Abstract
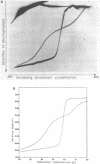
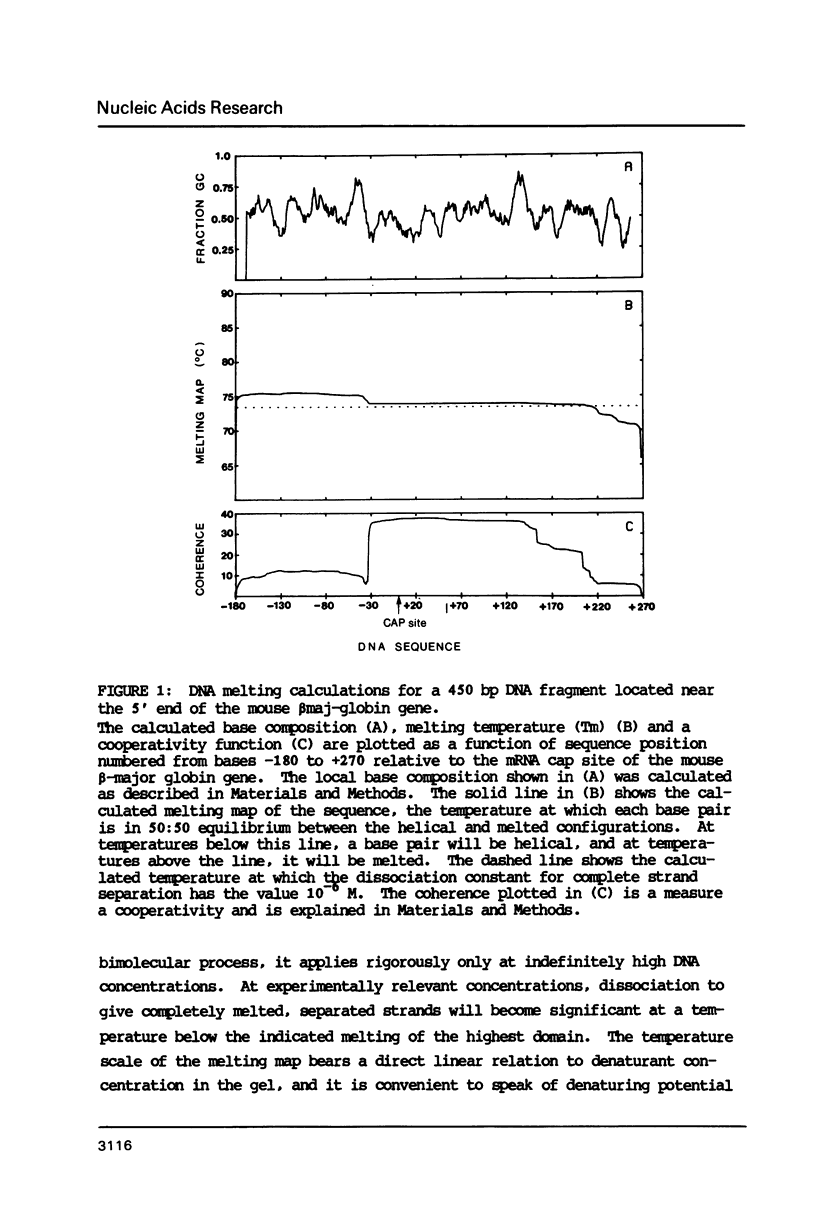
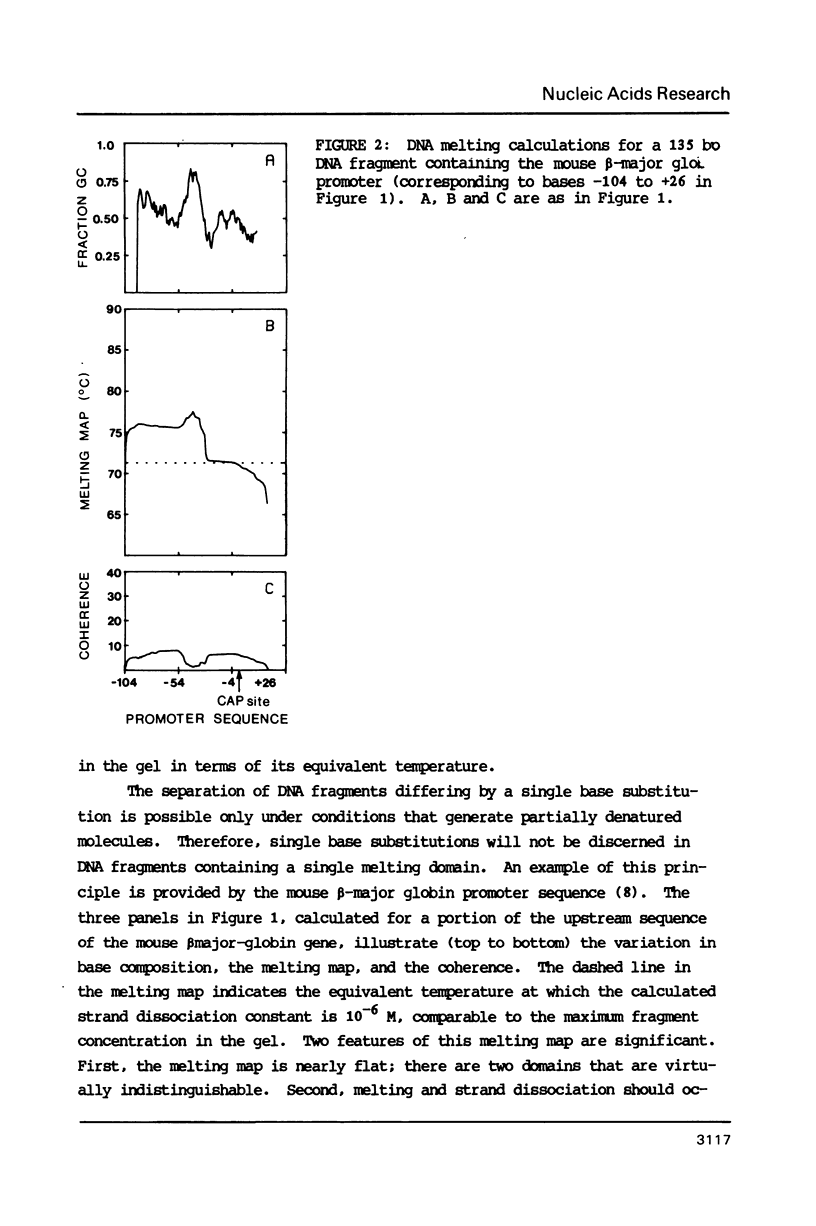
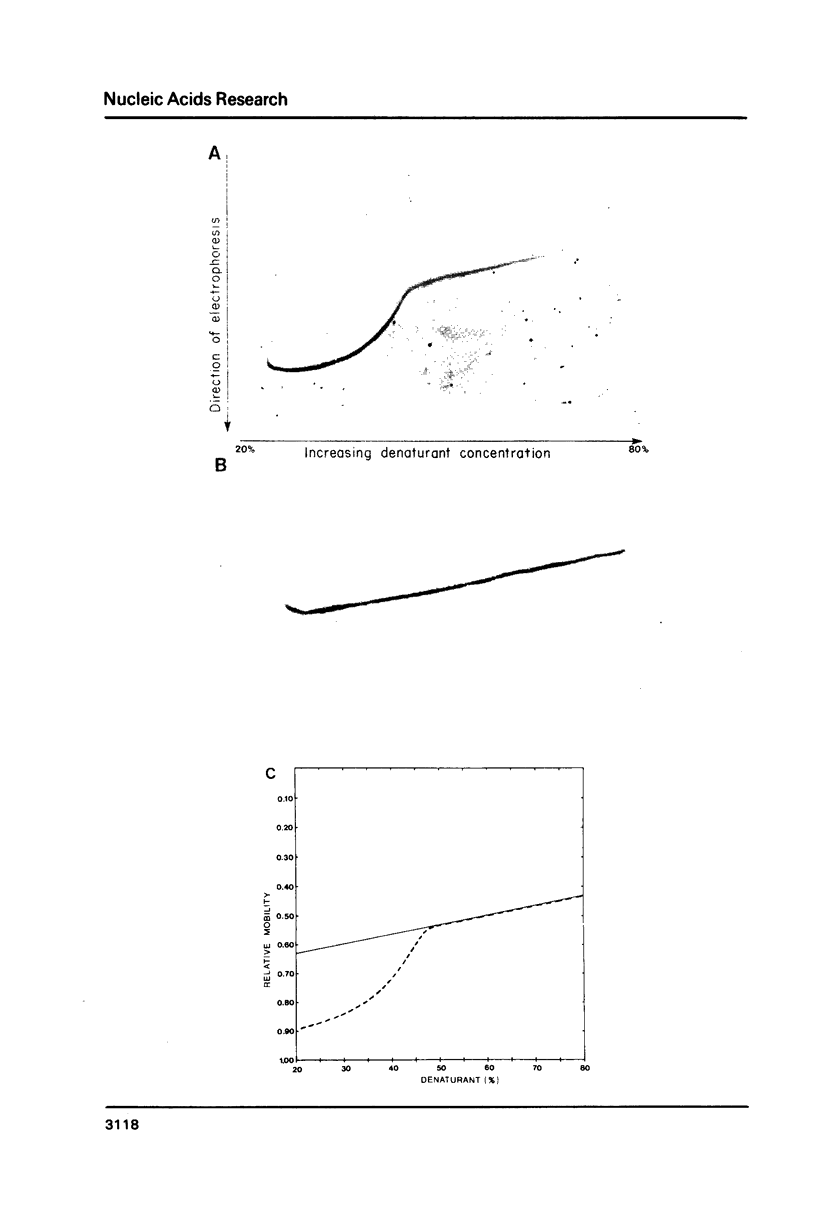
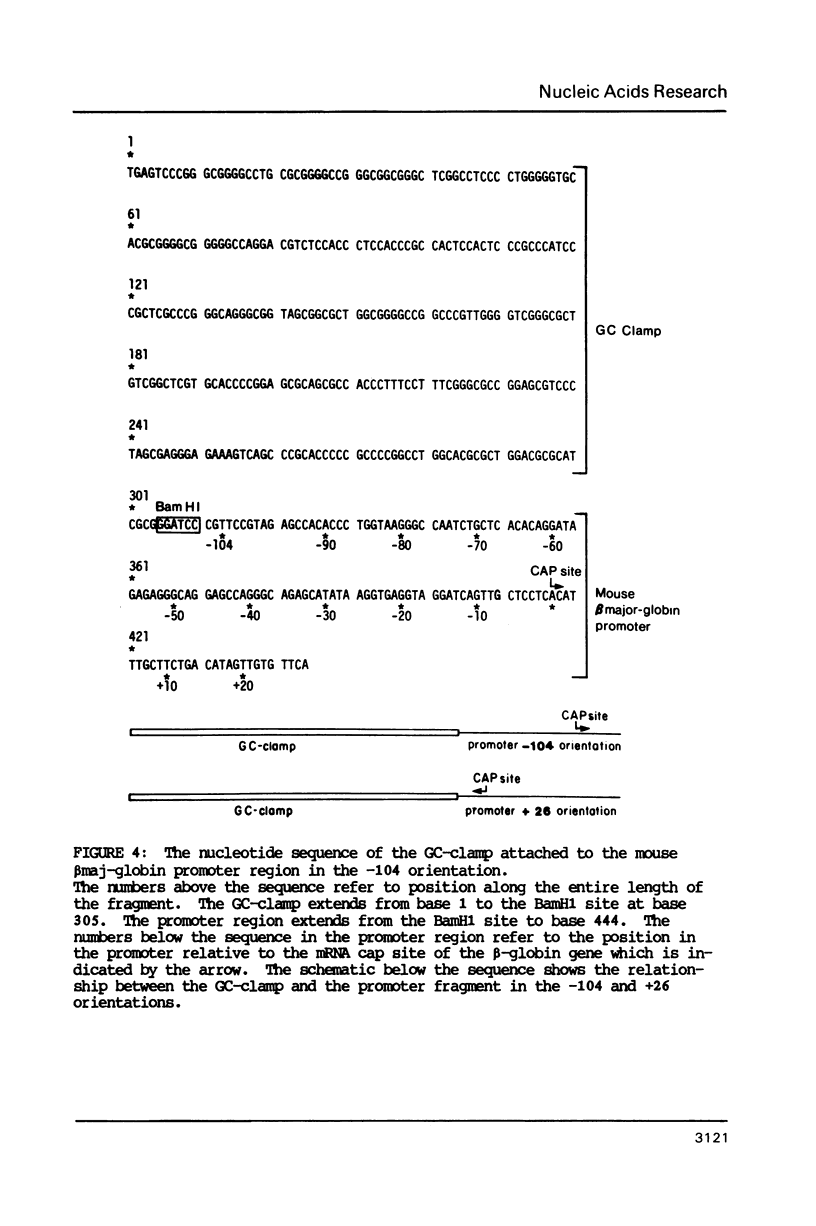
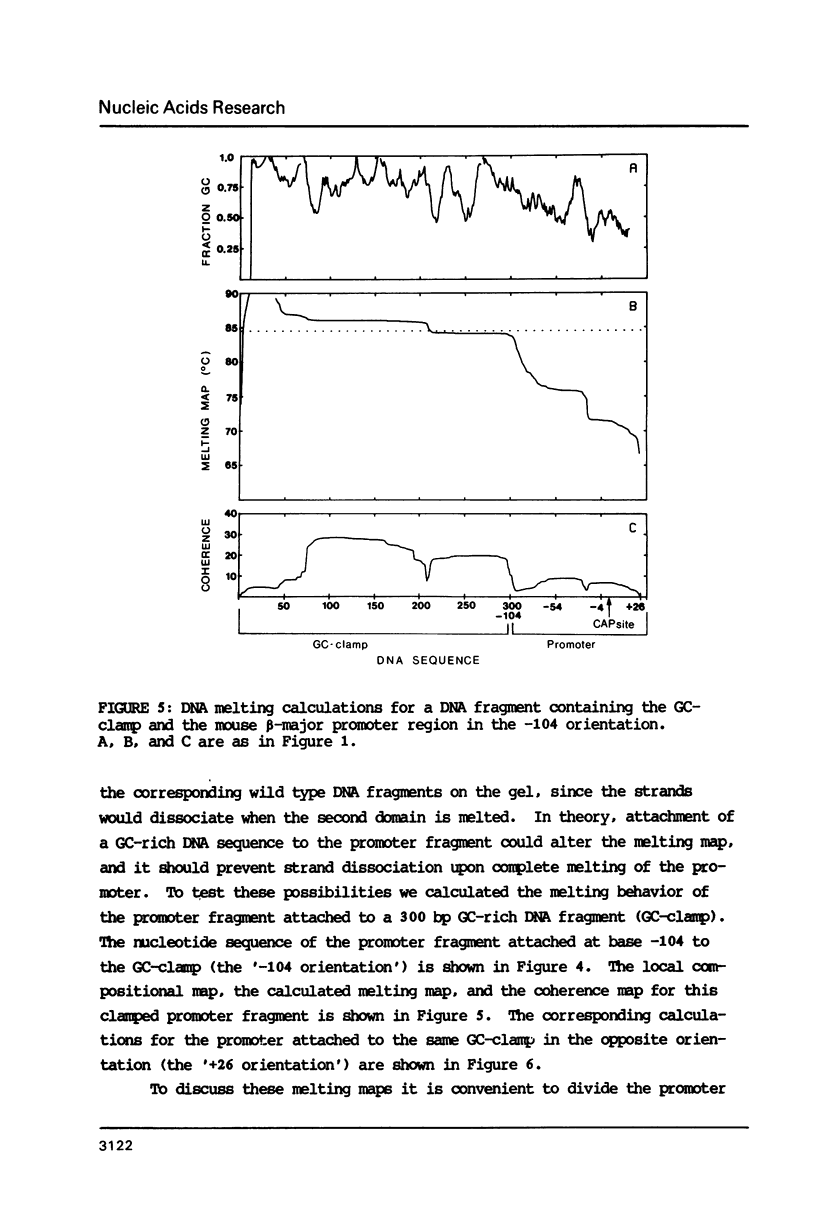
