Abstract
α-Ketoglutaramate (KGM) is the α-keto acid analogue of glutamine, which exists mostly in equilibrium with a lactam form (2-hydroxy-5-oxoproline) under physiological conditions. KGM was identified in human urine and its concentration quantified by gas chromatography/mass spectrometry (GC/MS). The keto acid was shown to be markedly elevated in urine obtained from patients with primary hyperammonemia due to an inherited metabolic defect in any one of the five enzymes of the urea cycle. Increased urinary KGM was also noted in other patients with primary hyperammonemia, including three patients with a defect resulting in lysinuric protein intolerance and one of two patients with a defect in the ornithine transporter I. These findings indicate disturbances in nitrogen metabolism, most probably at the level of glutamine metabolism in primary hyperammonemia diseases. Urinary KGM levels, however, were not well correlated with secondary hyperammonemia in patients with propionic acidemia or methylmalonic acidemia, possibly as a result, in part, of decreased glutamine levels. In conclusion, the GC/MS procedure has the required lower limit of quantification for analysis of urinary KGM, which is markedly increased in urea cycle disorders and other primary hyperammonemic diseases.
Keywords: α-Ketoglutaramate, 2-hydroxy-5-oxoproline, GC/MS analysis of urine, Primary hyperammonemia, Secondary hyperammonemia, Urea cycle disorders
Introduction
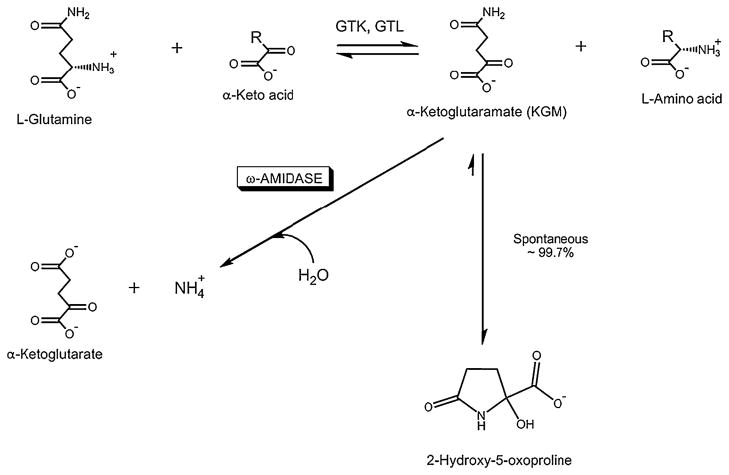
A major route for glutamine metabolism is via the well-known glutaminase reaction, which results in the hydrolysis of glutamine to glutamate and ammonia. However, tissues contain another, albeit less well studied, pathway by which ammonia is generated from the amide position of glutamine. This pathway, sometimes referred to as the glutaminase II pathway, consists of a glutamine transaminase (E.C. 2.6.1.64) that catalyzes transamination of glutamine to α-ketoglutaramate (KGM; 2-oxoglutaramate; Eq. 1) coupled to an ω-amidase (ω-dicarboxylate amidohydrolase, E.C. 3.5.1.3) that catalyzes the hydrolysis of KGM to α-ketoglutarate (2-oxoglutarate) and ammonia (Eq. 2) [1–10]. The net reaction is shown in Eq. (3). The glutaminase II pathway is shown in Fig. 1. Two forms of glutamine transaminase have been identified, namely a liver type (GTL) and a kidney type (GTK; also known as kynurenine aminotransferase I, KAT I) [4–6]. In the rat, the highest specific activity of GTK is in the kidney, but appreciable activity is also present in other organs, especially the liver. GTK activity is most prominent in the cytosolic fractions of kidney and liver, but the activity is also present in mitochondria [5]. In the rat, the highest specific activity of GTL is in the liver, with smaller amounts in other tissues. ω-Amidase is present in all rat tissues studied, but is especially prominent in both the cytosolic and mitochondrial fractions of rat kidney and liver [4, 5, 7].
Fig. 1.
