Abstract
Background
Depression in adolescence is common and early onset predicts worse outcome in adulthood. Studies in adults have suggested a link between higher total 25-hydroxyvitamin D [25(OH)D] concentrations and lower risk of depression.
Objectives
To investigate (a) the association between serum 25(OH)D2 and 25(OH)D3 concentrations and depressive symptoms in children, and (b) whether the associations of 25(OH)D2 and 25(OH)D3 are different from, and independent of, each other.
Methods
Prospective cohort study with serum 25(OH)D2 and 25(OH)D3 concentrations measured at mean age of 9.8 years and depressive symptoms assessed with the Mood and Feelings Questionnaire by a trained interviewer at the mean ages of 10.6 years (n = 2,759) and 13.8 years (n = 2,752).
Results
Higher concentrations of 25(OH)D3 assessed at mean age 9.8 years were associated with lower levels of depressive symptoms at age 13.8 years [adjusted risk ratio (RR; 95% confidence interval (CI)): 0.90 (0.86–0.95)], but not at age 10.6 years [adjusted RR (95% CI): 0.98 (0.93–1.03)] and with increased odds of decreasing symptoms between age 10.6 and 13.8 years [adjusted RR (95% CI): 1.08 (1.01–1.16)]. Serum 25(OH)D2 concentrations were not associated with depressive symptoms.
Conclusions
This is the first study in children to suggest that the association between 25(OH)D3 concentrations and depression emerges in childhood. The association is independent of a wide range of potential confounding factors, and appears to be stronger with greater time separation between assessment of 25(OH)D3 and assessment of depressive symptoms. Confirmation of our findings in large prospective studies and trials would be valuable.
Keywords: 25-Hydroxyvitamin D, calcium, parathyroid hormone, child, depression, ALSPAC
Introduction
Depression affects 1–6% of adolescents worldwide and early onset often predicts more serious disease manifestation in later life (Thapar, Collishaw, Potter, & Thapar, 2010). Characterisation of modifiable risk factors that could be used to prevent or delay the early onset of depression is important. It has been suggested that higher concentrations of vitamin D may protect against depression in adults. Depression rates are higher in winter than in summer months, which could support a role for vitamin D (Bertone-Johnson, 2009). Some (Armstrong et al., 2007; Berk et al., 2007; Eskandari et al., 2007; Ganji, Milone, Cody, McCarty, & Wang, 2010; Hoogendijk et al., 2008; Jorde, Waterloo, Saleh, Haug, & Svartberg, 2006, Lee et al., 2010; Schneider, Weber, Frensch, Stein, & Fritz, 2000; Wilkins, Sheline, Roe, Birge, & Morris, 2006) but not all (Annweiler et al., 2010; Herran et al., 2000; Michelson et al., 1996; Nanri et al., 2009; Pan et al., 2009; Zhao, Ford, Li, & Balluz, 2010) cross-sectional studies in adults have found an association between higher serum concentrations of 25-hydroxyvitamin D (25(OH)D) and lower risk of depression in adults. Two prospective studies found that higher concentration of 25(OH)D were associated with lower risk of depression in older adults (May et al., 2010; Milaneschi et al., 2010) and three randomised controlled trials in selective populations had inconsistent findings, with a trial of vitamin D3 supplements improving depression symptoms in overweight/obese adults (Jorde, Sneve, Figenschau, Svartberg, & Waterloo, 2008) but two further trials of D3 showing no effect on symptoms in older women with seasonal affective disorder (Dumville et al., 2006) or on preventing depression in older women (Sanders et al., 2011).
25(OH)D is a robust and reliable indicator of vitamin D status, reflecting both dietary intake and synthesis in skin, which normally accounts for most of the vitamin D in humans (Seamans & Cashman, 2009). Circulatory total 25(OH)D consists of 25(OH)D3 [metabolite of vitamin D3 synthesised in skin after ultraviolet B (UVB) exposure and obtained from animal food sources] and 25(OH)D2 (synthesised from vitamin D2 obtained from plant sources). 25(OH)D3 and 25(OH)D2 are converted to 1,25-dihydroxyvitamin D3 and D2, the steroid hormones that mediate the biological actions of vitamin D. The former is known to have higher affinity to vitamin D binding protein and receptor (Glendenning et al., 2009; Houghton & Vieth, 2006) and with respect to bone health, vitamin D3 has been suggested to be more potent than D2 (Finch, Brown, & Slatopolsky, 1999). However, to date no studies have examined whether associations between these two differ with respect to depression. Vitamin D, together with parathyroid hormone (PTH), regulates calcium and phosphate homoeostasis (Brown, Dusso, & Slatopolsky, 1999; Mundy & Guise, 1999) and some (Eskandari et al., 2007; Herran et al., 2000, Hoogendijk et al., 2008; Jorde et al., 2006; May et al., 2010) but not all (Michelson et al., 1996; Schneider et al., 2000) studies have reported higher serum PTH among adults with depression. As the previous studies have included only adults, it is unknown if serum 25(OH)D concentrations are associated with mood in childhood or early adolescence. Examining this association in childhood/adolescence is important because confounding by alcohol, smoking and mood-altering drugs is somewhat less likely than in adult studies and because it is increasingly recognised that depression can emerge in childhood/adolescence (Thapar et al., 2010) and its prevention may be best started at this age.
The aims of this study were (a) to investigate the prospective association between serum 25(OH)D2 and 25(OH)D3 concentrations and depressive symptoms in children, (b) to investigate if the associations of 25(OH)D2 and 25(OH)D3 are different, and (c) to examine whether any associations were independent of serum PTH, phosphate and calcium concentrations.
