Abstract
Use of stable isotope-labeled full-length proteins as an internal standard prior to multiple reaction monitoring (MRM) analysis enables pre-fractionation of the target proteins and quantification of those low-abundance proteins, which cannot be reached without biological sample enrichment. In terms of membrane proteins, this benefit can be used if a sample processing workflow allows entire solubilization of membrane proteins. We have developed a universal workflow for sample processing and enrichment by optimizing washing and solubilization conditions and implementing sample fractionation by Whole Gel Eluter. The optimized protocol was applied to various membrane-bound cytochromes P450 (CYPs) and their electron transferring protein partners, cytochrome P450 reductase (CPR), ferredoxin reductase (FdR), and ferredoxin (Fdx), all important proteins for cholesterol elimination from different organs. Both, weakly- (CPR and FdR) and tightly-associated (CYP7B1, CYP11A1, CYP27A1, and CYP46A1) membrane proteins were quantified. Measurements were performed on three human tissues (temporal lobe of the brain, retina and retinal pigment epithelium) obtained from multiple donors. The biological implications of our quantitative measurements are also discussed.
There are two general difficulties in quantification of membrane proteins using multiple reaction monitoring (MRM) mass spectrometry (MS): (i) membrane proteins predispose to incomplete digestion (trypsinolysis) and poor sequence coverage, and (ii) membrane proteins are largely low-abundance proteins.1 We have pioneered the use of 15N-labeled full-length protein standards for quantification of membrane proteins2, 3 and showed that this approach eliminates quantitative error originated from incomplete trypsinolysis and simplifies selection of available and appropriate peptides for MRM analysis. However, low abundance of membrane proteins remains a problem limiting broader application of MRM quantification. One successful approach to improving the number of low abundance proteins quantified has been termed SILAP.4, 5 Central to this approach is utilization of isotope labeled proteins as internal standards. Biological samples supplemented with SILAP standard are typically subjected to immunopurification and isoelectric focusing in order to remove high abundance proteins.4, 5 Indeed, full-length protein standards completely match the biochemical properties of the target proteins and can be added to the biological sample prior to sample processing.6, 7 This will establish the analyte/standard ratio and enable sample fractionation to obtain the desired level of membrane protein enrichment. However to achieve it, the membrane proteins must be first entirely solubilized and ready for fractionation. Protein association with biological membrane ranges from a weak interaction to essentially irreversible interaction. Consequently, the choice of solubilization procedure will vary depending on the group of membrane proteins targeted for quantification. The requirement of entire quantitative solubilization imposes a challenge because the strongest solubilizing and chaotropic agents (like SDS or urea) are not compatible with the MS analysis and require a removal step before the trypsinolysis. Hence, solubilization of membrane proteins with mild detergents is preferable as it provides better compatibility with many fractionation techniques. The disadvantage of mild detergents is that their application could be quantitative only for a group of weakly-associated membrane proteins.
Our research focuses on cholesterol-metabolizing cytochromes P450 (CYPs) and their redox partners, cytochrome P450 oxidoreductase (CPR), ferredoxin reductase (FdR) and ferredoxin (Fdx). Overall, these proteins represent a broad group of soluble and weakly- and tightly-associated membrane proteins.8–10 In the present work, we have developed a universal workflow for sample processing and enrichment by optimizing washing and solubilization conditions and implementing sample fractionation by Whole Gel Eluter. This optimized workflow was applied to quantification of CYP11A1, CYP27A1, CYP46A1, CYP7B1, CPR, FdR, and Fdx. For each of these proteins, the 15N-labelled human recombinant protein was expressed, purified, and used as internal standard in the MRM analysis. Measurements were performed on the specimens of human brain, retina, and retinal pigment epithelium (RPE).
EXPERIMENTAL SECTION
Materials
Ammonium chloride (15N, 99%) was from Cambridge Isotope Laboratories (Andover, MA). The DC Protein Assay kit was from Bio-Rad Laboratories (Hercules, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
15N-Labeled Full-Length Protein Standards
Human recombinant CYP11A1, CYP7B1, CYP27A1, CYP46A1, FdR, Fdx and CPR were expressed as described11, 12 using M9 minimal salt medium containing 1g/L of 15NH4Cl as the sole nitrogen source.3 Purification of 15N-labeled proteins was similar to that of the unlabeled analogs.11, 12 The percent incorporation of 15N in the recombinant proteins was determined at the peptide level as described.2 The experimental MALDI spectra for representative peptides from 15N-labeled proteins are shown in the Supporting Information (Figure S1).
