj.onc.1208049. [DOI] [PubMed] [Google Scholar]
- 149.Chakravarti A, Noll E, Black PM, et al. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20(4):1063–8. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 150.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3(1):46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 151.Kishi H, Igawa M, Kikuno N, Yoshino T, Urakami S, Shiina H. Expression of the survivin gene in prostate cancer: correlation with clinicopathological characteristics, proliferative activity and apoptosis. J Urol. 2004;171(5):1855–60. doi: 10.1097/01.ju.0000120317.88372.03. [DOI] [PubMed] [Google Scholar]
- 152.Zhang M, Mukherjee N, Bermudez RS, Latham DE, Delaney MA, Zietman AL, et al. Adenovirus-mediated inhibition of survivin expression sensitizes human prostate cancer cells to paclitaxel in vitro and in vivo. Prostate. 2005;64(3):293–302. doi: 10.1002/pros.20263. [DOI] [PubMed] [Google Scholar]
- 153.Zhang M, Ho A, Hammond EH, et al. Prognostic value of survivin in locally advanced prostate cancer: study based on RTOG 8610. Int J Radiat Oncol Biol Phys. 2009;73(4):1033–42. doi: 10.1016/j.ijrobp.2008.06.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fortugno P, Wall NR, Giodini A, et al. Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci. 2002;115(Pt 3):575–85. doi: 10.1242/jcs.115.3.575. [DOI] [PubMed] [Google Scholar]
- 155.Okera M, Bae K, Bernstein E, Cheng L, Lawton C, Wolkov H, et al. Evaluation of nuclear factor kappaB and chemokine receptor CXCR4 co-expression in patients with prostate cancer in the Radiation Therapy Oncology Group (RTOG) 8610. BJU Int. 2011;108(2 Pt 2):E51–8. doi: 10.1111/j.1464-410X.2010.09884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Torres-Roca JF, DeSilvio M, Mora LB, et al. Activated STAT3 as a correlate of distant metastasis in prostate cancer: a secondary analysis of Radiation Therapy Oncology Group 86-10. Urology. 2007;69(3):505–9. doi: 10.1016/j.urology.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 157.Abdel-Wahab M, Berkey BA, Krishan A, et al. Influence of number of CAG repeats on local control in the RTOG 86-10 protocol. Am J Clin Oncol. 2006;29(1):14–20. doi: 10.1097/01.coc.0000195085.34162.88. [DOI] [PubMed] [Google Scholar]
- 158.Roach M, 3rd, De Silvio M, Rebbick T, et al. Racial differences in CYP3A4 genotype and survival among men treated on Radiation Therapy Oncology Group (RTOG) 9202: a phase III randomized trial. Int J Radiat Oncol Biol Phys. 2007;69(1):79–87. doi: 10.1016/j.ijrobp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 159.Vergis R, Corbishley CM, Thomas K, et al. Expression of Bcl-2, p53, and MDM2 in localized prostate cancer with respect to the outcome of radical radiotherapy dose escalation. Int J Radiat Oncol Biol Phys. 2010;78(1):35–41. doi: 10.1016/j.ijrobp.2009.07.1728. [DOI] [PubMed] [Google Scholar]
- 160.Vergis R, Corbishley CM, Norman AR, et al. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9(4):342–51. doi: 10.1016/S1470-2045(08)70076-7. [DOI] [PubMed] [Google Scholar]
- 161.Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and Radiation Response in Human Tumors. Semin Radiat Oncol. 1996;6(1):3–9. doi: 10.1053/SRAO0060003. [DOI] [PubMed] [Google Scholar]
- 162.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2(10):803–11. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 163.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999;281(17):1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 164.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163(6):1632–42. [PubMed] [Google Scholar]
- 165.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–30. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 167.Van der Kwast TH, Bolla M, Van Poppel H, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25(27):4178–86. doi: 10.1200/JCO.2006.10.4067. [DOI] [PubMed] [Google Scholar]
- 168.Nielsen ME, Trock BJ, Walsh PC. Salvage or adjuvant radiation therapy: counseling patients on the benefits. J Natl Compr Canc Netw. 2009;8(2):228–37. doi: 10.6004/jnccn.2010.0015. [DOI] [PubMed] [Google Scholar]
- 169.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. Jama. 2008;299(23):2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25(15):2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cher ML, Bianco FJ, Jr, Lam JS, et al. Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J Urol. 1998;60(4):1387–91. [PubMed] [Google Scholar]
- 172.Buskirk SJ, Pisansky TM, Schild SE, et al. Salvage radiotherapy for isolated prostate specific antigen increase after radical prostatectomy: evaluation of prognostic factors and creation of a prognostic scoring system. J Urol. 2006;176(3):985–90. doi: 10.1016/j.juro.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 173.Rigaud J, Tiguert R, Decobert M, et al. Expression of p21 cell cycle protein is an independent predictor of response to salvage radiotherapy after radical prostatectomy. Prostate. 2004;58(3):269–76. doi: 10.1002/pros.10329. [DOI] [PubMed] [Google Scholar]