Participants and methods
Population
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a population-based birth cohort from South West England. The cohort consisted of 14,062 live births from 14,541 enrolled pregnant women who were expected to give birth between 1 April 1991 and 31 December 1992 (Golding, Pembrey, Jones and ALSPAC Study Team, 2001). From age 7, all children were invited for an annual assessment of physical and psychological development. Parents gave informed consent at enrolment, and ethical approval was obtained from the ALSPAC Law and Ethics Research Committee and the National Health Service (NHS) local research ethics committee.
Single and twin births were included in this study; the very small number of triplets and quadruplets were not included for reasons of confidentiality. Figure 1 shows how the numbers included in the analyses presented here was derived. Complete data on outcomes, exposures and confounders were available from 2,759 and 2,752 children, respectively, for assessment with outcomes at 10.6 years and with outcomes at 13.8 years.
Figure 1.
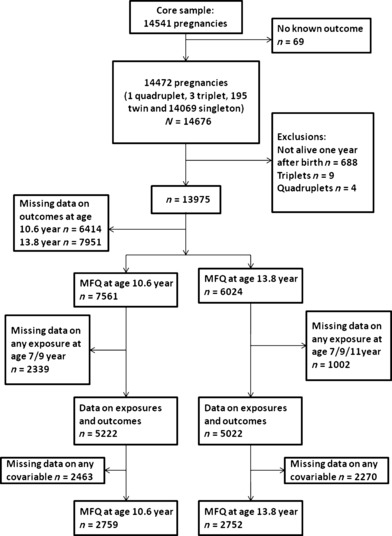
Flow of participants
Outcome
The prevalence of depressive symptoms was evaluated with the Mood and Feelings Questionnaire (MFQ) by a trained interviewer at the mean ages of 10.6 and 13.8 years. The MFQ is a 13-item depression inventory validated for use in 6- to 18-year-olds. Each item is scored between 0 and 2, resulting to a maximum score of 26 with higher scores indicating presence of symptoms of depression. MFQ correlates highly with more extensive evaluations like the Children’s Depression Inventory and the Diagnostic Interview Schedule for Children (Costello & Angold, 1988).
The MFQ scores were positively skewed even after various transformations so we generated three categories of the score (0–2, 3–5, ≥ 6), representing approximate thirds of the distribution. An ordinal categorical variable was derived to indicate change in MFQ score category (increase by two categories/increase by one category/no change/decrease by one category/decrease by two categories) between ages 10.6 and 13.8 years.
Exposures and phlebotomy-based covariables
Serum 25(OH)D3, 25(OH)D2, PTH, phosphate and calcium were assayed on nonfasting blood samples collected at mean age 9.9 years for the majority of participants (n = 2,130 for MFQ assessed at age 13.8 years and n = 2,493 for MFQ assessed at age 10.6 years). If no samples were available from the 9.9-year assessment, samples from mean age 11.8 years (n = 416) or, second, the 7.6-year assessments (n = 206 for MFQ assessed at age 13.8 years and n = 266 for MFQ assessed at age 10.6 years) were used. The mean age at sample collection in the whole study sample was 9.8 years (standard deviation: SD = 0.74). To keep the analyses prospective, we excluded exposure measurements that were taken at 11.8-year clinic when the outcome was measured at age 10.6 years.
Following collection, samples were immediately spun, frozen and stored at −80 °C. Assays were performed after a maximum of 12 years in storage with no previous freeze-thaw cycles. 25(OH)D3, 25(OH)D2 and deuterated internal standard were extracted from serum samples, following protein precipitation, using Isolute C18 solid phase extraction cartridges. Potential interfering compounds were removed by initial elution with 50% methanol followed by elution of the vitamins using 10% tetrahydrofuran in acetonitrile. Dried extracts were reconstituted prior to injection into a high-pressure liquid chromatography tandem mass spectrometer (Waters Acuity, Manchester, UK). The following transitions (mass to charge ratio) in multiple reaction mode were used: 413.2 > 395.3, 401.1 > 383.3 and 407.5 > 107.2 for 25(OH)D2, 25(OH)D3, and hexa-deuterated 25(OH)D3 respectively. Interassay coefficients of variation for the assay were < 10% across a working range of 1–250 ng/ml for both 25(OH)D3 and 25(OH)D2. Measurements were performed in a laboratory meeting the performance target set by the Vitamin D External Quality Assessment Scheme (DEQAS) Advisory Panel for 25(OH)D assays.
Total serum calcium, phosphate and albumin concentrations were measured by standard laboratory methods on Roche Modular analysers (Roche Diagnostics Ltd, West Sussex, UK). Serum calcium was adjusted for albumin using a normogram of calcium and albumin distributions of the samples analysed in the clinical chemistry laboratory where the measurements were performed and albumin-adjusted calcium was used in all statistical analyses. Intact parathyroid hormone [iPTH(1–84)] was measured by electrochemiluminescent immunoassay on Elecsys 2010 immunoanalyzer (Roche, Lewes, UK). Interassay coefficient of variation was < 6% from 2 to 50 pmol/L. The assay sensitivity was 1 pmol/L.
Confounding factors
We considered gender, age, ethnicity (white, nonwhite), head of household occupational social class, maternal and paternal education, family history of depression or schizophrenia, UVB exposure, body mass index (BMI) and cognitive function to be important confounders because of their known associations with 25(OH)D3 concentrations and depressive symptoms. We also adjusted for pubertal stage as this might affect depressive symptoms and 25(OH)D3. Data on head of household occupational social class, ethnicity, parents’ education and family history of depression and schizophrenia were obtained from parent-completed questionnaires. Time spent outdoors during summer months on school days, school weekends and holidays was reported as None, 1 hr/day, 1–2 hr/day and 3 or more hr/day in parent-completed questionnaires at mean age of 8.5 years. Responses were coded as follows: None = 0, 1 = 1, 1–2 = 1.5 and 3 = 5. Average hours spent outdoors per summer day (1 June–31 August) were calculated using term dates from Bristol City Council’s Education Committee term dates for 2001–2002 (summer term 1 June–24 July, holidays 24 July–31 August). Information on protection from UVB exposure (use of sunblock, covering clothing or hat and avoidance of midday sun) were obtained from the same questionnaires. A summary variable for UVB protection score was derived by scoring the responses to questions on use of sunblock, covering clothing or hat and avoidance of midday sun as Always = 3, Usually = 2, Sometimes = 1, Never = 0 and summing these scores. This gives a single variable that ranges from 0 to 12, with 0 indicating the least meticulous protection from UVB.