Human Tissues
Samples of gray matter from the temporal lobe were obtained from the Alzheimer's Disease Center, Boston University. Eyes were acquired through the Cleveland and Georgia Eye Bank. Information on the donors is summarized in the Supporting Information (Table S1).
Processing of Samples
The tissue was placed in 25 mM NH4HCO3 and homogenized by sonication on ice at 30 W using three 10 s continuous cycles (Sonicator 3000, Misonix Inc., Farmingdale, NY). The total protein concentration was measured in the presence of 2% SDS using the DC protein Assay kit and bovine serum albumin as a standard. The homogenates were then aliquoted into 0.2 mg-portions of total tissue protein per tube. One set of tubes was frozen at −80 °C, whereas the other set was subjected to centrifugation at 153 000g for 30 min. The resulting pellet was frozen at −80 °C. Thus, two types of fractions were obtained and used for subsequent processing steps: the whole tissue homogenate and the total membrane pellet. In some experiments, the total membrane pellet was washed with either 50 mM phosphate buffer/0.5 M NaCl or 100 mM Na2CO3. The washing step was repeated twice and included sonication of a sample in the washing buffer and centrifugation at 153 000g for 30 min to pellet the sample. In other experiments, the total membrane pellet was treated for 60 min with various solubilizing agents, such as 1% sodium cholate, 8 M urea, or 1% SDS, followed by centrifugation at 153 000g for 30 min. This generated supernatant with solubilized proteins and pellet with proteins remained attached to the membranes. During the following processing, both soluble and pelleted fractions were dissolved in 25 mM NH4HCO3/1% SDS and supplemented with 0.3 – 1.0 pmole of various 15N-labeled protein standards. The samples were then precipitated with chloroform/methanol.13 The pellets obtained were sonicated in 100 µL of 25 mM NH4HCO3 and treated with trypsin for 15 h at 37 °C. The substrate/trypsin ratio was 50:1 (w/w). After trypsinolysis, the samples were mixed with 50% acetonitrile/0.1% trifluoroacetic acid and dried using a Vacufuge (Eppendorf AG, Hamburg, Germany).
LC-MS/MS Analysis
Instrumental analyses were performed on a hybrid triple quadrupole/linear ion trap mass spectrometer (4000 QTRAP, ABI/MDS-Sciex) coupled to an Eksigent nanoLC-2D system (Dublin, CA). Separation of peptides was performed with an Eksigent cHiPLC- nanoflex system equipped with a nano cHiPLC column, 15 cm × 75 µm, packed with ReproSil-Pur C18-AQ, 3 µm (Dr. Maisch, Germany). Peptides were eluted over a 29 min-gradient from 15% to 35% acetonitrile, containing 0.1% formic acid and at a flow rate of 300 nL/min. The column effluent was continuously directed into the nanospray source of the mass spectrometer using a fused-silica tip (FS360-20-10-N20-C15, PicoTip Emitter, New Objective, Woburn, MA). All acquisition methods used the following parameters: an ion spray voltage of 2200 V, curtain gas of 15 psi, source gas of 20 psi, interface heating temperature of 170 °C, declustering potential of 76 V for +2 precursor ions and 65 V for +3 precursor ions, collision energy of 30 V for +2 precursor ions and 22 V for +3 precursor ions, and collision cell exit potential of 16 V for +2 precursor ions and 13V for +3 precursor ions. The dwell time for all transitions was 40 ms and the total cycle time was less than 2 sec.
Quantitative Analysis and Validation
The mass spectrometer monitored three transitions per peptide. The MRM transitions were selected as described2, 3 and listed in the Supporting Information (Table S2). The relative ratios of the three transitions monitored in the 25 mM NH4HCO3 for 15N-labeled standards were similar to those observed by spiking 15N-labeled standards into the biological samples. This confirms no significant interference for the quantification based on selected transitions. The identities of the measured peptides were confirmed based on the retention time of the three MRM peaks from a given peptide and the ratio among the three MRM peaks. Representative extracted ion chromatograms for quantification based on different signature peptides are shown in the Supporting Information (Figure S2) and demonstrate comparable heavy/light ratios. The mean and standard deviation for the protein concentrations were calculated by treating all three transitions for each of the target peptides and the three experimental replicates as independent measurements.