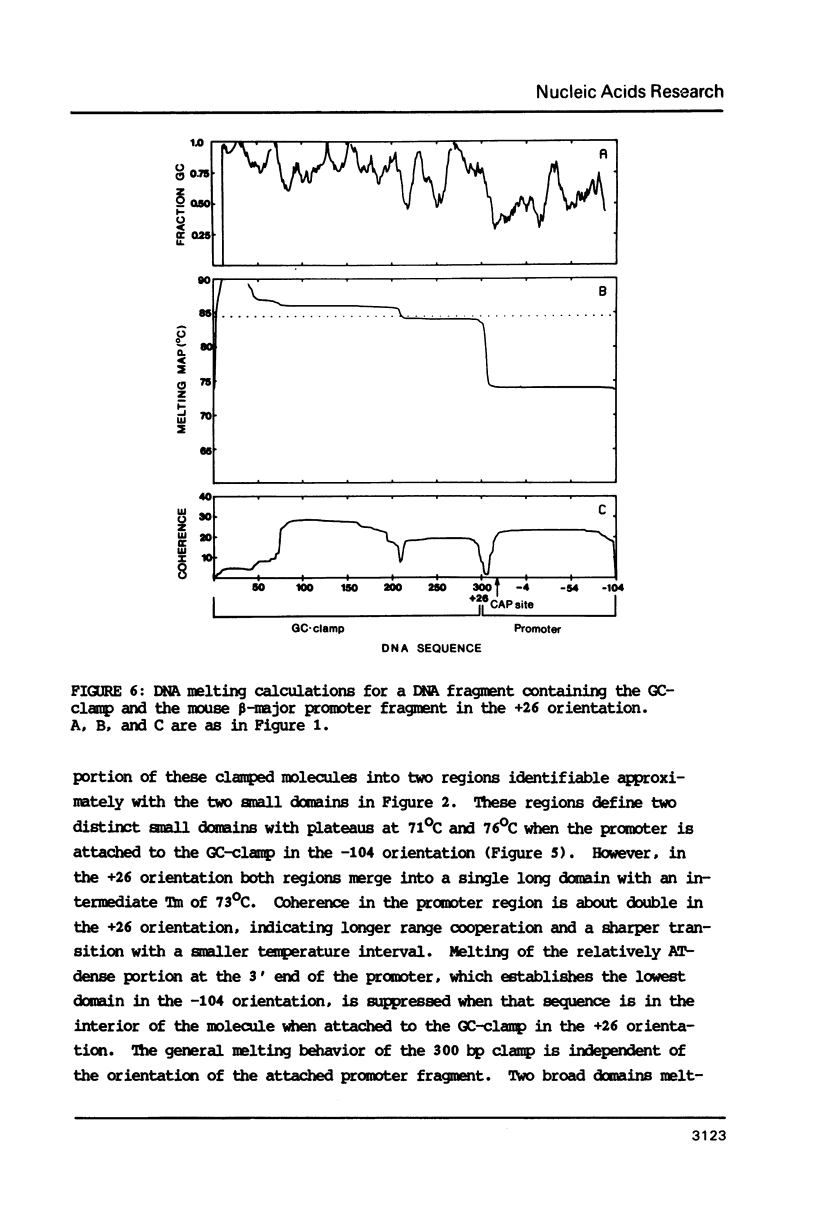
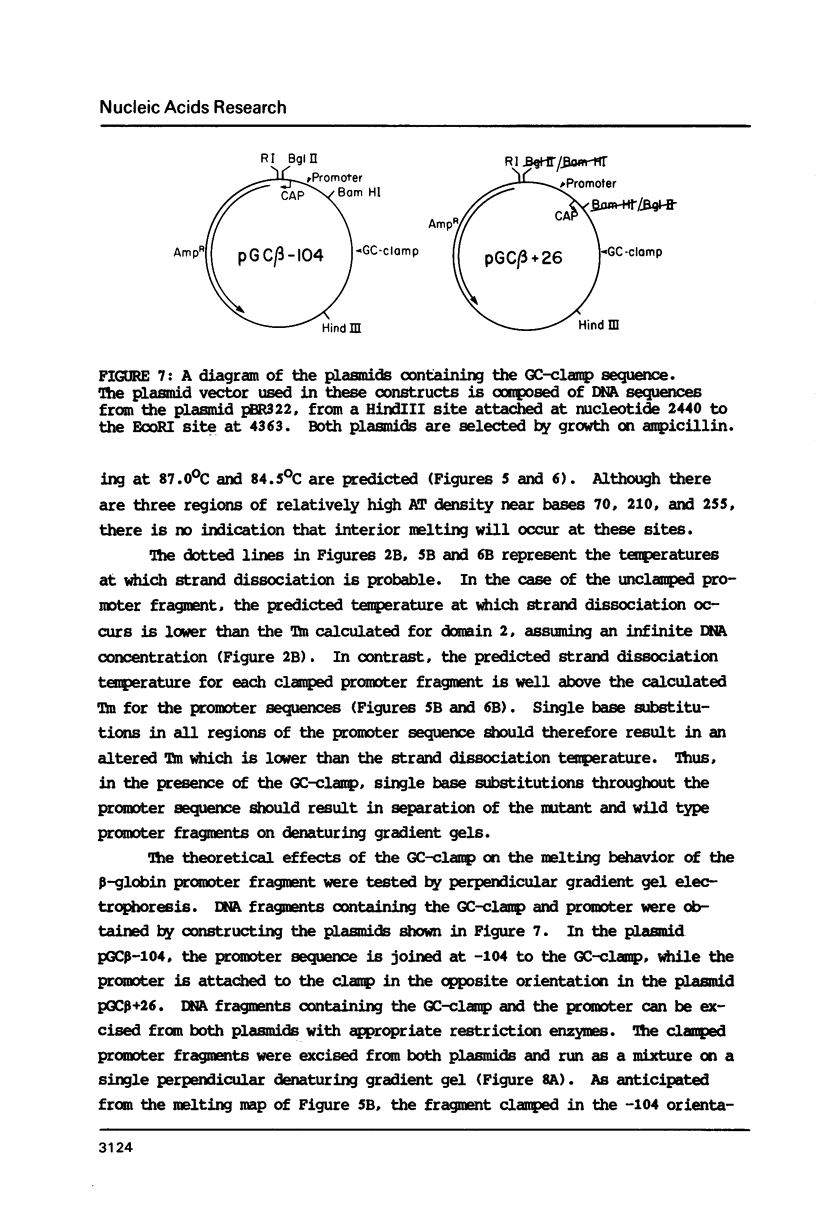
The melting behavior of a DNA fragment carrying the mouse beta maj-globin promoter was investigated as a means of establishing procedures for separating DNA fragments differing by any single base substitution using the denaturing gradient gel electrophoresis procedure of Fischer and Lerman (1,2). We find that attachment of a 300 base pair GC-rich DNA sequence, termed a GC-clamp, to a 135 bp DNA fragment carrying the mouse beta-globin promoter significantly alters the pattern of DNA melting within the promoter. When the promoter is attached to the clamp, the promoter sequences melt without undergoing strand dissociation. The calculated distribution of melting domains within the promoter differs markedly according to the relative orientation of the clamp and promoter sequences. We find that the behavior of DNA fragments containing the promoter and clamp sequences on denaturing gradient polyacrylamide gels is in close agreement with the theoretical melting calculations. These studies provide the basis for critical evaluation of the parameters for DNA melting calculations, and they establish conditions for determining whether all single base substitutions within the promoter can be separated on denaturing gradient gels.