Height and weight were measured at the same time as blood samples for obtaining 25(OH)D3 and other assays and were used to calculate BMI. Total IQ score in Wechsler Intelligence Scale for Children (WISC–III UK version) was assessed at mean age 8.5. Puberty stage was assessed by parental report using Tanner staging (Tanner, 1962) of pubic hair, breast and genitalia development on repeat occasions. In our analyses, we used data from the questionnaire closest to the time of phlebotomy for the exposures for each child.
25(OH)D3 concentrations had displayed a sinusoidal seasonal variation (Figure 2). In order to derive a value of 25(OH)D3 for each participant that was accurately adjusted for the seasonal effects of when the sample was taken, we used linear regression with date of blood sampling as the independent variable and loge 25(OH)D3 as the outcome (dependent) variable and with trigonometric sine and cosine functions. 25(OH)D3 was loge transformed in this regression model to ensure that the residuals in the sine–cosine regression were approximately normally distributed. The residuals from this regression are the participants logged 25(OH)D3 having adjusted for seasonal differences in when the sample was taken. This seasonal adjusted 25(OH)D3 variable represents a participants average 25(OH)D3 across seasons and is the main 25(OH)D3 exposure measurement used in our analyses [we also present associations without this adjustment]. There was no strong seasonal variation in 25(OH)D2 concentrations.
Figure 2.
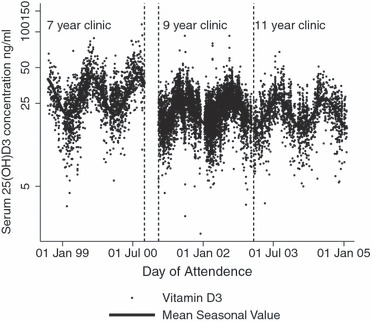
Seasonal variation in serum 25(OH)D3 concentrations
Statistical analyses
Statistical analyses were conducted with Stata 11.0 (Stata Corp LP, College Station, TX).
To include all participants on whom a 25(OH)D2 was assayed, those with a value below the detectable limit of the assay (0.5 ng/ml) were given a value of 0.5 ng/ml and indicated using a binary covariable in all regression models. Serum 25(OH)D3, 25(OH)D2, calcium, phosphate and PTH concentrations were age- and gender-standardised using the internal cohort data with age in 1-month categories.
The association of potential confounders with serum 25(OH)D3, 25(OH)D2, calcium, phosphate and PTH concentrations was assessed with linear regression and associations of confounders with depressive symptoms with ordered logistic regression. We investigated the linearity of associations between 25(OH)D3 and 25(OH)D2 and depressive symptoms by splitting the 25(OH)D variables into fifths of their distribution and graphically examining the odds of depressive symptoms across these fifths. We statistically tested for linearity by obtaining a p value for linear trend from a regression model in which these fifths were entered as a continuous score (1–5). Deviation from linearity was assessed with a likelihood ratio test comparing regression models in which the fifths were entered as categories (four indicator variables) and one in which they were entered as a continuous score.
The main analyses assessing associations of seasonally adjusted 25(OH)D3 and 25(OH)D2 with outcomes were done with a nonparametric bootstrap procedure (10,000 replications) in conjunction with ordered logistic regression using bsample and ologit commands in Stata. The bootstrapping procedure (Efron & Tibshirani, 1986) enabled us to statistically compare associations of 25(OH)D3 to those of 25(OH)D2. The difference between the effect of 25(OH)D3 and 25(OH)D2 was calculated from the bootstrap replicate distribution To numerically compare the associations of two forms of 25(OH)D, we scaled them the same by multiplying the beta coefficients from the regression models by loge(2). The results are interpreted as the difference in odds of MFQ between the lowest and middle or middle and highest MFQ score per doubling of exposure.
In addition to examining associations with 25(OH)D2 and 25(OH)D3 on a continuous scale, we also examined the association between total 25(OH)D deficiency and insufficiency and depressive symptoms. Total 25(OH)D was calculated by summing 25(OH)D2 and 25(OH)D3 and insufficiency was defined as a value below 30 ng/ml and deficiency below 20 ng/ml (Holick, 2007). Finally, in a sensitivity analysis we examined the association of 25(OH)D3 that was not adjusted for seasonal variation with outcomes. All association analyses were performed for both genders combined as there was no strong statistical evidence for Gender × Exposure interaction (p ≥ .20).
Results
The mean (interquartile range) serum concentrations of season-adjusted 25(OH)D3 and 25(OH)D2 were 24.9 (24.7–25.1) and 1.3 (0.5–2.7) ng/ml, respectively. The median (interquartile range) of serum phosphate, calcium and PTH were 1.54 (1.43–1.64) mmol/L, 2.38 (2.31–2.44) mmol/L and 4.5 (3.4–5.8) pmol/L respectively. Other characteristics of the participants are shown in Table S1.