The linearity of the optimum transitions was verified by spiking temporal lobe samples with 0.05 – 8.0 pmoles of the internal standards. The data were plotted for every individual transition and showed linearity and low scatter (Supporting Information, Figure S3). Limit of quantification (LOQ) was defined as the lowest calibration point of the curve that could be measured with a coefficient of variance less than 20%. The LOQ was 0.1 pmol/mg of tissue protein for CPR, FdR, CYP27A1, and CYP46A1.
RESULTS AND DISCUSSION
Solubilization of Membrane Proteins
Solubilization is a necessary step in sample processing if subsequent enrichment is required to quantify low-abundance membrane proteins. To develop solubilization procedures yielding maximum recovery of target proteins, samples of human brain were placed in 25 mM NH4HCO3 and homogenized by sonication in the presence of different additives. Tissue homogenates were then subjected to high speed centrifugation (153,000g), and the extent of solubilization was estimated by quantifying target proteins in the supernatant and pellet versus that in homogenate before centrifugation. Sonication in ammonium bicarbonate containing no additives led to only partial detachment FdR and CPR from the brain membranes as indicated by appearance of ≈44% of FdR and ≈24% of CPR in the 153,000g supernatant (Table 1). When 1% sodium cholate was added prior to sonication, almost complete solubilization of FdR (≈95%) was achieved, yet this treatment did not seem to increase the amount of CPR in the soluble fraction. Differential effects of sodium cholate on solubilization of FdR and CPR suggest that attachment of CPR to the membrane via the transmembrane anchor9 is stronger than that of FdR which interacts with the lipid bilayer only peripherally.10
Table 1.
Solubilization of FdR and CPRa
| Protein (pmol/mg tissue protein) | |||||
|---|---|---|---|---|---|
| Whole homogenate | |||||
| Protein/Peptide | without treatment |
sonication | 1% sodium cholate | ||
| supernatant | pellet | supernatant | pellet | ||
| FdR | |||||
| LTELLLR | 4.59 ± 0.73 | 2.00 ± 0.21 | 2.64 ± 0.34 | 4.35 ± 0.78 | ND |
| TATEKPGVEEAAR | 4.64 ± 0.77 | 2.06 ± 0.19 | 2.62 ± 0.34 | 4.44 ± 0.80 | ND |
| consensus | 4.62 ± 0.79 (100%) |
2.03 ± 0.23 (44%) |
2.63 ± 0.36 (57%) |
4.40 ± 0.82 (95%) |
ND |
| CPR | |||||
| GVATNWLR | 2.96 ± 0.51 | 0.67 ± 0.09 | 1.70 ± 0.26 | 0.80 ± 0.11 | 1.94 ± 0.31 |
| NPFLAAVTTVR | 3.16 ± 0.50 | 0.78 ± 0.10 | 2.17 ± 0.22 | 0.84 ± 0.10 | 2.08 ± 0.29 |
| consensus | 3.06 ± 0.54 (100%) |
0.73 ± 0.11 (24%) |
1.94 ± 0.26 (63%) |
0.82 ± 0.12 (27%) |
2.01 ± 0.32 (66%) |
Measurements were performed on the temporal lobe (patient ID 9517, Table S1) whole homogenate and on the supernatant and pellet fractions of the whole homogenate generated by centrifugation at 153 000g for 30 min after sonication or 1% sodium cholate treatment. The concentration was calculated for three experimental replicates by monitoring three transitions per individual peptide and presented as mean ± SD. The monitored transitions are summarized in the Supporting Information (Table S2). For the consensus, the data for two peptides from FdR or CPR were combined and presented as mean ± SD. For comparison, the concentrations in the whole homogenate were taken as a 100%, and the other relevant concentrations were presented as a percentile of the concentration in the whole homogenate. ND, not detected.
Unlike FdR and CPR, sonication in only 25 mM NH4HCO3 did not detach CYPs from biological membranes.2, 3 No CYP46A1 was solubilized in the presence of 1% sodium cholate as no enzyme was detected in the high speed supernatant (Table 2). This result suggests that attachment of CYP46A1 to the membrane through the N-terminal anchor and peripheral associations8 is stronger than membrane interactions of CPR. In contrast to CYP46A1, 1% sodium cholate partially extracted CYP27A1, but the extent of solubilization was only ≈35%.
Table 2.