The glutaminase II pathway. Glutamine transaminases catalyze a freely reversible transamination reaction between L-glutamine and a suitable α-keto acid substrate to generate the α-keto acid analogue of L-glutamine (α-ketoglutaramate; KGM) and an L-amino acid. Two glutamine transaminases have been described, namely glutamine transaminase K (GTK) and glutamine transaminase L (GTL). Both glutamine transaminases have a broad specificity toward α-keto acids. However, an especially prominent α-keto acid substrate for both enzymes is α-keto-γ-methiolbutyrate, an important component of the methionine salvage pathway [5, 6, 8]. Note that although the glutamine transaminase reaction is freely reversible, the reaction is drawn in the direction of glutamine utilization by the cyclization of the product KGM to a lactam (2-hydroxy-5-oxoproline) and by hydrolysis of KGM to α-ketoglutarate. Under physiological conditions ~99.7% of KGM is in the cyclized (lactam) form and only 0.3% is in the open-chain form (i.e., the substrate form of ω-amidase) [10]. Modified from [8]
| (1) |
| (2) |
| (3) |
Despite the widespread occurrence of glutamine transaminases and ω-amidase in mammalian tissues and the inherently high activity of ω-amidase, there has been some uncertainty as to the relative contribution of the glutaminase II pathway to the production of ammonia from the amide position of glutamine. For example, Nissim et al. [11] suggested that the glutaminase II pathway is not a significant source of ammonia in human kidney cells in culture. Nevertheless, glutamine transamination must occur in vivo because KGM occurs normally at micromolar concentrations in rat tissues, including liver, brain and kidney [12], rat cerebrospinal fluid (CSF) [13], and human CSF [12–14]. The presence of glutamine transamination has not been well studied in human tissues, but the glutaminase II pathway is known to be present in human brain [15], and GTK is known to be present in human kidney [16]. Moreover, mass balance studies using L-[15N]glutamate, L-[2-15N]glutamine, and L-[5-15N]glutamine infused intravenously into adult volunteers showed that glutamine transamination is an active pathway in humans in vivo [17].
KGM concentrations in CSF were previously found to be markedly increased in encephalopathic patients with hyperammonemia associated with liver disease [13, 14]. Based on (a) the finding that CSF KGM is increased in hyperammonemic patients with liver disease, (b) glutamine transaminase activity is present in human tissues, and (c) CSF is an ultrafiltrate of plasma produced by the choroid plexus, we hypothesized that KGM may be systemically elevated in other hyperammonemic diseases. In the present pilot study, we show that KGM can readily be detected in human urine by a GC/MS technique, and that urinary KGM is markedly increased in defects of the urea cycle and in other primary hyperammonemic diseases.
Patients and methods
Study patients
Urine specimens from patients with inborn errors of metabolism resulting in primary or secondary hyperammonemic syndromes were collected by doctors in clinics and hospitals from many parts of Japan. The urine specimens were immediately frozen, sent on dry ice to the Department of Biochemistry, Division of Human Genetics, Medical Research Institute, Kanazawa Medical University, and stored at −20 °C until analyzed by GC/MS-based metabolomics. Among the patient population studied here, causes of primary hyperammonemia included: carbamoylphosphate synthetase-I (CPS-I) deficiency (OMIM 237300), ornithine transcarbamylase (OTC) deficiency (OMIM 311250), argininosuccinate synthetase (ASS) deficiency (OMIM 603470), argininosuccinate lyase (ASL) deficiency (OMIM 608310), arginase deficiency (OMIM 207800), lysinuric protein intolerance (LPI; OMIM 222700), and hyperornithinemia–hyperammonemia–hyperhomocitrullin-uria syndrome (HHH; OMIM 238970). Causes of secondary hyperammonemia included: propionyl CoA carboxylase deficiency (OMIM 606054) and methylmalonyl-CoA mutase deficiency (OMIM 251000). Control urine specimens were collected during pilot studies of newborn screening procedures. In other cases, urine specimens were from volunteers. Blood ammonia levels were measured at clinics and hospitals from where the urine samples were sent. Normal blood ammonia levels obtained in the Clinical Laboratory of Kanazawa Medical University Hospital are <40 μM.
Precise collection time differences between blood drawn for determination of ammonia and urine drawn for metabolomic analysis were not obtained, but the blood and urine specimens were obtained on the same day, usually within a few hours. Among the patients with urea cycle defects studied in the present work, no patients at the time of sampling were being treated with phenylbutyrate or phenylacetate as a means of increasing glutamine excretion. No patients in this group were described as being under continuous protein restriction (except where noted), but the patients were, however, on low protein intake prior to urine sampling. Patient CPS4 was on sodium benzoate and exchange transfusion.