- 174.Schipper JH, Frixen UH, Behrens J, Unger A, Jahnke K, Birchmeier W. E-cadherin expression in squamous cell carcinomas of head and neck: inverse correlation with tumor dedifferentiation and lymph node metastasis. Cancer Res. 1991;51(23 Pt 1):6328–37. [PubMed] [Google Scholar]
- 175.Oka H, Shiozaki H, Kobayashi K, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53(7):696–701. [PubMed] [Google Scholar]
- 176.Umbas R, Isaacs WB, Bringuier PP, et al. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54(14):3929–33. [PubMed] [Google Scholar]
- 177.Wu TT, Hsu YS, Wang JS, Lee YH, Huang JK. The role of p53, bcl-2 and E-cadherin expression in predicting biochemical relapse for organ confined prostate cancer in Taiwan. J Urol. 2003;170(1):78–81. doi: 10.1097/01.ju.0000065802.92406.a6. [DOI] [PubMed] [Google Scholar]
- 178.Brewster SF, Oxley JD, Trivella M, Abbott CD, Gillatt DA. Preoperative p53, bcl-2, CD44 and E-cadherin immunohistochemistry as predictors of biochemical relapse after radical prostatectomy. J Urol. 1999;161(4):1238–43. [PubMed] [Google Scholar]
- 179.Richmond PJ, Karayiannakis AJ, Nagafuchi A, Kaisary AV, Pignatelli M. Aberrant E-cadherin and alpha-catenin expression in prostate cancer: correlation with patient survival. Cancer Res. 1997;57(15):3189–93. [PubMed] [Google Scholar]
- 180.Umbas R, Schalken JA, Aalders TW, et al. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992;52(18):5104–9. [PubMed] [Google Scholar]
- 181.De Marzo AM, Knudsen B, Chan-Tack K, Epstein JI. E-cadherin expression as a marker of tumor aggressiveness in routinely processed radical prostatectomy specimens. Urology. 1999;53(4):707–13. doi: 10.1016/s0090-4295(98)00577-9. [DOI] [PubMed] [Google Scholar]
- 182.Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329(6137):341–3. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- 183.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6(8):622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 184.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–59. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13(23):7003–11. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 186.Ray ME, Mehra R, Sandler HM, Daignault S, Shah RB. E-cadherin protein expression predicts prostate cancer salvage radiotherapy outcomes. J Urol. 2006;176(4 Pt 1):1409–14. doi: 10.1016/j.juro.2006.06.014. discussion 1414. [DOI] [PubMed] [Google Scholar]
- 187.Parker AS, Heckman MG, Wu KJ, et al. Evaluation of ki-67 staining levels as an independent biomarker of biochemical recurrence after salvage radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75(5):1364–70. doi: 10.1016/j.ijrobp.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 188.Seliger B, Marincola FM, Ferrone S, Abken H. The complex role of B7 molecules in tumor immunology. Trends Mol Med. 2008;14(12):550–9. doi: 10.1016/j.molmed.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 190.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100(18):10388–92. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 192.Prasad DV, Nguyen T, Li Z, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–6. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 193.Suh WK, Gajewska BU, Okada H, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 194.Wang L, Fraser CC, Kikly K, et al. B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol. 2005;35(2):428–38. doi: 10.1002/eji.200425518. [DOI] [PubMed] [Google Scholar]
- 195.Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–74. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 196.Roth TJ, Sheinin Y, Lohse CM, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67(16):7893–900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 197.Parker AS, Heckman MG, Sheinin Y, et al. Evaluation of B7-H3 expression as a biomarker of biochemical recurrence after salvage radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys. 2011;79(5):1343–9. doi: 10.1016/j.ijrobp.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 198.Beer AJ, Eiber M, Souvatzoglou M, Schwaiger M, Krause BJ. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol. 2011;12(2):181–91. doi: 10.1016/S1470-2045(10)70103-0. [DOI] [PubMed] [Google Scholar]
- 199.Ideker T, Galitski T, Hood L. A new approach to decoding life: systems biology. Annu Rev Genomics Hum Genet. 2001;2:343–72. doi: 10.1146/annurev.genom.2.1.343. [DOI] [PubMed] [Google Scholar]
- 200.Taylor BS, .Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ross AE, Marchionni L, Vuica-Ross M, et al. Gene expression pathways of high grade localized prostate cancer. Prostate. 2011;71(14):1568–77. doi: 10.1002/pros.21373. [DOI] [PubMed] [Google Scholar]
- 202.Goo YA, Goodlett DR. Advances in proteomic prostate cancer biomarker discovery. J Proteomics. 2010;73(10):1839–50. doi: 10.1016/j.jprot.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 203.Sreekumar A, Poisson LM, Rajendiran TM, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–4. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]