Solubilization of CYP27A1 and CYP46A1a
| Protein (pmol/mg tissue protein) | |||||||
|---|---|---|---|---|---|---|---|
| Total membrane pellet | |||||||
| Protein/Peptide | without treatment |
1% sodium cholate | 8 M urea | 1% SDS | |||
| supernatant | pellet | supernatant | pellet | supernatant | pellet | ||
| CYP27A1 | |||||||
| LYPVVPTNSR | 0.19 ± 0.04 | 0.06 ± 0.01 | 0.14 ± 0.02 | 0.12 ± 0.02 | 0.07 ± 0.01 | 0.19 ± 0.03 | ND |
| IQHPFGSVPFGYGVR | 0.15 ± 0.03 | 0.05 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.02 | 0.06 ± 0.01 | 0.15 ± 0.03 | ND |
| consensus | 0.17 ± 0.04 (100%) |
0.06 ± 0.01 (35%) |
0.12 ± 0.02 (71%) |
0.11 ± 0.02 (65%) |
0.07 ± 0.01 (41%) |
0.17 ± 0.03 (100%) |
- |
| CYP46A1 | |||||||
| LLEEETLIDFVR | 0.39 ± 0.07 | ND | 0.42 ± 0.07 | 0.19 ± 0.03 | 0.20 ± 0.03 | 0.37 ± 0.07 | ND |
| VIDLAFSR | 0.39 ± 0.06 | ND | 0.38 ± 0.07 | 0.19 ± 0.03 | 0.22 ± 0.03 | 0.40 ± 0.07 | ND |
| consensus | 0.39 ± 0.08 (100%) |
- | 0.40 ± 0.08 (103%) |
0.19 ± 0.04 (49%) |
0.21 ± 0.04 (53%) |
0.39 ± 0.09 (100%) |
- |
Measurements were performed on the temporal lobe (patient ID 9517, Table S1) total membrane pellet and on the supernatant and pellet fractions of the total membrane pellet generated by centrifugation at 153 000g for 30 min after treatment with various solubilizing agents. The concentration was calculated for three experimental replicates by monitoring three transitions per individual peptide and presented as mean ± SD. The monitored transitions are summarized in the Supporting Information (Table S2). For the consensus, the data for two peptides from CYP27A1 or CYP46A1 were combined and presented as mean ± SD. For comparison, the concentrations in the total membrane pellet were taken as a 100%, and the other concentrations were presented as a percentile of the concentration in the total membrane pellet. ND, not detected.
We tested sodium cholate because this anionic detergent does not denature membrane proteins and is compatible with various fractionation procedures used for enrichment of folded proteins. Experiments with the two reductases and two CYPs, however, demonstrated that only FdR was entirely solubilized with this mild detergent suggesting that only a limited set of weakly-associated membrane proteins can be quantitatively solubilized under non-denaturating conditions. Therefore, we next tested the agents that provide better solubilization efficiency, but unfold proteins and require subsequent protein fractionation under denaturating conditions. Using denaturating conditions limits the opportunities for protein fractionation, yet is absolutely necessary for quantitative solubilization of tightly-associated membrane proteins. Table 2 shows that inclusion of 8 M urea to the sonication buffer increased detachment of CYP27A1 and CYP46A1 from the membrane to ≈65% and ≈49%, respectively, however, still did not result in a complete protein solubilization. Hence, a strong denaturating detergent, 1% SDS, was used which enabled complete transfer of both CYPs from the membranes to the aqueous phase (Table 2).
Sample Enrichment Prior to Solubilzation
Previously, we have demonstrated that tightly-associated membrane proteins can be quantified from the total membrane pellet.2, 3 In the present work, we investigated whether total membrane pellet could be further enriched by additional washing and centrifugation steps. Two washing conditions were used: (i) 50 mM phosphate buffer/0.5 M NaCl, which can disrupt protein-phospholipid interactions and (ii) 100 mM Na2CO3, which opens membrane vesicles and releases trapped cytosolic proteins.14 Table 3 shows that both washings of the total membrane pellet did not result in a loss of CYP27A1 or CYP46A1. On the level of total protein, washing with 50 mM phosphate buffer/0.5 M NaCl removed only 7% of the total protein and contributed little in the enrichment. However, washing with 100 mM Na2CO3 removed 40% of the total protein while retaining 100% of CYPs, thus demonstrating approximately 2-fold sample enrichment, a useful step in the sample processing of low-abundance membrane proteins.
Table 3.