Full text
PDF






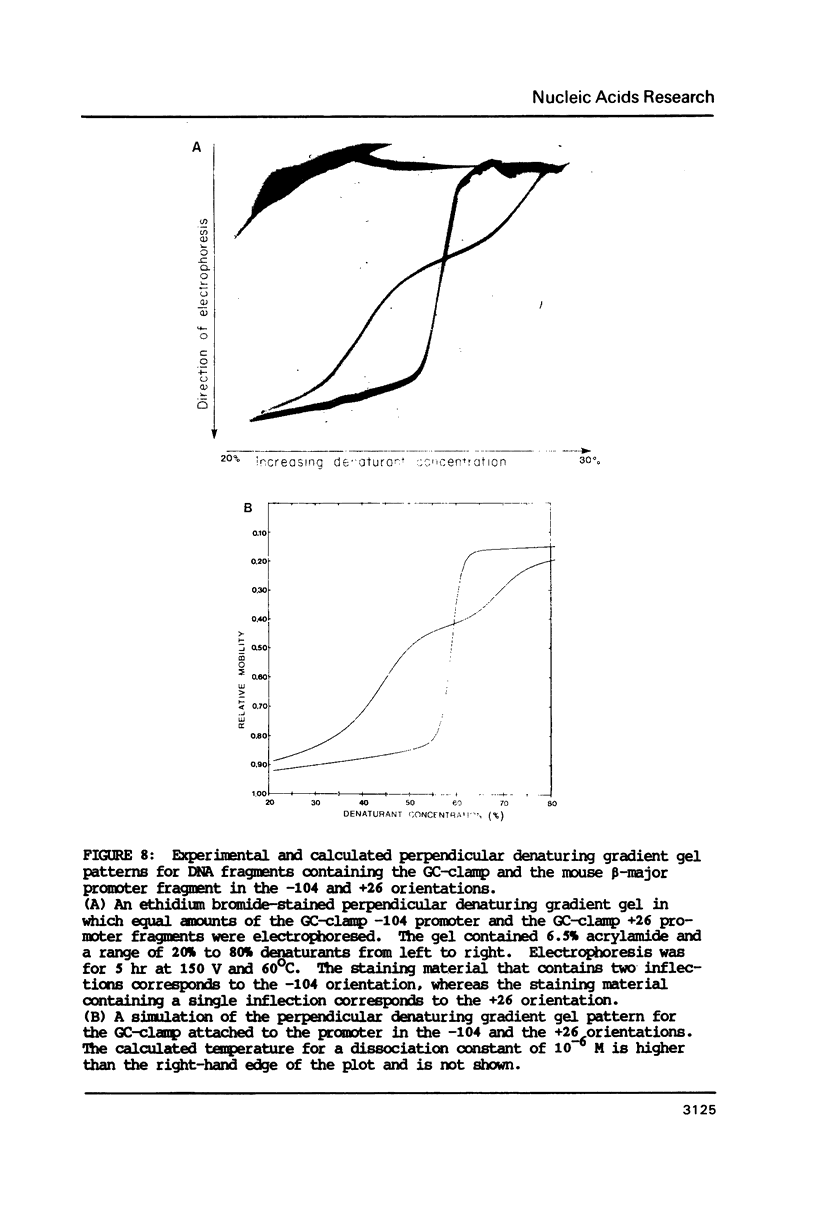

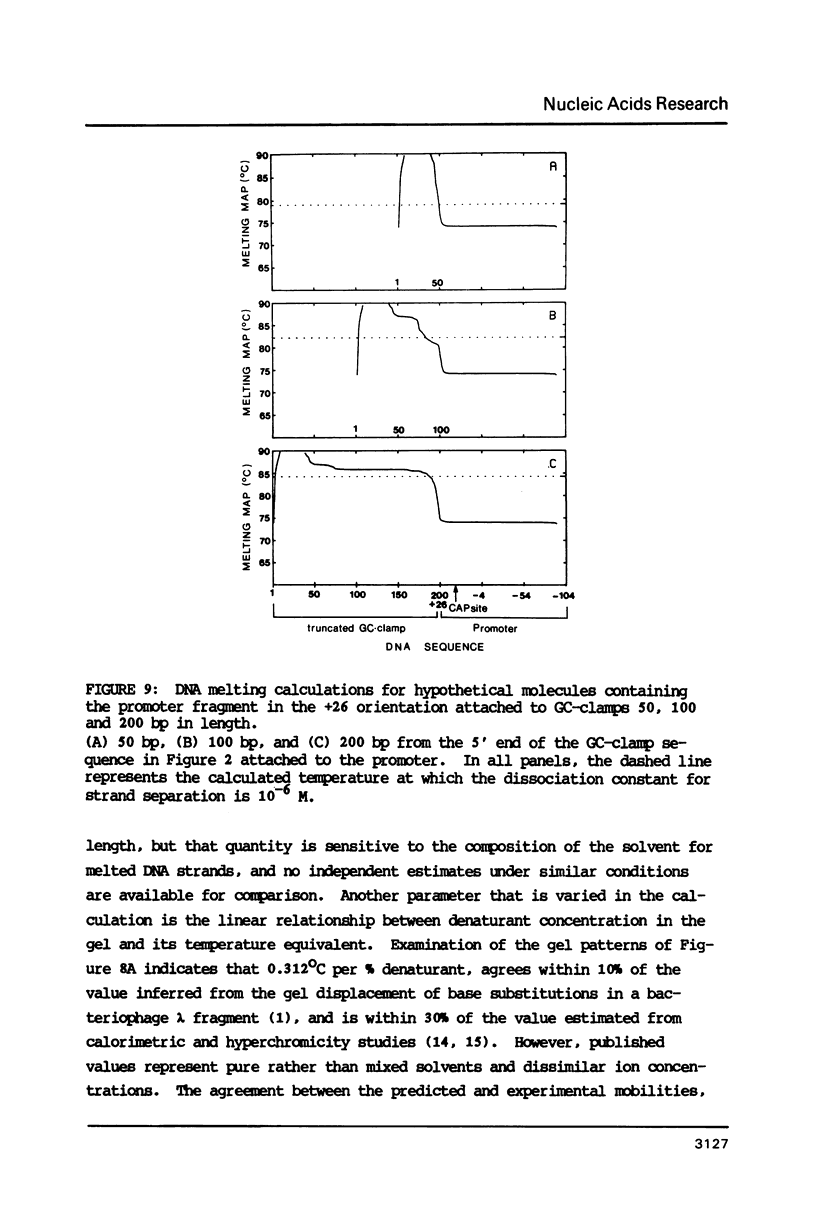


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. Length-independent separation of DNA restriction fragments in two-dimensional gel electrophoresis. Cell. 1979 Jan;16(1):191–200. doi: 10.1016/0092-8674(79)90200-9. [DOI] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. Two-dimensional electrophoretic separation of restriction enzyme fragments of DNA. Methods Enzymol. 1979;68:183–191. doi: 10.1016/0076-6879(79)68013-8. [DOI] [PubMed] [Google Scholar]
- Fixman M., Freire J. J. Theory of DNA melting curves. Biopolymers. 1977 Dec;16(12):2693–2704. doi: 10.1002/bip.1977.360161209. [DOI] [PubMed] [Google Scholar]
- Gotoh O. Prediction of melting profiles and local helix stability for sequenced DNA. Adv Biophys. 1983;16:1–52. doi: 10.1016/0065-227x(83)90007-2. [DOI] [PubMed] [Google Scholar]
- Hutton J. R. Renaturation kinetics and thermal stability of DNA in aqueous solutions of formamide and urea. Nucleic Acids Res. 1977 Oct;4(10):3537–3555. doi: 10.1093/nar/4.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump H., Burkart W. Calorimetric measurements of the transition enthalpy of DNA in aqueous urea solutions. Biochim Biophys Acta. 1977 Apr 19;475(4):601–604. doi: 10.1016/0005-2787(77)90320-3. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Tilghman S. M., Leder P. The sequence of the chromosomal mouse beta-globin major gene: homologies in capping, splicing and poly(A) sites. Cell. 1978 Dec;15(4):1125–1132. doi: 10.1016/0092-8674(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Fischer S. G., Hurley I., Silverstein K., Lumelsky N. Sequence-determined DNA separations. Annu Rev Biophys Bioeng. 1984;13:399–423. doi: 10.1146/annurev.bb.13.060184.002151. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Goossens M. J., Kan Y. W. Cloning and complete nucleotide sequence of human 5'-alpha-globin gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7054–7058. doi: 10.1073/pnas.77.12.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. The 3' untranslated regions of the duplicated human alpha-globin genes are unexpectedly divergent. Cell. 1980 Nov;22(2 Pt 2):371–377. doi: 10.1016/0092-8674(80)90347-5. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Lerman L. S., Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland D. Recursion relation generation of probability profiles for specific-sequence macromolecules with long-range correlations. Biopolymers. 1974;13(9):1859–1871. doi: 10.1002/bip.1974.360130916. [DOI] [PubMed] [Google Scholar]