Standards and reagents
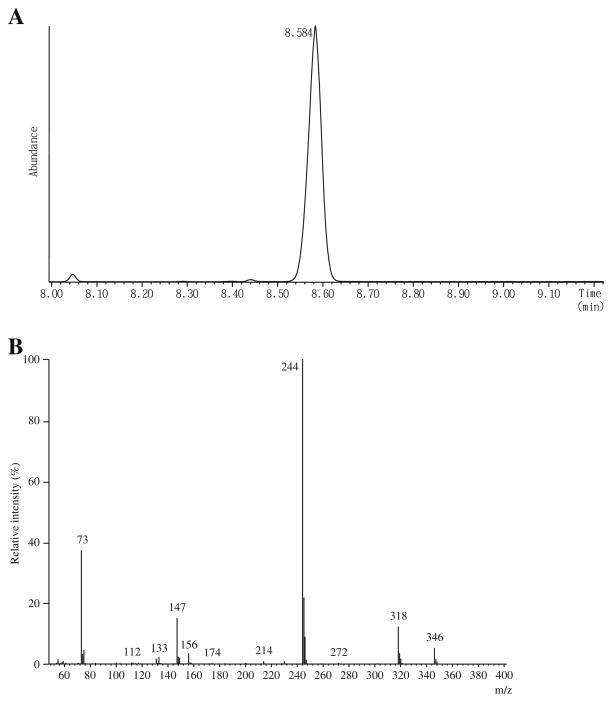
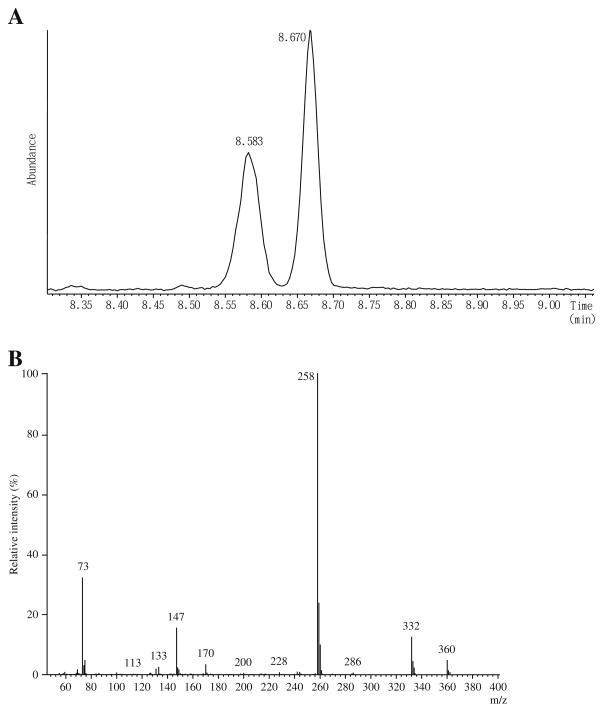
[2H3]Creatinine was purchased from ISOTEC INC., Miamisburg, Ohio, USA. [15N2]Orotate was obtained from Cambridge Isotope Laboratory, Andover, MA, USA. 2-Hydroxyundecanoate (2HC11) was obtained from Wako Pure Chemical Industries, Ltd, Osaka, Japan, and N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) containing 1% trimethylchlorosilane (TMCS) was purchased from Thermo Scientific, Rockford, IL, USA. Urease type-C3 was purchased from Sigma Chemical Co. St. Louis, MO, USA. A standard solution of KGM was made by oxidizing L-glutamine with snake venom L-amino acid oxidase in the presence of catalase by the method of Meister [2] as modified by Krasnikov et al. [18]. Authentic KGM standards in 10 mM potassium phosphate buffer (pH 7.2) are stable for at least 6 months when stored at −20 °C. The solid barium salt of L-γ-methyl KGM (M-KGM; internal standard, IS) was made by the method of Meister et al. [3]. Both compounds were >98% pure as judged by GC/MS analysis (Figs. 2A and 3A). An M+ ion was not detected, but an ion with an m/z value of 346 (M+-15) corresponding to a loss of CH3 from the tris-trimethylsilyl (TMS) derivative of KGM was detected. The major ion detected has an m/z value of 244 (M+-117), ascribed to loss of CO2Si(CH3)3 from the tris-TMS compound.
Fig. 2.
GC/MS of KGM. A Elution profile of the TMS derivative of KGM analyzed according to the method of Kuhara [20]. The small peak at 8.05 min is due to 5-oxoproline, resulting from cyclization of glutamine during the preparation of KGM from glutamine. The y axis represents the TIC. B The mass spectrum of the TMS derivative of KGM. The spectrum is fully consistent with a tris-TMS derivative of KGM lactam (see the text)
Fig. 3.