Washing Steps for Total Membrane Pelleta
| Protein (pmol/mg tissue protein) | |||
|---|---|---|---|
| Protein/Peptide | washing steps for total membrane pellet | ||
| none | 50 mM phosphate buffer/0.5 M NaCl |
100 mM Na2CO3 |
|
| CYP27A1 | |||
| LYPVVPTNSR | 0.19 ± 0.03 | 0.19 ± 0.03 | 0.20 ± 0.03 |
| IQHPFGSVPFGYGVR | 0.18 ± 0.03 | 0.17 ± 0.02 | 0.20 ± 0.03 |
| CYP46A1 | |||
| LLEEETLIDFVR | 0.40 ± 0.04 | 0.39 ± 0.04 | 0.41 ± 0.05 |
| VLQDVFLDWAK | 0.38 ± 0.05 | 0.42 ± 0.05 | 0.43 ± 0.06 |
| Total protein | 100% | 93% | 60% |
Measurements were performed on the temporal lobe (patient ID 9499, Table S1) total membrane pellet and on the pellet fractions generated by centrifugation at 153 000g for 30 min after washing the total membrane pellet with 50 mM phosphate buffer/0.5 M NaCl or 100 mM Na2CO3. The concentration was calculated for three transitions per individual peptide for two individual peptides per protein and presented as mean ± SD. ND, not detected. For comparison, the total protein concentration in membrane pellet before washing was taken as a 100%, and the total protein concentrations after washings were presented as a percentile respectively.
Whole Gel Elution
The measurements for CYPs 27A1 and 46A1 in the total membrane pellet and in the total membrane pellet after Na2CO3 washes result in similar concentrations (Tables 3 and 4). In contrast, to detect CYP 7B1, the membranes must to be washed with Na2CO3 (Table 4). In addition, all of these enrichment procedures were still insufficient to quantify CYP11A1. The quantification of CYP11A1 was achieved using an additional enrichment technique called “whole gel elution”.
Table 4.
Quantification Using Whole Gel Elutiona
| Protein (pmol/mg tissue protein) | |||
|---|---|---|---|
| Protein | Total membrane pellet | Total membrane pellet after Na2CO3 washes |
Whole gel elution, fraction #8 |
| CYP27A1 | 0.18 ± 0.03 | 0.20 ± 0.03 | 0.20 ± 0.03 |
| CYP46A1 | 0.40 ± 0.07 | 0.41 ± 0.07 | 0.46 ± 0.08 |
| CYP7B1 | ND | 0.29 ± 0.05 | 0.26 ± 0.06 |
| CYP11A1 | ND | ND | 0.016 ± 0.005 |
The temporal lobe sample (patient ID 10337, Table S1) was used to generate total membrane pellet, total membrane pellet washed with 100 mM Na2CO3, and a whole gel elution fractions. The concentration was calculated for three transitions per individual peptide for two individual peptides per protein and presented as mean ± SD. The monitored transitions are summarized in the Supporting Information (Table S2). ND, not detected.
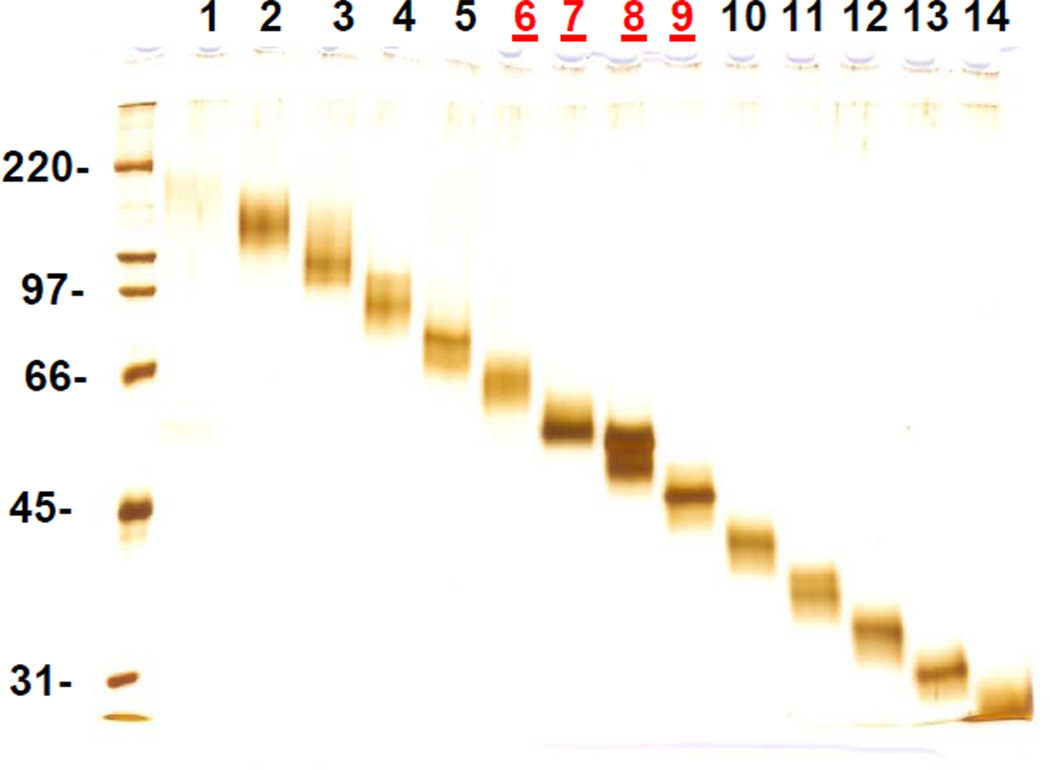
The Whole Gel Eluter is a preparative electrophoresis instrument which allows simultaneous electro-elution of multiple protein bands separated on a polyacrylamide gel into individual fractions. This procedure results in protein purification based on molecular mass and could be performed on protein samples containing SDS. We applied whole gel elution to the membrane pellet from human temporal lobe after the pellet was washed with 100 mM Na2CO3, supplemented with 15N-labeled CYPs 7B1, 11A1, 27A1, and 46A1 and solubilized with 1% SDS. The solubilized sample was separated into 14 