Abstract
Background:
Glucagon like peptide-1 (GLP-1) mimetic therapy induces medullary thyroid neoplasia in rodents. We sought to establish whether C cells in human medullary thyroid carcinoma, C cell hyperplasia, and normal human thyroid express the GLP-1 receptor.
Methods:
Thyroid tissue samples with medullary thyroid carcinoma (n = 12), C cell hyperplasia (n = 9), papillary thyroid carcinoma (n = 17), and normal human thyroid (n = 15) were evaluated by immunofluorescence for expression of calcitonin and GLP-1 receptors.
Results:
Coincident immunoreactivity for calcitonin and GLP-1 receptor was consistently observed in both medullary thyroid carcinoma and C cell hyperplasia. GLP-1 receptor immunoreactivity was also detected in 18% of papillary thyroid carcinoma (three of 17 cases). Within normal human thyroid tissue, GLP-1 receptor immunoreactivity was found in five of 15 of the examined cases in about 35% of the total C cells assessed.
Conclusions:
In humans, neoplastic and hyperplastic lesions of thyroid C cells express the GLP-1 receptor. GLP-1 receptor expression is detected in 18% papillary thyroid carcinomas and in C cells in 33% of control thyroid lobes. The consequence of long-term pharmacologically increased GLP-1 signaling on these GLP-1 receptor-expressing cells in the thyroid gland in humans remains unknown, but appropriately powered prospective studies to exclude an increase in medullary or papillary carcinomas of the thyroid are warranted.
Glucagon like peptide-1 (GLP-1) is an incretin hormone released by L cells in the ileum in response to food ingestion (1). Actions of GLP-1 include amplification of glucose-mediated insulin secretion, delayed gastric emptying, and increased satiety, attributes that are beneficial in the treatment of type 2 diabetes mellitus. Circulating GLP-1 has a short life, being degraded by the enzyme dipeptidyl-peptidase-4 (DPP-4). To surmount this, GLP-1-based therapies for type 2 diabetes have been developed that involve either inhibition of the DPP-4 enzyme, which augments circulating GLP-1 levels arising from endogenous secretion, or injection of GLP-1 mimetics that are resistant to DPP-4 degradation. Since 2005, five drugs in this category have been approved by the U.S. Food and Drug Administration. These include the GLP-1 receptor agonists, exenatide (Byetta) and liraglutide (Victoza), and the DPP-4 inhibitors, sitagliptin (Januvia), saxagliptin (Onglyza), and linagliptin (Tradjenta) (2).
In routine preclinical animal testing studies of liraglutide, an increase in the frequency of C cell hyperplasia and thyroid tumors was observed (3). Although GLP-1 receptor stimulation induced calcitonin release and C cell proliferation in rodents, these effects were not observed in nonhuman primates, implying possible species-specific differences in GLP-1 receptor expression and activation in the thyroid (4). C cells are sparsely distributed within the normal human thyroid, being located in the middle and upper third of the lateral lobes. They are often difficult to identify on routine hematoxylin-and-eosin-stained sections (5). In contrast, C cells are much more abundant in the rodent thyroid (3). Because GLP-1 mimetic therapy is now widely used for type 2 diabetes mellitus, it is important to establish whether GLP-1 receptor expression occurs in human thyroid, in particular in the C cells within the thyroid, in health as well as in the in the setting of C cell hyperplasia and medullary thyroid carcinoma.
Available data in this regard are limited. A prior study using GLP-1 receptor scintigraphy reported relatively abundant GLP-1 receptor expression in 28% of medullary thyroid carcinomas examined and in 6% of normal human thyroid glands (6). In another study, C cells identified in 10 thyroid glands from humans were negative by in situ hybridization for GLP-1 receptor mRNA and in situ receptor ligand binding by the GLP-1 receptor antagonist [125I]exendin (9–39) (4). To date, there are no data reporting the presence of GLP-1 receptor expression by immunoreactivity in normal human thyroid gland, medullary thyroid carcinoma, papillary thyroid carcinoma, or C cell hyperplasia. The latter, considered a premalignant condition by some, may be more common than previously appreciated (7).
In the present study, we examined thyroid tissue samples procured at surgery from individuals with C cell hyperplasia and those with medullary thyroid carcinoma. Moreover, C cells within relatively normal tissue without any hyperplastic or neoplastic changes were evaluated for GLP-1 receptor expression. For comparison, we also examined papillary thyroid carcinomas (non-C cell lineage) for the presence of GLP-1 receptor expression.
Materials and Methods
Human subjects
Individuals for inclusion in the present studies were identified from the Department of Pathology and Laboratory Medicine at the University of California, Los Angeles (Los Angeles, CA) database for cases that were submitted for pathological evaluation between 2002 and 2010. Thyroid was evaluated from 53 individuals as follows.
C cell hyperplasia
Nine individuals with a histopathological diagnosis of C cell hyperplasia were identified, four of which were classified as having reactive/physiological C cell hyperplasia and five with C cell hyperplasia due to RET (rearranged during transfection) germline mutations [four multiple endocrine neoplasia (MEN)-2 and one familial medullary thyroid carcinoma]. C cell hyperplasia was defined by the presence of at least 50 C cells per one low-power field (×10 objective) (8).
Medullary thyroid carcinoma
Twelve individuals with medullary thyroid carcinoma were identified. There was no overlap between cases of C cell hyperplasia and medullary thyroid cancer.
Papillary thyroid carcinoma
Seventeen cases of papillary thyroid carcinoma were selected at random from the same database.
Controls (Table 1)
Table 1.