Table S2 shows the associations of 25(OH)D3, 25(OH)D2 phosphate, calcium and PTH concentrations with potential confounders. Those of nonwhite ethnicity had higher PTH and lower 25(OH)D3 concentrations. BMI was negatively associated with serum 25(OH)D3 and 25(OH)D2 concentrations and positively with PTH concentrations. Higher socioeconomic position of parents was associated with lower concentrations of calcium and 25(OH)D2 and higher concentrations of 25(OH)D3. Less meticulous protection from UVB was associated with lower PTH concentrations and higher 25(OH)D3 concentrations. Children who spent more time outdoors during summer had higher 25(OH)D3 and 25(OH)D2 concentrations. Children with family history of psychiatric problems had lower 25(OH)D3 and calcium concentrations. Those with more advanced puberty stage had lower 25(OH)D3 concentrations and higher calcium concentrations.
Table S3 summarises the associations of confounders and depressive symptoms at ages 10.6 and 13.8 years. Higher socioeconomic position and IQ were associated with lower risk of depressive symptoms at age 10.6 years and higher BMI with higher risk of depressive symptoms at age 13 years. Children who spent more time outdoors during summer had lower risk of depressive symptoms at age 13.8 years. Children with family history of psychiatric problems had higher risk of depressive symptoms at both ages.
The associations of 25(OH)D3 and 25(OH)D2 with depressive symptoms were either linear or null, with no strong evidence of deviation from linearity or suggestion of a threshold association (Figure 3). Table 1 shows the prospective associations between serum 25(OH)D3, 25(OH)D2, phosphate, calcium and PTH concentrations and depressive symptoms at ages 10.6 and 13.8 years. Higher concentrations of 25(OH)D3 were associated with lower risk of depressive symptoms at age 13.8 years but not at age 10.6 years. 25(OH)D2 was not associated with depressive symptoms at either age. There was statistical evidence that the association of 25(OH)D3 with depressive symptoms at age 13.8 years differed from the association of 25(OH)D2 with the same outcome (p ≤ .001), but there was no evidence of difference in associations of 25(OH)D3 and 25(OH)D2 with symptoms at age 10.6 years (p = .81). There was some suggestion that higher concentrations of PTH were associated with lower risk of depressive symptoms at age 13.8 years after adjusting for all confounders and other measured analytes, but confidence intervals were wide and included the null value. Other exposures were not associated with depressive symptoms.
Figure 3.
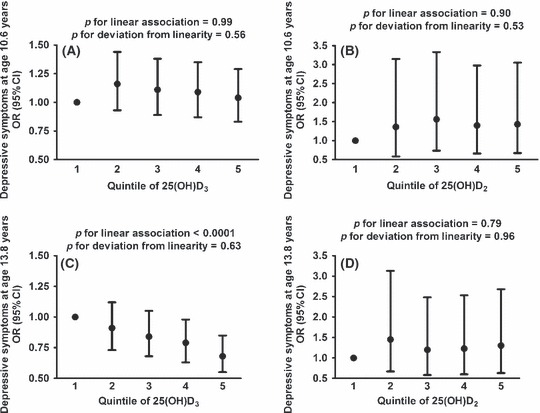
Odds of depressive symptoms at age 10.6 years (A and B) and age 13.8 years (C and D) by fifths of the distribution of 25(OH)D3 (A and C) and 25(OH)D2 (B and D)
Table 1.
Prospective association of 25(OH)D3, 25(OH)D2, phosphate, albumin-adjusted calcium and parathyroid hormone concentrations with depressive symptoms assessed by short Mood and Feelings Questionnaire at age 10.6 years (n = 2,759; exposures assessed at mean age 9.2 years) and age 13.8 years (n = 2,752; exposures assessed at mean age 9.8 years)
| OR for category change per doubling of exposure (95% CI) | ||||
|---|---|---|---|---|
| Outcome | Exposure | Model 1a | Model 2b | Model 3c |
| Depressive symptoms at age 10.6 years | 25(OH)D3 | 0.98 (0.93, 1.03) | 0.98 (0.93, 1.03) | 0.98 (0.93, 1.03) |
| 25(OH)D2 | 1.02 (0.94, 1.10) | 1.01 (0.93, 1.10) | 0.99 (0.94, 1.04) | |
| Albumin-adjusted calcium | 1.02 (0.97, 1.07) | 1.01 (0.97, 1.06) | 1.00 (0.95, 1.05) | |
| Phosphate | 1.03 (0.98, 1.08) | 1.04 (0.99, 1.09) | 1.04 (0.98, 1.09) | |
| Parathyroid hormone | 0.98 (0.93, 1.03) | 0.98 (0.94, 1.03) | 0.97 (0.93, 1.03) | |
| Depressive symptoms at age 13.8 years | 25(OH)D3 | 0.90 (0.86, 0.94) | 0.91 (0.86, 0.95) | 0.90 (0.86, 0.95) |
| 25(OH)D2 | 1.02 (0.95, 1.11) | 1.03 (0.95, 1.12) | 1.02 (0.97, 1.08) | |
| Albumin-adjusted calcium | 0.99 (0.94, 1.04) | 1.00 (0.95, 1.04) | 0.99 (0.94, 1.04) | |
| Phosphate | 1.01 (0.97, 1.06) | 1.02 (0.97, 1.06) | 1.02 (0.98, 1.07) | |
| Parathyroid hormone | 0.99 (0.94, 1.03) | 0.98 (0.93, 1.02) | 0.96 (0.91, 1.01) | |
OR, odds ratio.
Model 1 is unadjusted (the exposures are standardised for age and gender and 25OHD3 is adjusted for season and ethnicity).
Model 2 is adjusted for ethnicity, head of household social class, mothers and partners education, time spent outdoors during summer (age 8.5 years), UVB protection score, WISC IQ score at 8.5 years, BMI, family history of psychiatric problems and puberty stage.
Model 3 is adjusted for Model 2 plus serum concentrations of other hormones/metabolites which are related vitamin D homeostasis [e.g. association of 25(OH)D3 is adjusted for 25(OH)D2, phosphate, albumin-adjusted calcium and parathyroid hormone].