fractions (Figure 1). The fractions 6, 7, 8, and 9 encompassing the expected molecular mass of CYPs were individually precipitated with chloroform/methanol. The precipitate was treated with trypsin and subjected to MRM analysis. As is seen from comparison of protein amounts in untreated and washed membrane pellets (Table 4), the whole gel elution does not introduce any quantitative error; the concentrations for CYPs 27A1, 46A1, and 7B1 remains similar to those determined without whole gel elution. At the same time, it enabled quantification of low-abundance CYP11A1 in the temporal lobe (Table 4). Thus, whole gel elution can be a useful technique for quantification of low-abundance proteins.
Figure 1.
Whole gel elution. Total membrane pellet from temporal lobe was first washed with 100 mM Na2CO3 and supplemented with 15N-labeled full-length protein standards. It was then separated into 14 fractions using Whole Gel Eluter. The SDS-PAGE for these fractions is shown. Molecular mass standards (in kDa) are shown on the left. Fractions # 6–9 (marked in red) encompass the molecular mass range expected for CYPs. The proteins in these fractions were precipitated with chloroform/methanol, treated with trypsin, and subjected to MRM analysis.
Quantification in Different Human Tissues
Table 5 shows summarized data for human brain, retina and RPE. In addition to membrane-bound CPR, FdR, CYP27A1, CYP7B1 and CYP46A1, we also quantified Fdx, a soluble protein directly interacting with mitochondrial CYPs. Current quantifications confirmed our previous measurements showing that in the eye (retina and RPE) CYP27A1 is more abundant than CYP46A1, whereas in the brain (temporal lobe) the more abundant protein is CYP46A1.3 Quantification of CYP7B1 was novel and demonstrated that CYP7B1 is expressed at intermediate between CYP27A1 and CYP46A1 level in the brain and higher than CYPs 27A1 and 46A1 level in the eye. Also novel were the measurements of the CYP redox partners, FdR, Fdx, and CPR, which along with the quantification of CYPs provided information on the redox partner ratios in different tissues.
Table 5.
Quantification of Different Cholesterol-Metabolizing CYPs and Their Redox Partners in the Human Temporal Lobe, Retina, and RPEa
| Protein (pmol/mg tissue protein) | ||||||
|---|---|---|---|---|---|---|
| Mitochondria | Microsomes | |||||
| Tissue | FdR | Fdx | CYP27A1 | CPR | CYP7B1 | CYP46A1 |
| Temporal lobe | 4.35 ± 0.87 | 1.40 ± 0.25 | 0.19 ± 0.05 | 3.24 ±0.62 | 0.29 ± 0.06 | 0.40 ± 0.08 |
| Retina | 1.35 ± 0.24 | 1.37 ± 0.23 | 0.50 ± 0.07 | 2.52 ± 0.40 | 0.90 ± 0.16 | 0.10 ± 0.02 |
| RPE | 2.29 ± 0.47 | 2.38 ± 0.48 | 1.24 ± 0.40 | 2.52 ± 0.54 | 1.79 ± 0.40 | ND |
Proteins are grouped as mitochondrial and microsomal. Measurements were performed on tissues obtained from four human donors (Table S1). The concentration was calculated for three experimental replicates by monitoring three transitions per individual peptide for two individual peptides per protein and presented as mean ± SD. The monitored transitions are summarized in the Supporting Information (Table S2). ND, not detected.