Controls
| Subject no. | Age (yr) | Sex | Pathology | GLP-1 receptor | Calcitonin | Colocalization |
|---|---|---|---|---|---|---|
| No inflammation | ||||||
| 1 | 65 | M | Rare hyperplastic and colloid nodules | – | + | – |
| 2 | 62 | M | Normal thyroid tissue | – | + | – |
| 3 | 26 | F | Normal thyroid tissue | – | + | – |
| 4 | 50 | M | Normal thyroid tissue | – | + | – |
| 5 | 63 | M | Rare hyperplastic and colloid nodules | – | + | – |
| 6 | 66 | F | Normal thyroid tissue | – | + | – |
| 7 | 51 | F | Normal thyroid tissue | – | + | – |
| 8 | 20 | F | Multinodular goiter | ++ | + | + |
| 9 | 27 | M | Normal thyroid tissue | – | + | – |
| 10 | 36 | F | Multinodular goiter | ++ | + | + |
| 11 | 77 | M | Normal thyroid tissue | – | + | – |
| Mild inflammation | ||||||
| 12 | 36 | M | Normal thyroid tissue | ++ | + | + |
| 13 | 61 | F | Normal thyroid tissue | – | + | – |
| 14 | 28 | F | Multinodular goiter | ++ | + | + |
| 15 | 45 | F | Normal thyroid tissue | ++ | + | + |
Table represents a summary of immunohistochemistry and immunofluorescence results. −, Negative; ++, 10–35% positive; + less than 10% positive; M, male; F, female.
Histologically normal thyroid lobes from 15 individuals that had undergone total thyroidectomy for papillary thyroid cancer in the other lobe were used as control thyroid tissue, being selected randomly.
All slides and paraffin-embedded tissue blocks as well as fresh frozen tissue samples were retrieved with institutional review board approval (no. 09-11-052-01) and in a manner compliant with the Health Insurance Portability and Accountability Act. Informed consent was obtained for procurement of fresh frozen tissue; use of archived specimens met criteria for exemption from informed consent.
Tissue processing
Thyroidectomy specimens were fixed in 10% buffered formalin and either representative sections or the entire specimen, depending on its size, was submitted, routinely processed, and embedded in paraffin. Serial 4-μm-thick sections were prepared for routine hematoxylin-and-eosin (H&E) and immunohistochemical/fluorescent staining. For Western blot analysis, fresh frozen samples were used.
GLP-1 receptor antibody validation
Plasmid transfection experiments with COS-7 and Chinese hamster ovary (CHO) cells, which do not express the GLP-1 receptor, were used to validate the GLP-1 receptor antibodies. These cell lines were kindly provided by Dr. B. Lee (University of California, Los Angeles, Los Angeles, CA) (9). Cells were grown in a complete growth medium DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (Gemini, West Sacramento, CA), 100 IU/ml penicillin, and 100 mg/ml streptomycin (Invitrogen) at 37 C in a humidified 5% CO2 atmosphere. INS 823/13 rat insulinoma cells served as positive controls and were grown in RPMI 1640 medium supplemented with 10 mm HEPES, 1 mm sodium pyruvate, 100 IU/ml penicillin, and 100 mg/ml streptomycin (Invitrogen); 10% heat-inactivated fetal bovine serum (Gemini); and 50 mm β-mercaptoethanol (Sigma, St. Louis, MO) under the same conditions.
For transfections, cells were plated on 12-well plates at a density of 25,000 cells/well (cells were plated on glass coverslips for immunofluorescent staining). The next day, cells were transfected with human GLP-1 receptor cDNA (using lipofectamine2000-plasmid complexes prepared according to the manufacturer's instructions; Invitrogen) in Opti-MEM (Invitrogen) for 6 h and then cultured in complete medium for 72 h. The GLP-1 receptor cDNA was kindly provided by Dr. C. Unson (Rockefeller University, New York, NY) (10). The transfected cells were used for Western blotting and experiments and immunohistochemistry as described below.
Western blotting (cell lines)
After transfection, cells were washed with ice-cold PBS and then lysed in lysis buffer (50 mm HEPES, 1% Nonidet P-40, 2 mm Na3VO4, 100 mm NaF, 10 mm PyrPO4, 4 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 mg/ml leupeptin, and 1 mg/ml aprotinin). Samples were stored at −80 C until use for subsequent protein determination by BCA assay (Bio-Rad, Hercules, CA) and Western blotting.
Proteins (25–50 μg/lane) were separated on a 4–12% Bis-Tris NuPAGE gel (Invitrogen) and blotted onto a polyvinylidene fluoride membrane (FluoroTrans; Pall Life Sciences, Ann Arbor, MI). Membranes were probed overnight at 4 C with primary antibodies against human GLP-1 receptor (both polyclonal rabbit antihuman GLP-1 receptor, dilution 1:500, NLS1206; Novus Biologicals, Littleton, CO; and ab39072; Abcam, Cambridge, MA). After incubation with horseradish peroxidase-conjugated secondary antibody (1:5000; Jackson Laboratories), proteins were visualized using enhanced chemiluminescence (Millipore, Bedford, MA). Validation of the antisera NLS1206 was also performed and published previously (11). In this publication, ab13181 is identical with NLS1206 because both have been licensed to the corresponding companies by a developing laboratory.
Western blotting with either anti-GLP-1 receptor antibody detected a 53-kDa band in INS 832/13 cells, which were used as positive control and were kindly provided by Dr. C. Newgard (Duke University, Durham, NC) (12) but not in untransfected CHO or COS cells (Fig. 1). In contrast, COS cells and CHO cells transfected with GLP-1 receptor cDNA also had a 53-kDa band. These results were consistent with the immunofluorescent staining as described below that was positive in cells transfected with GLP-1 receptor cDNA but not in untransfected cells (illustrated for each antibody in CHO cells in Fig. 1).
Fig. 1.