The associations of 25(OH)D3 concentrations which were not adjusted for seasonality (Table S4) and of total 25(OH)D (Table S5) were identical to those of seasonal adjusted 25(OH)D3. Consistent with these findings depressive symptoms at age 10.6 years did not differ between those with total 25(OH)D deficiency or total 25(OH)D insufficiency, whereas those with either deficiency or insufficiency had increased risk of depressive symptoms of 20–30% at age 13.8 (Table 2).
Table 2.
Prospective association of total 25(OH)D deficiency and insufficiency with depressive symptoms assessed by short Mood and Feelings Questionnaire at age 10.6 years (n = 2,759; vitamin D status assessed at mean age 9.2 years) and age 13.8 years (n = 2,752; vitamin D status assessed at mean age 9.8 years)
| OR for category decrease per doubling of exposure (95% CI) | ||||
|---|---|---|---|---|
| Outcome | Exposure | Model 1a | Model 2b | Model 3c |
| Depressive symptoms at age 10.6 years | Vitamin D deficiency | 0.97 (0.87, 1.08) | 0.97 (0.88, 1.09) | 0.98 (0.87, 1.09) |
| Vitamin D insufficiency | 1.00 (0.90, 1.11) | 1.02 (0.91, 1.16) | 1.01 (0.90, 1.13) | |
| Depressive symptoms at age 13.8 years | Vitamin D deficiency | 1.20 (1.08, 1.33) | 1.19 (1.07, 1.33) | 1.21 (1.08, 1.36) |
| Vitamin D insufficiency | 1.26 (1.12, 1.42) | 1.26 (1.13, 1.41) | 1.27 (1.12, 1.42) | |
OR, odds ratio.
Model 1 is unadjusted.
Model 2 is adjusted for ethnicity, age, gender, head of household social class, mothers and partners education, time spent outdoors during summer (age 8.5 years), UVB protection score, WISC IQ score at 8.5 years, BMI, maternal history of psychiatric problems and puberty stage.
Model 3 is adjusted for Model 2 plus serum concentrations of phosphate, albumin-adjusted calcium and parathyroid hormone.
Table 3 shows change in depressive symptoms between age 10.6 and 13.8 years. We present these for all participants with valid responses to the MFQ at both ages (N = 4,487) and also those with complete data on MFQ at both ages and all covariables used in any multivariable models (N = 1,815). The distributions of the change variables are very similar in these two groups. The most common occurrence was for participants to remain stable over time or for depressive symptoms to have increased by one category. The least common was for a decrease in depressive symptoms by two categories.
Table 3.
Changes in depressive symptom categories assessed by change in Mood and Feelings Questionnaire (MFQ) score category between age 10.6 and 13.8 years (in all participants with MFQ data at both ages (N = 4,487) and those with complete data on MFQ at both ages and on all other variables included in any multivariable analyses (N = 1,815)
| Category | All participants with valid MFQ data at both ages, n (%) | Participants with complete data on MFQ at both ages and all other covariables, n (%) |
|---|---|---|
| Increase by two categories | 582 (13.0) | 247 (13.6) |
| Increase by one category | 1,285 (28.7) | 534 (29.4) |
| No change | 1,285 (28.7) | 520 (28.7) |
| Decrease by one category | 1,037 (23.1) | 421 (23.2) |
| Decrease by two categories | 298 (6.6) | 93 (5.2) |
Table 4 shows the associations of serum concentrations of 25(OH)D3, 25(OH)D2, calcium, phosphate and PTH with changes in depressive symptom category. A doubling in 25(OH)D3 concentrations was associated with a 9% increased odds of depressive symptoms decreasing between age 10.6 and 13.8 years, with little effect of adjustment for potential confounding factors. 25(OH)D2, calcium, phosphate or PTH were not associated with change in depressive symptoms over time and there was statistical evidence of a different association of 25(OH)D3 and 25(OH)D2 with change in depressive symptoms (p = .012).
Table 4.
Prospective association of 25(OH)D3, 25(OH)D2, phosphate, albumin-adjusted calcium and parathyroid hormone concentrations (assessed at mean age 9.2 years) with decrease in depressive symptoms [N = 1,815; five categories: increase by two categories (reference); increase by one category; no change, decrease by one category: decrease by two categories]
| OR for category decrease per doubling of exposure (95% CI) | |||
|---|---|---|---|
| Exposure | Model 1a | Model 2b | Model 3c |
| 25(OH)D3 | 1.09 (1.02, 1.16) | 1.08 (1.01, 1.16) | 1.08 (1.01, 1.16) |
| 25(OH)D2 | 0.97 (0.88, 1.07) | 0.96 (0.86, 1.06) | 0.95 (0.90, 1.01) |
| Albumin-adjusted calcium | 1.03 (0.97, 1.09) | 1.01 (0.96, 1.08) | 1.01 (0.96, 1.08) |
| Phosphate | 0.99 (0.94, 1.05) | 0.99 (0.94, 1.05) | 0.99 (0.93, 1.05) |
| Parathyroid hormone | 0.97 (0.92, 1.03) | 0.98 (0.93, 1.04) | 0.99 (0.93, 1.05) |
OR, odds ratio.
Model 1 is unadjusted (the exposures are standardised for age and gender and 25OHD3 is adjusted for season and ethnicity).
Model 2 is adjusted for ethnicity, head of household social class, mothers and partners education, time spent outdoors during summer (age 8.5 years), UVB protection score, WISC IQ score at 8.5 years, BMI, maternal history of psychiatric problems and puberty stage.