Biological Implications
About 140 single nucleotide polymorphisms and over 50 mutations have been currently identified in human CPR,15 however, it is not yet known how these genetic variants affect the CPR protein levels. Thus, in the future one can use our protocol and quantify CPR in humans especially when it will be necessary to assess the basis of the Antley-Bixler syndrome, a severe developmental disorder caused by the mutations in the CPR gene.16
Perhaps the most significant was the measurements of CYP7B1. In the brain, CYP7B1 is suggested to inactivate two neurosteroids, pregnenolone and dehydroepiandrosterone,17 and also metabolize 27-hydroxycholesterol.18 However, the amounts of CYP11A1 (Table 4) and its product pregnenolone19 are low in the brain suggesting that the proposed inactivation of pregnenolone may not be the most important function of CYP7B1 in this organ. In contrast, the metabolism of 27-hydroxycholesterol could be of importance.18 Indeed, cerebral expression of CYP27A1 is comparable to that of CYP7B1, yet the level of 27-hydroxycholesterol is very low and comparable to the level of pregnenolone.19 Low level of 27-hydroxycholesterol suggests its efficient local metabolism,18 and is in agreement with abundant CYP7B1 expression in the temporal lobe.
CYP7B1 is also more abundant than CYP27A1 in the retina and RPE (Table 4). Like in the brain, the level of 27-hydroxycholesterol is low in these tissues.19 Yet, in contrast to the brain, the retina and RPE metabolize 27-hydrohycholesterol to 5-cholestenoic acid19, likely via the action of CYP27A1.20 Further studies are required to identify endogenous substrates for CYP7B1 in the retina and RPE and the role of this CYP in the eye.
Overall, CYP7B1 is an important human enzyme whose deficiency leads to liver failure in newborns and progressive neuropathy in children and adults.21, 22 The availability of an analytical tool to precisely quantify CYP7B1 in different tissues will facilitate unraveling the physiological roles of this CYP.
CONCLUSIONS
We continue to demonstrate the benefits of isotope-labeled full-length proteins as internal standards in MRM quantification of membrane proteins. We show that when internal standards are added at the very beginning of sample processing, the analyte to standard ratio is preserved during subsequent sample enrichment steps, thus enabling quantification of low-abundance proteins. We also developed enrichment protocols for weakly- and tightly-associated membrane proteins and applied these protocols to quantification of a group of important enzymes involved in cholesterol elimination from different tissues. Our measurements on samples from human donors provide further insights into cholesterol maintenance in different organs and the role of CYP enzymes in this process. The developed workflow for sample processing can be used for the analysis of other membrane proteins.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. P.F. Guengerich (Vanderbilt University, Nashville, TN) for expression construct for human CPR; Dr. R.C. Tuckey (the University of Western Australia, Crawley, WA, Australia) for expression constructs for human CYP11A1, FdR and Fdx, and Dr. D.W. Russell (UT Southwestern, Dallas, TX) for cDNA for human CYP7B1. This work was supported in part by grant from the National Institutes of Health (EY018383 to I.A.P.). I.A.P is also a recipient of the Jules and Doris Stein Professorship from the Research to Prevent Blindness Foundation.