Detection of GLP-1 receptor by Western blot and immunostaining. A and B, GLP-1 receptor immunoreactivity in the lysates of INS 832/13, CHO, and COS-7 cells shown by immunoblot. The cells were transfected with the indicated concentrations of human GLP-1R cDNA (0, nontransfected cells), and cell lysates were collected after 72 h. After separation on acrylamide gels and subsequent transfer, polyvinylidene fluoride membranes were labeled with antisera the GLP-1 receptor (GLP-1R) antisera NLS1206 (A) or ab39072 (B). Both antisera detect specific bands at 53 kDa in the presence of the GLP-1 receptor (INS 832/13 cells as a positive control and transfected CHO as well as COS-7 cells) but not in untransfected cells. A 63-kDa band was also present in all lysates with NLS1206. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. C and D, To ensure that this reactivity does not interfere with immunostaining, transfected CHO cells (1.0 μg/ml GLP-1R cDNA, 72 h) as well as control cells were immunostained with NLS1206 (red, upper panel) and counterstained with DAPI (blue, lower panel). As can be seen, no immunofluorescence for GLP-1 receptor was present in nontransfected cells but was present in transfected cells (C). Similar immunostaining of transfected CHO cells (1.0 μg/ml GLP-1R cDNA, 72 h) was observed with ab39072. Antisera ab39072-labeled GLP-1R (red, upper panel) only in transfected cells, counterstained with DAPI, is shown in lower panel (blue) (D). E and F, Specific detection of the GLP-1R (53-kDa band) with two antisera (NLS1206, E; ab39072, F) in lysates of fresh frozen control thyroid lobes (subjects 29 and 30) as well as medullary thyroid carcinoma samples (two separate tissue samples; subject 25).
Immunofluorescent staining (cell lines)
CHO cells were washed and then fixed with ice-cold 4% PFA for 10 min and permeabilized (0.4% Triton X-100 in TBS) for 30 min on ice. After rinsing with PBS, primary GLP-1 receptor antibodies (NLS1206, dilution 1:100; ab39072, dilution 1:250) were applied and incubated overnight at 4 C, followed by 1 h treatment with secondary antibody conjugated to Cy3 [1:100, room temperature (RT, 20–25 C); Jackson ImmunoResearch Laboratories, West Grove, PA]. Cells were then mounted with Vectashield (Vector Laboratories, Burlingame, CA) with 4′,6′-diamino-2-phenylindole (DAPI).
Western blotting (thyroid tissue)
Western blot experiments were carried out with fresh frozen tissue from two control thyroid lobes and one medullary thyroid carcinoma case (two separate tissue samples). Tissue was transferred to lysis buffer [20 mm Tris acetate (pH 7.0), 0.27 m sucrose, 1 mm EDTA, 50 mm NaF, 1% Triton X-100, 5 mm sodium pyrophosphate, 10 mm β-glycerophosphate], homogenized in a Tissue LyserII (QIAGEN, Valencia, CA), and stored for further analysis. Proteins (50–70 μg/lane) were separated on an 8% Bis-Tris Novex gel (Invitrogen). Subsequent steps were carried out as described for cells earlier (Fig. 1).
Immunofluorescent staining (thyroid sections)
Unstained thyroid sections were deparaffinized in toluene and rehydrated in an ethanol gradient. One section per case was stained in Harris hematoxylin solution (HHS16; Sigma) and eosin Y solution (HT110132; Sigma). Adjacent sections were used for immunofluorescent labeling as follows. Antigen retrieval was performed via microwave heating in citrate buffer (pH 8) (H-3300; Vector Laboratories). Slides were blocked in Tris-buffered saline (3% BSA, 0.2% Triton X-100, and 2% bovine serum) for 1 h. GLP-1 receptor antibody as described above (NLS1206, dilution 1:100 in 3% BSA/0.2% Tween-20/Tris-buffered saline) was used for overnight incubation (4 C), followed by colabeling for calcitonin (rabbit anticalcitonin, no. A0676, dilution 1:3000; Dako, Carpinteria, CA) for 4 h at RT. Secondary antibodies labeled with Cy3 and fluorescein isothiocyanate were obtained from Jackson ImmunoResearch Laboratories and applied at dilutions of 1:100 for 1 h incubation at RT. Vectashield with DAPI (Vector Laboratories) was used to counterstain the nuclei.
As an independent source, a second GLP-1 receptor antibody was also used (ab39072, dilution 1:250) as validated above. We compared the use of the two GLP-1 receptor antibodies for immunostaining in adjacent sections of all reported cases. As shown in Supplemental Figs. 1–5, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org, the pattern of immunostaining was comparable with the two anti-GLP-1 receptor antibodies. As a further validation and positive control, immunostaining with both of the GLP-1 receptor antibodies was compared in pancreatic islets present in sections of normal human pancreas (Supplemental Fig. 6). The majority of islet cells were positive for GLP-1 receptor, showing a comparable pattern with either antibody.
Finally, normal rabbit serum (X0902; Dako) as well as the corresponding blocking peptides (NLS1206PEP; Novus Biologicals; and ab39071, peptide sequence: KYLYEDEGCWTRNSNMN; Abcam) to the GLP-1 receptor antibodies were also used as a primary antibody control. Omission of the primary GLP-1 receptor antibody served as a negative control for the secondary antibody (Supplemental Fig. 6).