Model 3 is adjusted for Model 2 plus serum concentrations of other hormones/metabolites which are related vitamin D homeostasis (e.g. association of 25(OH)D3 is adjusted for 25(OH)D2, phosphate, albumin-adjusted calcium and parathyroid hormone.
Discussion
In our prospective study of children, we have found higher concentrations of season-adjusted 25(OH)D3 assessed at mean age 9.8 years to be associated with lower levels of depressive symptoms at age 13.8 years and with increased odds of decreasing symptoms between age 10.6 and 13.8 years. These associations were independent of a wide range of potential confounders, as well as of 25(OH)D2, calcium, phosphate and PTH concentrations, which were not strongly associated with depressive symptoms at either age. We also found statistical evidence that the association of 25(OH)D3 with depressive symptoms was stronger than that of 25(OH)D2. The association of 25(OH)D3 with depressive symptoms was linear across the distribution of 25(OH)D3 concentrations, suggesting that even amongst those with what would be considered normal concentrations, an increase might result in somewhat lower depressive symptoms (if our findings are in future studies shown to represent causal effects). Consistent with these findings, and reflecting the fact that 25(OH)D3 is the biggest contributor to total 25(OH)D, we found that risk of depressive symptoms was greater at 13.8 years in those with total 25(OH)D deficiency or total 25(OH)D insufficiency.
To our knowledge, this is the first prospective study to examine this association in children. Our findings in this cohort of children are consistent with findings from the two prospective studies in adults (May et al., 2010; Milaneschi et al., 2010) and from one randomised controlled trial that examined the effect of vitamin D3 supplementation on depressive symptoms in adults (Jorde et al., 2008).
The association of 25(OH)D3 with depressive symptoms in children only emerged with symptoms measured 3 years after exposure assessment, and was not present when symptoms were assessed just 1 year after exposure assessment. One might expect a stronger association with the earlier age, possibly in part because of reverse causality [i.e. depressive symptoms resulting in less outdoor activity and hence reduced vitamin 25(OH)D3 concentrations]. It is possible that children with higher concentrations of 25(OH)D3 at age 9.8 years have on average higher concentrations over the subsequent 3 years and that the inverse association with depressive symptoms requires an accumulation of consistent concentrations. Alternatively, factors other than 25(OH)D3 but that are associated with it and accumulate over time (e.g. outdoor physical activity) might explain the association. The risk factors for childhood–onset depression may also be different from those of adolescence–onset depression (Jaffee et al., 2002). Lastly, it is possible that the biological pathways linking 25(OH)D3 to depression involve a chain of effects that take some time to emerge.
The stronger association of 25(OH)D3 compared to 25(OH)D2 could be a chance finding. The difference could also reflect possible greater residual confounding by, for example, different dietary patterns associated with 25(OH)D2 and 25(OH)D3 or outdoor physical activity, which will affect D3 more than D2; whilst we have attempted to adjust for a wide range of potential confounding factors residual confounding is possible. Lastly, the different associations could be explained by D3 being truly more potent at preventing depressive symptoms than D2. This finding requires further replication in other studies before we can conclude that D3 is more strongly associated with depressive symptoms than is D2.
Vitamin D receptors are expressed throughout the brain and both 25(OH)D3 and 25(OH)D2 cross the blood–brain barrier (Eyles, Smith, Kinobe, Hewison, & McGrath, 2005). Animal studies have shown that vitamin D is essential for normal neurogenesis (Cui, McGrath, Burne, Mackay-Sim, & Eyles, 2007), learning ability and behaviour in rodents (Becker, Eyles, McGrath, & Grecksch, 2005; Eyles et al., 2006; Harms, Eyles, McGrath, Mackay-Sim, & Burne, 2008), but currently it is unknown if the neural actions of vitamin D metabolites affect monoamine concentrations, hypothalamic–pituitary–adrenal axis responsiveness to stress or other mechanisms involved in depression (Belmaker & Agam, 2008).
Contrary to previous studies showing an association between higher PTH concentrations and depression in adults (Hoogendijk et al., 2008; May et al., 2010), serum concentrations of PTH were not strongly associated with depressive symptoms in children in our study. The differences between our results and these previous studies in adults could be explained by differences in outcome measurement and study sample or could reflect real differences in these associations by age.
Study strengths and limitations
To our knowledge, this is the first study to examine these associations in children and is one of the few studies to examine them prospectively. We had a large sample size and were able to examine potential confounding by a wide range of characteristics and study the different effects of 25(OH)D3 and 25(OH)D2. We also used self-reported, rather than parent-reported depressive symptoms. This is important because parent-reported scores do not reveal depressive symptoms as early as self-reported measures (Cole et al., 2002). Consistent with other prospective cohort studies there has been substantial attrition over time with those who continued to attend the follow-up clinics being more likely to be from higher socioeconomic backgrounds (Golding, Pembrey, Jones and ALSPAC Study Team, 2001). Twenty-seven per cent of our study population had total 25(OH)D concentration below 20 ng/ml so the results are likely to apply to other populations with high prevalence of low 25(OH)D concentrations (Lips, 2010; Mansbach, Ginde, & Camargo, 2009).
Depressive symptoms were analysed as a categorical variable instead of a continuous score. This might have lost some refinement, but this was necessary due to highly skewed distribution. Serum 25(OH)D3, D2, phosphate, calcium and PTH were measured on a single occasion and this may not accurately reflect usual status (Schram et al., 2007). However, previous epidemiological studies in adults (including with bone phenotypes for which these exposures have established biological relationships) also use single measurements and for season-specific vitamin D status over a longer time, a single measure is likely to be adequate (Hofmann, Yu, Horst, Hayes, & Purdue, 2010) and serum calcium concentrations are normally maintained within relatively narrow limits in humans (Parfitt, 1987). As noted above, our results do not imply causality and the association of 25(OH)D3 with depressive symptoms 3 years later might be explained by residual confounding.