Certain commercial materials, instruments, and equipment are identified in this manuscript in order to specify the experimental procedure as completely as possible. In no case does such identification imply a recommendation or endorsement by the National Institute of Standards and Technology nor does it imply that the materials, instruments, or equipment identified are necessarily the best available for the purpose.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Lu B, McClatchy DB, Kim JY, Yates JR., III Proteomics. 2008;8:3947–3955. doi: 10.1002/pmic.200800120. [DOI] [PubMed] [Google Scholar]
- 2.Liao WL, Heo GY, Dodder NG, Pikuleva IA, Turko IV. Anal. Chem. 2010;82:5760–5767. doi: 10.1021/ac100811x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao WL, Heo GY, Dodder NG, Reem RE, Mast N, Huang S, DiPatre PL, Turko IV, Pikuleva IA. J. Proteome Res. 2011;10:241–248. doi: 10.1021/pr1008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu KH, Barry CG, Austin D, Busch CM, Sangar V, Rustgi AK, Blair IA. J. Proteome Res. 2009;8:1565–1576. doi: 10.1021/pr800904z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rangiah K, Tippornwong M, Sangar V, Austin D, Tetreault MP, Rustgi AK, Blair IA, Yu KH. J. Proteome Res. 2009;8:5153–5164. doi: 10.1021/pr900518v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janecki DJ, Bemis KG, Tegeler TJ, Sanghani PC, Zhai L, Hurley TD, Bosron WF, Wang M. Anal. Biochem. 2007;369:18–26. doi: 10.1016/j.ab.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Brun V, Dupuis A, Adrait A, Marcellin M, Thomas D, Court M, Vandenesch F, Garin J. Mol. Cell. Proteomics. 2007;6:2139–2149. doi: 10.1074/mcp.M700163-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.von Wachenfeldt C, Johnson EF. In: Cytochrome P450: Structure, Mechanism, and Biochemistry. Ortiz de Montellano PR, editor. New York: Plenum Press; 1995. pp. 183–244. [Google Scholar]
- 9.Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ. Proc. Natl. Acad. Sci. USA. 1997;94:8411–8416. doi: 10.1073/pnas.94.16.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler GA, Vonrhein C, Hanukoglu I, Schulz GE. J. Mol. Biol. 1999;289:981–990. doi: 10.1006/jmbi.1999.2807. [DOI] [PubMed] [Google Scholar]
- 11.Mast N, Norcross R, Andersson U, Shou M, Nakayama K, Bjorkhem I, Pikuleva IA. Biochemistry. 2003;42:14284–14292. doi: 10.1021/bi035512f. [DOI] [PubMed] [Google Scholar]
- 12.Sagara Y, Wada A, Takata Y, Waterman MR, Sekimizu K, Horiuchi T. Biol. Pharm. Bull. 1993;16:627–630. doi: 10.1248/bpb.16.627. [DOI] [PubMed] [Google Scholar]
- 13.Liao WL, Turko IV. Anal. Biochem. 2008;377:55–61. doi: 10.1016/j.ab.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Fujiki Y, Hubbard AL, Fowles S, Lazarow PB. J. Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller WL, Agrawal V, Sandee D, Tee MK, Huang N, Choi JH, Morrissey K, Giacomini KM. Mol. Cell. Endocrinol. 2010;336:174–179. doi: 10.1016/j.mce.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, van Vliet G, Sack J, Fluck CE, Miller WL. Am. J. Hum. Genet. 2005;76:729–749. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiles AR, McDonald JG, Bauman DR, Russell DW. J. Biol. Chem. 2009;284:28485–28489. doi: 10.1074/jbc.R109.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meaney S, Heverin M, Panzenboeck U, Ekstrom L, Axelsson M, Andersson U, Diczfalusy U, Pikuleva I, Wahren J, Sattler W, Bjorkhem I. J. Lipid Res. 2007;48:944–951. doi: 10.1194/jlr.M600529-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Mast N, Reem R, Bederman I, Huang S, DiPatre PL, Bjorkhem I, Pikuleva IA. Invest. Ophthalmol. Vis. Sci. 2011;52:594–603. doi: 10.1167/iovs.10-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pikuleva IA, Babiker A, Waterman MR, Bjorkhem I. J. Biol. Chem. 1998;273:18153–18160. doi: 10.1074/jbc.273.29.18153. [DOI] [PubMed] [Google Scholar]
- 21.Setchell KD, Schwarz M, O'Connell NC, Lund EG, Davis DL, Lathe R, Thompson HR, Weslie Tyson R, Sokol RJ, Russell DW. J. Clin. Invest. 1998;102:1690–1703. doi: 10.1172/JCI2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsaousidou MK, Ouahchi K, Warner TT, Yang Y, Simpson MA, Laing NG, Wilkinson PA, Madrid RE, Patel H, Hentati F, Patton MA, Hentati A, Lamont PJ, Siddique T, Crosby AH. Am. J. Hum. Genet. 2008;82:510–515. doi: 10.1016/j.ajhg.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.