Immunohistochemical staining
Thyroid tissue sections were deparaffinized and rehydrated in an ethanol gradient. After antigen retrieval and quenching of endogenous peroxidase activity (10% methanol, 10% H2O2 in Tris-buffered saline), a rabbit antihuman calcitonin antibody (dilution 1:3000, no. A0676; Dako) was used for calcitonin labeling. Subsequent detection was performed with Envision+ antirabbit horseradish peroxidase (no. K4003; Dako) with 3′,3′-diaminobenzidine used as the chromogen and hematoxylin as the counterstain. Representative examples of calcitonin staining for reactive C cell hyperplasia, nodular/neoplastic C cell hyperplasia, and medullary thyroid carcinoma are shown in Supplemental Fig. 7. GLP-1 receptor labeling by immunohistochemistry was performed with NLS1206 (Novus Biologicals; and ab39072; Abcam) as specified above (Supplemental Figs. 1–5).
Image analysis
Fluorescent slides were analyzed and imaged using a Leica microscope DM6000 (Leica Microsystems, Bannockburn, IL), and images were acquired using Open Lab microscope software (Improvision, Lexington, MA). All other slides were analyzed with a Olympus Ix70 (Olympus, Melville, NY).
Results
Expression of GLP-1 receptor in medullary thyroid carcinoma (Table 2, cases 16–27)
Table 2.
Tumor and C cell hyperplasia
| Subject number | Age (yr) | Sex | GLP-1 receptor | Calcitonin | Colocalization | Calcitonin levels (pg/ml) |
|---|---|---|---|---|---|---|
| Medullary thyroid cancer | ||||||
| 16 | 23 | F | + | + | + | 139.6 |
| 17 | 24 | M | + | + | + | 137254 |
| 18 | 46 | M | +++ | + | + | 3850 |
| 19 | 43 | F | + | + | + | 3260 |
| 20 | 25 | M | +++ | + | + | 507 |
| 21 | 63 | M | + | + | + | 1072 |
| 22 | 76 | M | +++ | + | + | |
| 23 | 41 | F | – | + | – | 2824 |
| 24 | 64 | F | +++ | + | + | 6412 |
| 25 | 64 | F | +++ | + | + | 25248 |
| 26 | 74 | F | +++ | + | + | 134 |
| 27 | 49 | F | + | – | – | 8164 |
| C cell hyperplasia with RET germline mutations | ||||||
| 28 MEN-2A | 67 | F | ++ | + | + | 29.1 |
| 29 MEN-2A | 25 | M | ++ | + | + | 165 |
| 30 MEN-2A | 19 | F | ++ | + | + | 1193 |
| 31 MEN-2B | 2 | F | ++ | + | + | 66.2 |
| 32 FMTC | 3 | F | +++ | + | + | 10.6 |
| Reactive C-cell hyperplasia | ||||||
| 33 | 40 | F | +++ | + | + | |
| 34 | 43 | F | + | + | + | |
| 35 | 59 | F | + | + | + | |
| 36 | 59 | F | + | + | + | |
| Papillary thyroid cancer | ||||||
| 37 | 36 | M | – | – | – | |
| 38 | 42 | M | – | – | – | |
| 39 | 40 | F | – | – | – | |
| 40 | 40 | F | – | – | – | |
| 41 | 34 | F | +++ | – | – | |
| 42 | 22 | F | – | – | – | |
| 43 | 46 | F | – | – | – | |
| 44 | 31 | F | ++ | – | – | |
| 45 | 38 | M | – | – | – | |
| 46 | 48 | F | – | – | – | |
| 47 | 53 | M | – | – | – | |
| 48 | 54 | M | – | – | – | |
| 49 | 41 | F | – | – | – | |
| 50 | 37 | F | – | – | – | |
| 51 | 48 | F | – | – | – | |
| 52 | 63 | M | – | – | – | |
| 53 | 55 | F | +++ | – | – |
Table represents a summary of immunohistochemistry and immunofluorescence results. GLP-1 receptor immunoreactivity was comparable in areas of C cell hyperplasia and medullary thyroid cancer. −, Negative; +++, more than 70% of cells positive; ++, 10–35% positive; +, less than 10% positive; M, male; F, female; FMTC, familial medullary thyroid carcinoma.
To investigate presence of GLP-1 receptor protein, we analyzed lysates from a case of medullary thyroid carcinoma (two separate tissue samples) and compared it with two control thyroid lobes. Figure 1 illustrates specific detection of GLP-1 receptor protein (53-kDa band) by Western blot with two different GLP-1 receptor antibodies in the medullary thyroid carcinoma case, which is absent in controls. Having established that GLP-1 receptor protein is present in medullary thyroid carcinoma, we examined thyroid obtained from 12 individuals for GLP-1 receptor expression by immunostaining. In six of these cases, a high proportion (>70%) of the medullary thyroid carcinoma cells were immunoreactive for GLP-1 receptor (Fig. 2). In five of the cases of medullary thyroid carcinoma, GLP-1 receptor immunoreactivity was readily detected, being present in approximately 10–30% of the tumor cells in these cases; however, there was clear heterogeneity, with many calcitonin immunoreactive C cells being negative for GLP-1 receptor. In one of the cases, no GLP-1 receptor labeling was present in the medullary thyroid carcinoma. There was no immunoreactivity for either calcitonin or GLP-1 receptor in normal thyroid follicles identified in the same sections. There was no correlation between the extent of GLP-1 receptor immunoreactivity and either tumor size (range 0.6–6.5 cm, mean 3.4 ± 0.5 cm) or plasma calcitonin levels (range 134–137,124 pg/ml, mean 17,172 ± 12,201 pg/ml).
Fig. 2.