Conclusions
Our results suggest that the association between 25(OH)D3 concentrations and depression emerges in childhood. The association is independent of a wide range of potential confounding factors, and appears to be stronger with greater time separation between assessment of 25(OH)D3 and that of depressive symptoms. Given the importance of depression in childhood and adolescence and the relative ease with which 25(OH)D3 could be increased through supplementation, randomised controlled trials to assess the effectiveness of this for prevention of depressive symptoms in this age group would be appropriate.
Key points
Depression in adolescence is common and early onset predicts worse outcome in adulthood.
Previous, mainly cross-sectional studies in adults have suggested a link between higher total 25-hydroxyvitamin D [25(OH)D] concentrations and lower risk of depression.
Findings from this first prospective study in children suggest that the linear association between 25(OH)D concentrations and depression emerges in childhood/early adolescence and is driven by the 25(OH)D3 form.
25(OH)D2 concentrations were not associated with depressive symptoms.
Acknowledgments
Work on this study is funded by an UK Medical Research Council (MRC; Grant G0701603), which also pays AMT’s salary. Salary support for A.S. is provided by Wellcome Trust grant ref 079960. MRC, the Wellcome Trust and the University of Bristol provide core funding support for ALSPAC. The MRC and the University of Bristol provide core funding for the MRC Centre of Causal Analyses in Translational Epidemiology (Grant G0600705). We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The views expressed in this article are those of the authors and not necessarily those of any funding body or others whose support is acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1 Sample characteristics (only complete cases for depressive symptoms at age 13.8 years are included)
Table S2 Univariable associations between potential confounders and age- and gender-standardised serum 25-hydroxyvitamin D3, D2, phosphate, calcium and PTH concentrations
Table S3 Univariable associations between potential confounders, exposures and depressive symptoms
Table S4 Prospective association of unadjusted 25(OH)D3 with depressive symptoms assessed by short Moodand Feelings Questionnaire at age 10.6 years (n = 2,759; exposures assessed at mean age 9.2 years) and age 13.8 years (n = 2,752; exposures assessed at mean age 9.8 years)
Table S5 Prospective association of total 25(OH)D [25(OH)D3 + 25(OH)D2] with depressive symptoms assessed by short Mood and Feelings Questionnaire at age 10.6 years (n = 2,759; exposures assessed at mean age 9.2 years) and age 13.8 years (n = 2,752; exposures assessed at mean age 9.8 years)
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Annweiler C, Schott AM, Allali G, Bridenbaugh SA, Kressig RW, Allain P, Herrmann FR, Beauchet O. Association of vitamin D deficiency with cognitive impairment in older women: Cross-sectional study. Neurology. 2010;74:27–32. doi: 10.1212/WNL.0b013e3181beecd3. [DOI] [PubMed] [Google Scholar]
- Armstrong DJ, Meenagh GK, Bickle I, Lee AS, Curran ES, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clinical Rheumatology. 2007;26:551–554. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]
- Becker A, Eyles DW, McGrath JJ, Grecksch G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behavioural Brain Research. 2005;161:306–312. doi: 10.1016/j.bbr.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. New England Journal of Medicine. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Berk M, Sanders KM, Pasco JA, Jacka FN, Williams LJ, Hayles AL, Dodd S. Vitamin D deficiency may play a role in depression. Medical Hypotheses. 2007;69:1316–1319. doi: 10.1016/j.mehy.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson ER. Vitamin D and the occurrence of depression: Causal association or circumstantial evidence? Nutrition Reviews. 2009;67:481–492. doi: 10.1111/j.1753-4887.2009.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Dusso A, Slatopolsky E. Vitamin D. American Journal of Physiology. 1999;277:F157–F175. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- Cole DA, Tram JM, Martin JM, Hoffman KB, Ruiz MD, Jacquez FM, Maschman TL. Individual differences in the emergence of depressive symptoms in children and adolescents: A longitudinal investigation of parent and child reports. Journal of Abnormal Psychology. 2002;111:156–165. [PubMed] [Google Scholar]
- Costello EJ, Angold A. Scales to assess child and adolescent depression: Checklists, screens, and nets. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27:726–737. doi: 10.1097/00004583-198811000-00011. [DOI] [PubMed] [Google Scholar]
- Cui X, McGrath JJ, Burne TH, Mackay-Sim A, Eyles DW. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. International Journal of Developmental Neuroscience. 2007;25:227–232. doi: 10.1016/j.ijdevneu.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dumville JC, Miles JN, Porthouse J, Cockayne S, Saxon L, King C. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. Journal of Nutrition, Health and Aging. 2006;10:151–153. [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science. 1986;1:54–77. [Google Scholar]
- Eskandari F, Martinez PE, Torvik S, Phillips TM, Sternberg EM, Mistry S, Ronsaville D, Wesley R, Toomey C, Sebring NG, Reynolds JC, Blackman MR, Calis KA, Gold PW, Cizza G, Premenopausal, Osteoporosis Women, Alendronate, Depression (POWER) Study Group Low bone mass in premenopausal women with depression. Archives of Internal Medicine. 2007;167:2329–2336. doi: 10.1001/archinte.167.21.2329. [DOI] [PubMed] [Google Scholar]
- Eyles DW, Rogers F, Buller K, McGrath JJ, Ko P, French K, Burne TH. Developmental vitamin D (DVD) deficiency in the rat alters adult behaviour independently of HPA function. Psychoneuroendocrinology. 2006;31:958–964. doi: 10.1016/j.psyneuen.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. Journal of Chemical Neuroanatomy. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Finch JL, Brown AJ, Slatopolsky E. Differential effects of 1,25-dihydroxy-vitamin D3 and 19-nor-1,25-dihydroxy-vitamin D2 on calcium and phosphorus resorption in bone. Journal of the American Society of Nephrology. 1999;10:980–985. doi: 10.1681/ASN.V105980. [DOI] [PubMed] [Google Scholar]
- Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: The Third National Health and Nutrition Examination Survey. International Archives of Medicine. 2010;3:29. doi: 10.1186/1755-7682-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendenning P, Chew GT, Seymour HM, Gillett MJ, Goldswain PR, Inderjeeth CA, Vasikaran SD, Taranto M, Musk AA, Fraser WD. Serum 25-hydroxyvitamin D levels in vitamin D-insufficient hip fracture patients after supplementation with ergocalciferol and cholecalciferol. Bone. 2009;45:870–875. doi: 10.1016/j.bone.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Golding J, Pembrey M, Jones R, ALSPAC Study Team ALSPAC – The Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatric and Perinatal Epidemiology. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behavioural Brain Research. 2008;187:343–350. doi: 10.1016/j.bbr.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Herran A, Amado JA, Garcia-Unzueta MT, Vazquez-Barquero JL, Perera L, Gonzalez-Macias J. Increased bone remodeling in first-episode major depressive disorder. Psychosomatic Medicine. 2000;62:779–782. doi: 10.1097/00006842-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiology, Biomarkers and Prevention. 2010;19:927–931. doi: 10.1158/1055-9965.EPI-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. The New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Archives of General Psychiatry. 2008;65:508–512. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. American Journal of Clinical Nutrition. 2006;84:694–697. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin J. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Archives of General Psychiatry. 2002;59:215–222. doi: 10.1001/archpsyc.59.3.215. [DOI] [PubMed] [Google Scholar]
- Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. Journal of Internal Medicine. 2008;264:599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. The Tromso study. Journal of Neurology. 2006;253:464–470. doi: 10.1007/s00415-005-0027-5. [DOI] [PubMed] [Google Scholar]
- Lee DM, Tajar A, O’Neill TW, O’Connor DB, Bartfai G, Boonen S, Bouillon R, Casanueva FF, Finn JD, Forti G, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Lean ME, Punab M, Silman AJ, Vanderschueren D, Wu FC, Pendleton N. Lower vitamin D levels are associated with depression among community-dwelling European men. Journal of Psychopharmacology. 2010;10:1320–1328. doi: 10.1177/0269881110379287. [DOI] [PubMed] [Google Scholar]
- Lips P. Worldwide status of vitamin D nutrition. Journal of Steroid Biochemistry and Molecular Biology. 2010;121:297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: Do children need more vitamin D? Pediatrics. 2009;124:1404–1410. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May HT, Bair TL, Lappe DL, Anderson JL, Horne BD, Carlquist JF, Muhlestein JB. Association of vitamin D levels with incident depression among a general cardiovascular population. American Heart Journal. 2010;159:1037–1043. doi: 10.1016/j.ahj.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P. Bone mineral density in women with depression. New England Journal of Medicine. 1996;335:1176–1181. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Shardell M, Corsi AM, Vazzana R, Bandinelli S, Guralnik JM, Ferrucci L. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. Journal of Clinical Endocrinology and Metabolism. 2010;95:3225–3233. doi: 10.1210/jc.2010-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR, Guise TA. Hormonal control of calcium homeostasis. Clinical Chemistry. 1999;45:1347–1352. [PubMed] [Google Scholar]
- Nanri A, Mizoue T, Matsushita Y, Poudel-Tandukar K, Sato M, Ohta M, Mishima N. Association between serum 25-hydroxyvitamin D and depressive symptoms in Japanese: Analysis by survey season. European Journal of Clinical Nutrition. 2009;63:1444–1447. doi: 10.1038/ejcn.2009.96. [DOI] [PubMed] [Google Scholar]
- Pan A, Lu L, Franco OH, Yu Z, Li H, Lin X. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. Journal of Affective Disorders. 2009;118:240–243. doi: 10.1016/j.jad.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Bone and plasma calcium homeostasis. Bone. 1987;8(Suppl. 1):S1–S8. [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Jacka FN, Dodd S, Nicholson G, Berk M. Annual high-dose vitamin D3 and mental well-being: Randomised controlled trial. British Journal of Psychiatry. 2011;198:357–364. doi: 10.1192/bjp.bp.110.087544. [DOI] [PubMed] [Google Scholar]
- Schneider B, Weber B, Frensch A, Stein J, Fritz J. Vitamin D in schizophrenia, major depression and alcoholism. Journal of Neural Transmission. 2000;107:839–842. doi: 10.1007/s007020070063. [DOI] [PubMed] [Google Scholar]
- Schram MT, Trompet S, Kamper AM, de Craen AJ, Hofman A, Euser SM, Breteler MM, Westendorp RG. Serum calcium and cognitive function in old age. Journal of the American Geriatrics Society. 2007;55:1786–1792. doi: 10.1111/j.1532-5415.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: A systematic review. American Journal of Clinical Nutrition. 2009;89:1997S–2008S. doi: 10.3945/ajcn.2009.27230D. [DOI] [PubMed] [Google Scholar]
- Tanner J. Oxford, UK: Blackwell Scientific Publications; 1962. Growth at adolescence. [Google Scholar]
- Thapar A, Collishaw S, Potter R, Thapar AK. Managing and preventing depression in adolescents. British Medical Journal. 2010;340:c209. doi: 10.1136/bmj.c209. [DOI] [PubMed] [Google Scholar]
- Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. The American Journal of Geriatric Psychiatry. 2006;14:1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- Zhao G, Ford ES, Li C, Balluz LS. No associations between serum concentrations of 25-hydroxyvitamin D and parathyroid hormone and depression among US adults. British Journal of Nutrition. 2010;104:1696–1702. doi: 10.1017/S0007114510002588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