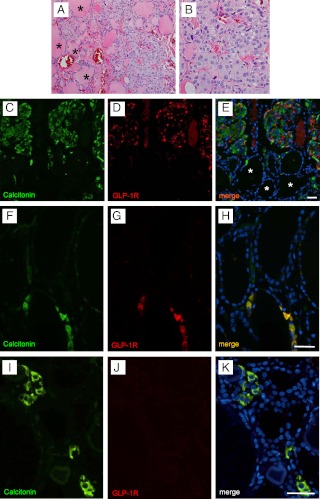
GLP-1 receptor expression in medullary thyroid carcinoma (subject 26). A, Low-power photomicrograph (original magnification, ×100) of an H&E-stained section of medullary thyroid carcinoma (top) adjacent to nonneoplastic thyroid tissue (bottom). The tumor exhibits an organoid growth pattern and entraps thyroid follicles (asterisks). B, At higher magnification (original magnification, ×400), the tumor cells demonstrate an epithelioid to spindle cell morphology. The nuclei contain stippled chromatin giving a salt-and-pepper appearance. Nucleoli are relatively inconspicuous. C and D, Immunohistochemical staining from the same medullary thyroid carcinoma showing immunoreactivity for GLP-1R (brown; NLS1206), hematoxylin was used to counterstain the nuclei (C). As illustrated in high-power magnification, the typical staining pattern of the GLP-1 receptor (GLP-1R) is present in approximately 70% of the calcitonin-expressing medullary thyroid carcinoma C cells (D). Scale bars, 100 μm (C) and 50 μm (D). E–G, Higher-power immunofluorescent images from the same medullary thyroid carcinoma case stained for calcitonin (green, E) and GLP-1R (NLS1206; red, F). In the merged image with DAPI (blue, G), colocalization of the GLP-1R and calcitonin is indicated by the orange color. Scale bar, 50 μm.
Expression of GLP-1 receptor in C cell hyperplasia (Table 2, cases 28–36)
We examined thyroid specimens from nine individuals with C cell hyperplasia, four of which were cases of reactive C cell hyperplasia, and in five cases C cell hyperplasia was due to the presence of RET germline mutations.
Reactive C cell hyperplasia was noted incidentally in the tumor-free thyroid lobe after thyroid resection for papillary thyroid carcinoma (three cases) and Hurthle cell adenoma (one case). In the four cases of reactive C cell hyperplasia, GLP-1 receptor immunoreactivity was present in most calcitonin positive cells (Fig. 3).
Fig. 3.
GLP-1 receptor expression in C cell hyperplasia (subject 32). A and B, Clusters of C cells in nodular/neoplastic C cell hyperplasia in a patient with familial medullary thyroid carcinoma syndrome and the presence of a germline mutation in the RET protooncogene (H&E stain; original magnification, ×200). Note that at least 50 C cells, located between thyroid follicles (asterisks), are present in this photomicrograph. A high-power image from the same cluster of C cells is shown in B. C–E, Immunofluorescent staining of the same thyroid showing a cluster of C cells (immunoreactive for calcitonin; green, C) coexpressing the GLP-1 receptor (GLP-1R; NLS1206; red, D). Thyroid follicular cells in the lower pole (thyroid follicles marked by asterisks) apparent with DAPI nuclear staining (blue, E) are negative for both calcitonin and GLP-1 receptor. Scale bar, 50 μm. GLP-1 receptor expression in C cells of control thyroid gland lobes (subjects 14 and 5). F and I, C cells were identified in control thyroid lobes by immunodetection for calcitonin (green). A total of 1797 C cells typically present as individual cells or microclusters were thus identified in the sections of the 15 control thyroid gland lobes (i.e. normal thyroid lobes). G and J, In five controls, C cells that were also immunoreactive for GLP-1 receptor (NLS1206; red, G) were identified. In 10 of the analyzed cases, the microclusters of C cells (calcitonin, green, I) were negative for the GLP-1 receptor labeling (J). H and K, In the merged image (DAPI, blue), adjacent thyroid follicular cells that are negative for both calcitonin and GLP-1 receptor are present. Subjects 14 (F–H), 5 (I–K). Scale bars, 50 μm.
In the five cases of C cell hyperplasia secondary to RET germline mutations, areas of nodular/neoplastic C cell hyperplasia and medullary thyroid microcarcinomas were detected. GLP-1 receptor immunoreactivity was invariably present in both the microcarcinomas and areas of nodular/neoplastic C cell hyperplasia in all five cases (Fig. 4). Although calcitonin immunoreactivity was relatively homogeneous in areas of C cell hyperplasia, GLP-1 receptor immunoreactivity was more heterogeneous in both reactive C cell hyperplasia and C cell hyperplasia secondary to RET germline mutations.
Fig. 4.
GLP-1 receptor expression in C cell hyperplasia in the presence of MEN (subject 28). A, H&E-stained histological section of a medullary thyroid microcarcinoma (<1 cm) arising from a background of nodular/neoplastic C cell hyperplasia in patient with MEN-2A (original magnification, ×40). The neoplastic C cells have extended beyond the basal lamina and are infiltrating the normal thyroid parenchyma. Note the presence of entrapped thyroid follicles (asterisks). B, Immunofluorescent image of the same medullary thyroid microcarcinoma showing relatively uniform expression of calcitonin (green) with more heterogeneous expression of GLP-1 receptor (GLP-1R; NLS1206; red). Nuclei are counterstained with DAPI (blue). C–E, Higher magnification of boxed area of tumor shown in B that emphasizes the relative homogeneity of calcitonin immunoreactivity (green, C) but heterogeneity of GLP-1R immunoreactivity (NLS1206; red, D) in C cells (merged image with DAPI blue and orange shows colocalization, E). Scale bar, 50 μm. F–H, A region of nodular C cell hyperplasia from the same thyroid gland. The C cells express calcitonin (green, F) and to a varying degree GLP-1R (NLS1206; red, G). In the merged image (DAPI, blue, H), it is apparent that the adjacent thyroid follicular cells are negative for both calcitonin and GLP-1R. Scale bar, 50 μm.
Expression of GLP-1 receptor in C cells from control thyroid glands (Table 1)
We detected a total of 1797 C-cells within the 15 cases examined (range 12–433 per section) by immunofluorescence for calcitonin on sections of control thyroid lobes (see Materials and Methods). In five of these cases, we identified C cells that stained positive for both calcitonin and GLP-1 receptor (Fig. 3, F and G). However, in 10 cases C cells were negative for GLP-1 receptor immunoreactivity (Fig. 3, I and J). Mild inflammation, as indicated by the presence of a lymphocytic infiltrate, was present in four of the control thyroid lobes. In three of these cases, GLP-1R was detected in C cells. In total, GLP-1 receptor immunoreactivity was found in five of 15 of the control thyroid lobes evaluated and in 35% of all C cells assessed.
Expression of GLP-1 receptor in papillary thyroid carcinoma (Table 2, cases 37–53)
We examined thyroid tissue obtained from 17 individuals with papillary thyroid carcinoma by both immunostaining for calcitonin and GLP-1 receptor. As expected, papillary thyroid carcinoma cells were not immunoreactive for calcitonin. GLP-1 receptor immunoreactivity was present in papillary thyroid carcinoma cells in three of the 17 cases (Fig. 5). C cells positive for calcitonin, but not GLP-1 receptor, were identified in the same sections surrounding normal thyroid follicles remote from the papillary thyroid carcinoma, thus serving as a positive control for calcitonin labeling.
Fig. 5.
GLP-1 receptor expression in papillary thyroid carcinoma (subject 41). A, High-magnification photomicrograph of a conventional-type papillary thyroid carcinoma exhibiting papillary structures with fibrovascular cores lined by follicular-derived epithelial cells exhibiting characteristic nuclear changes. These include nuclear enlargement and elongation, overlapping nuclei, fine chromatin, hypochromasia, intranuclear grooves (arrowheads), and intranuclear pseudoinclusions (arrows). A psammoma body is also seen within the circled area. B, A focal area of the same papillary thyroid carcinoma illustrates the specific immunoreactivity for GLP-1 receptor (GLP-1R; NLS1206; red) in some but not all cells of this papillary thyroid carcinoma (DAPI, blue) but no immunoreactivity for calcitonin (green). Scale bar, 50 μm. C–E, An area of adjacent normal (tumor free) thyroid from the same section as B revealed a normal small cluster of C cells immunoreactive for calcitonin (green, C) that were not labeled for GLP-1R (NLS1206; red, D). Adjacent thyroid follicular cells apparent in the merged image (DAPI, blue, E) were negative for both calcitonin and GLP-1R. The specific immunoreactivity of these C cells in the same section as papillary thyroid carcinoma cells in A and B serves as a positive control for calcitonin labeling and assures that GLP-1R (B) is indeed present in papillary thyroid carcinoma cells and not a mixture of papillary thyroid carcinoma and C cells. Scale bar, 50 μm.
Discussion
Our objective in these studies was to establish the pattern of GLP-1 receptor expression in human thyroid, with a particular focus on C cells across the range of normal C cells, C cell hyperplasia, and medullary thyroid carcinoma. The motivation to address this objective is the recent widespread use of long-term, high-dose GLP-1 receptor agonist therapy in the treatment of type 2 diabetes mellitus and the need to consider the possible unintended off-target consequences of this therapy.
We report that GLP-1 receptor expression is common in medullary thyroid carcinoma and C cell hyperplasia but also not infrequently present in normal C cells. Unexpectedly, we also identified GLP-1 receptor expression to be present in neoplastic cells in three of 17 papillary thyroid carcinomas examined. These studies are therefore in broad agreement with the work of Korner et al. (6), who found GLP-1 receptor expression in approximately 30% of medullary thyroid carcinomas by use of GLP-1 receptor scintigraphy, and Bjerre Knudsen et al. (4), who reported that GLP-1 receptors are rarely present in normal human thyroid C cells. The data lend mechanistic plausibility to the apparent signal of increased reported thyroid tumors in patients treated with exenatide in the Federal Drug Administration Adverse Event Reporting database (13). Such a signal would seem unlikely to arise exclusively from medullary thyroid cancer, given the rarity of this tumor, but it would be less surprising if long-term pharmacological exposure to GLP-1-based therapy was to promote the growth of the relatively common small papillary thyroid carcinomas present in the general population (14).
There were originally high hopes of GLP-1 receptor activation to promote reversal of the β-cell deficit in diabetes by the proproliferative actions of GLP-1 to induce β-cell replication, as described in juvenile mice. Subsequent studies revealed that epigenetic silencing of β-cell replication after the juvenile period of β-cell growth in mice rendered β-cells relatively unresponsive to GLP-1 induced replication (15). By analogy, thyroid cells that express GLP-1 receptor in humans (C cells or follicular cells) may not proliferate in response to GLP-1 therapy as C cells have been shown to do in rodents. On the other hand, by definition, papillary thyroid carcinoma or C cells with dysregulated cell cycle control due to germline mutations presumably are competent to proliferate.
The heterogeneity of GLP-1 receptor expression we report here in both C cell hyperplasia and medullary thyroid carcinoma likely accounts for the lower frequency of detection of GLP-1 receptor expression by scintigraphy vs. immunofluorescence in medullary thyroid carcinomas. In six of the 12 medullary thyroid carcinomas in which we were able to detect GLP-1 receptor labeling, the majority of C cells were immunoreactive for GLP-1 receptor. In the remaining five cases, a much lower proportion of cells (10–30%) was positive for GLP-1 receptor. Given the known proproliferative actions of GLP-1 (16) and based on these findings, there would appear to be reasonable concern to avoid GLP-1-based therapies in individuals with a known history or risk for medullary thyroid carcinoma.
Fortunately, medullary thyroid carcinoma is relatively uncommon. Approximately 37,000 new cases of thyroid cancer are diagnosed in the United States each year, of which 5–10% are medullary thyroid carcinomas (17). Twenty-five percent of medullary thyroid carcinomas are related to germline activating mutations in the RET protooncogene, which affect approximately one in 30,000 individuals and are associated with the hereditary cancer syndromes including MEN-2A, MEN-2B, and familial medullary thyroid carcinoma (18).
In the present study, we also established that GLP-1 receptor expression is present in C cell hyperplasia. C cell hyperplasia may be found in thyroid glands resected prophylactically in individuals with known predisposing RET mutations as a result of family screening. In the setting of an activating RET mutation, C cell hyperplasia is considered a preneoplastic lesion that constitutes carcinoma in situ of the thyroid C cells (19). Again, given the known actions of GLP-1 on cell proliferation, it would seem undesirable to expose individuals with a preneoplastic form of C cell hyperplasia to long-term, high-dose GLP-1 mimetic therapy. The more complex issue that arises is whether C cell hyperplasia is present in individuals without RET mutations. Sporadic C cell hyperplasia may be identified as an incidental finding on histopathology and has been described with aging, hyperparathyroidism, hypergastrinemia, chronic lymphocytic thyroiditis, and adjacent to follicular-derived thyroid tumors (19). The overall incidence of this more pedestrian form of C cell hyperplasia is uncertain, although it has been reported to occur in one third of supposedly normal thyroid glands at autopsy (7). Although C cell hyperplasia in the setting of activating RET mutations is accepted to be a premalignant condition, the potential of sporadic C cell hyperplasia to progress to invasive malignancy remains debated (20). In a recent study, sporadic C cell hyperplasia in patients with elevated serum calcitonin levels was shown to exhibit cytological atypia and morphological features substantially overlapping with those found in premalignant familial lesions (21).
Given the present data, it is plausible that GLP-1 receptor expression occurs only in C cells in humans as a consequence of dysregulated cell cycle control present in C cell hyperplasia or medullary thyroid carcinoma. Alternatively, it is possible that there is heterogeneity with respect to GLP-1 receptor expression, with most C cells not expressing the GLP-1 receptor but with a small subset that do. There is some indirect evidence to support the latter postulate. To date, the potential actions of GLP-1 receptor agonist therapy on the thyroid gland have been studied most extensively in relation to liraglutide. In six prospective trials, no differences in plasma calcitonin levels were observed between treated subjects and controls (22–27). However, pooled data revealed that liraglutide increased plasma calcitonin levels in a dose-dependent fashion (3), but a subsequent analysis of six phase 3 clinical trials of exposure to liraglutide for 26 wk or longer in 5698 patients did not reveal any detectable change in calcitonin levels (28). In the present study, we identified normal C cells that were immunoreactive for the GLP-1 receptor in 33% of normal human thyroid glands. Taken together with the minimal or absent calcitonin response in clinical studies with liraglutide, these data imply that, in the majority of individuals, pharmacological activation of GLP-1 receptor in C cells (when present) does not lead to C cell hyperplasia in the short term, at least as adjudged by calcitonin levels.
Concern with regard to GLP-1 therapy and thyroid C cell tumors was first raised by detection of such tumors in toxicology-screening studies in rodents treated with the GLP-1 receptor agonist liraglutide (3). Important species-specific differences in C cell histology and physiology do exist. In rodents, C cells are present in greater densities within the thyroid when compared with humans and nonhuman primates (4), which correlates with the fact that calcitonin plays a more important role in the regulation of serum calcium levels in rodents compared with primates (29, 30). It is common for rats to spontaneously develop proliferative C cell lesions as they age (3). In humans, C cells comprise only 0.1% of the total mass of the thyroid gland (31). In a recent study of human thyroid tissue, Bjerre Knudsen et al. (4) reported that chronic liraglutide administration increased calcitonin levels in rats but not in nonhuman primates. They also reported that in human thyroid glands, C cells were negative for GLP-1 receptor expression by in situ hybridization and in situ ligand binding. Given the small number of C cells identified by Bjerre Knudsen et al., these negative findings must be treated with caution.
In the present study, we also identified GLP-1 receptor expression in three of 17 papillary thyroid carcinomas, implying that aberrant GLP-1 receptor expression may arise from follicular cells in this relatively common thyroid cancer. This was an unexpected finding because our focus here was on the C cell lineage. If GLP-1 receptor activation were to promote growth of papillary thyroid carcinoma, the absence of an increase in calcitonin levels would be of little comfort. Papillary thyroid carcinoma comprises 90% of all thyroid cancer cases and has a rising incidence (32). Thus, our finding of GLP-1 receptor immunopositivity in a fraction of papillary thyroid carcinomas is likely of greater epidemiological significance than our findings related to the C cell lineage. It will be important to address the question of GLP-1 receptor expression in other thyroid tumors arising from follicular cells in the future. Given the prior report, using receptor scintigraphy, that GLP-1 receptor expression is present in a variety of other tumor types (for example, tumors of the central nervous system) (6), more detailed evaluation of GLP-1 receptor status in other tissues may also be warranted. Moreover, it may be prudent to monitor individuals exposed to long-term GLP-1 mimetic therapy for any increased incidence of carcinomas of the thyroid gland and other cell types in which the GLP-1 receptor has been demonstrated (33).
Supplementary Material
Acknowledgments
We thank Bonnie Lui for editorial assistance and Dr. Andre Van Herle for helpful suggestions. We are grateful to Dr. Jianyu Rao and Yusheng Jin for their assistance with critical revision.
This work was supported by the Larry L. Hillblom Foundation. The study was performed with approval from the University of California, Los Angeles, Institutional Review Board, protocol 09-11-052-01.
Disclosure Summary: The authors have no conflicts of interest to declare.
Footnotes
- CHO
- Chinese hamster ovary
- DAPI
- 4′,6′-diamino-2-phenylindole
- DPP-4
- dipeptidyl-peptidase-4
- GLP-1
- glucagon like peptide-1
- H&E
- hematoxylin and eosin
- MEN
- multiple endocrine neoplasia
- RET
- rearranged during transfection
- RT
- room temperature (20–25 C).
References
- 1. Aaboe K, Krarup T, Madsbad S, Holst JJ. 2008. GLP-1: physiological effects and potential therapeutic applications. Diabetes Obes Metab 10:994–1003 [DOI] [PubMed] [Google Scholar]
- 2. Peters A. 2010. Incretin-based therapies: review of current clinical trial data. Am J Med 123:S28–S37 [DOI] [PubMed] [Google Scholar]
- 3. 2009. Victoza (R) (liraglutide injection): human relevance of rodent thyroid C-cell tumors. http://www.fda.gov/downloads/AdvisoryCommittees/Committees%20MeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM151129.pdf Accessed October 5, 2010
- 4. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Mølck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M. 2010. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 151:1473–1486 [DOI] [PubMed] [Google Scholar]
- 5. Rosai J, Carcangiu ML, DeLellis RA. 1992. Tumors of the thyroid gland. In: Rosai J, ed. Atlas of tumor pathology. Bethesda, MD: Armed Forces Institute of Pathology [Google Scholar]
- 6. Körner M, Stöckli M, Waser B, Reubi JC. 2007. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 48:736–743 [DOI] [PubMed] [Google Scholar]
- 7. Guyétant S, Rousselet MC, Durigon M, Chappard D, Franc B, Guerin O, Saint-André JP. 1997. Sex-related C cell hyperplasia in the normal human thyroid: a quantitative autopsy study. J Clin Endocrinol Metab 82:42–47 [DOI] [PubMed] [Google Scholar]
- 8. Perry A, Molberg K, Albores-Saavedra J. 1996. Physiologic versus neoplastic C-cell hyperplasia of the thyroid: separation of distinct histologic and biologic entities. Cancer 77:750–756 [DOI] [PubMed] [Google Scholar]
- 9. Negrete OA, Levroney EL, Aguilar HC, Bertolotti-Ciarlet A, Nazarian R, Tajyar S, Lee B. 2005. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 436:401–405 [DOI] [PubMed] [Google Scholar]
- 10. Unson CG. 2008. Expression of glucagon receptors in tetracycline-inducible HEK293S GnT1-stable cell lines: an approach toward purification of receptor protein for structural studies. Biopolymers 90:287–296 [DOI] [PubMed] [Google Scholar]
- 11. Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. 2007. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148:4965–4973 [DOI] [PubMed] [Google Scholar]
- 12. Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. 2000. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430 [DOI] [PubMed] [Google Scholar]
- 13. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. 2011. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bondeson L, Ljungberg O. 1984. Occult papillary thyroid carcinoma in the young and the aged. Cancer 53:1790–1792 [DOI] [PubMed] [Google Scholar]
- 15. Tschen SI, Dhawan S, Gurlo T, Bhushan A. 2009. Age-dependent decline in β-cell proliferation restricts the capacity of β-cell regeneration in mice. Diabetes 58:1312–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z, Habener JF. 2008. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic β cell proliferation. J Biol Chem 283:8723–8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. 2009. Cancer statistics, 2009. CA Cancer J Clin 59:225–249 [DOI] [PubMed] [Google Scholar]
- 18. Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA., Jr 2009. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19:565–612 [DOI] [PubMed] [Google Scholar]
- 19. LiVolsi VA. 1997. C cell hyperplasia/neoplasia. J Clin Endocrinol Metab 82:39–41 [DOI] [PubMed] [Google Scholar]
- 20. Livolsi VA, Feind CR. 1979. Incidental medullary thyroid carcinoma in sporadic hyperparathyroidism. An expansion of the concept of C-cell hyperplasia. Am J Clin Pathol 71:595–599 [DOI] [PubMed] [Google Scholar]
- 21. Verga U, Ferrero S, Vicentini L, Brambilla T, Cirello V, Muzza M, Beck-Peccoz P, Fugazzola L. 2007. Histopathological and molecular studies in patients with goiter and hypercalcitoninemia: reactive or neoplastic C-cell hyperplasia? Endocr Relat Cancer 14:393–403 [DOI] [PubMed] [Google Scholar]
- 22. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. 2009. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374:39–47 [DOI] [PubMed] [Google Scholar]
- 23. Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B. 2009. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373:473–481 [DOI] [PubMed] [Google Scholar]
- 24. Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S. 2009. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 26:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M, Matthews DR. 2009. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 32:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simó R. 2009. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 52:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L. 2009. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 32:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. 2011. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 96:853–860 [DOI] [PubMed] [Google Scholar]
- 29. Hirsch PF, Baruch H. 2003. Is calcitonin an important physiological substance? Endocrine 21:201–208 [DOI] [PubMed] [Google Scholar]
- 30. Wang W, Lewin E, Olgaard K. 2002. Role of calcitonin in the rapid minute-to-minute regulation of plasma Ca2+ homeostasis in the rat. Eur J Clin Invest 32:674–681 [DOI] [PubMed] [Google Scholar]
- 31. Albores-Saavedra JA, Krueger JE. 2001. C-cell hyperplasia and medullary thyroid microcarcinoma. Endocr Pathol 12:365–377 [DOI] [PubMed] [Google Scholar]
- 32. Davies L, Welch HG. 2006. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 33. Butler PC, Dry S, Elashoff R. 2010. GLP-1-based therapy for diabetes: what you do not know can hurt you. Diabetes Care 33:453–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.