GC/MS of M-KGM (internal standard). A Elution profile of the tris-TMS derivative of M-KGM analyzed according to the method of Kuhara [21]. Note the formation of a pair of diastereoisomers resulting from cyclization of the α-keto acid to a lactam. The y axis represents the TIC. B The mass spectrum of the TMS derivative of KGM. The spectrum is fully consistent with a tris-TMS derivative of KGM lactam (see the text)
Analyses of KGM in urine samples
The urine samples (100 μl; or 50 μl plus 50 μl water) were spiked with the IS (M-KGM; 10 nmol dissolved in 50 μl of water) and treated with urease to remove interfering urea by a procedure [19] extensively modified from that of Shoemaker and Elliott [20]. Protein was precipitated with ethanol and removed by centrifugation. The deproteinized solution was then evaporated to dryness. Compounds of interest in the dried residue were converted to TMS derivatives with 100 μl of BSTFA/TMCS (10:1) and analyzed by GC/MS as described previously [21]. The tris-TMS derivative of KGM (KGM-3TMS) elutes as a single peak at 8.58 min (Fig. 2A). M-KGM is a mixture of two diastereoisomers (see the “Results and discussion” section). The tris-TMS derivatives of the two M-KGM diastereoisomers (M-KGM-3TMS) elute at 8.58 and 8.67 min (Fig. 3A). The ratio of the total ion current (TIC) of the earlier eluting peak to the later eluting peak was about 0.88. For quantitation, ions at m/z 244 and m/z 258, corresponding to [M+-COOTMS] were selected for KGM and the two diastereoisomers of M-KGM, respectively. In a separate experiment, a standard curve was constructed by adding 10 nmol of M-KGM to 100-μl aliquots of a control urine sample containing varying amounts of added KGM (0.00, 0.53, 2.12, 5.3, 10.6, and 21.2 nmol; two determinations in which n=3 for each case, for a total n=6), followed by GC/MS analysis. The intensity of the fragment ion at m/z 244 increased linearly over this range relative to the intensity of the fragment ion at m/z 258 (R2=0.998) according to the equation y=21.1x+0.885, where y is the concentration of KGM and x is the ratio of the intensity of the m/z ion fragment at 244 divided by the intensity of the ion fragment at m/z 258. The intersection value on the y axis is the endogenous level of KGM in the urine sample (0.885 nmol/100 μl of urine sample). The lower limit of quantitation for KGM in human urine is ≤5.3 nmol/ml. The concentrations of creatinine and creatine in urine specimens were determined by means of a Beckman CX5 autoanalyzer and the relative ratio of creatinine-to-creatine in urine was obtained. The level of KGM in urine is reported relative to that of creatinine and expressed as mmol KGM/mol creatinine. However, because creatine in urine is converted to creatinine during sample preparation, creatinine determined by GC/MS using D3-creatinine as an IS (retention time of creatinine-3TMS, 8.3 min) is actually the sum of creatinine and creatine. To correct for this conversion, creatinine, and creatine values obtained in a separate determination by the autoanalyzer technique were used in the calculation of mmol KGM/mol creatinine. Urinary orotate was determined by stable isotope dilution as described [22].
Statistical analyses
Data are presented as the mean±S.D. The one-tailed unpaired t test was used to compare urinary KGM levels among different age groups (GraphPad software). The one-tailed Mann–Whitney U test was used to compare urinary KGM levels in hyperammonemic patients to those of controls. A value of p≤0.05 is considered significant. Median and quartile values were calculated using MS Excel.
Results and discussion
At neutral pH, underivatized KGM exists overwhelmingly (~99.7%) as the lactam, 2-hydroxy-5-oxoproline (synonyms: 5-hydroxypyroglutamate; 2-pyrrolidone-5-hydroxy-5-carboxylate) [2, 10]. The mass spectrum of the TMS derivative of KGM (KGM-3TMS; Fig. 2B) is consistent with a lactam structure, and is remarkably similar to that published previously by Cooper et al. [23] in which KGM-TMS was analyzed by electron impact mass spectrometry. Later work by Nissim et al. [11] showed the t-butyldimethylsilyl derivative of KGM is a lactam. The mass spectrum of M-KGM-3TMS (Fig. 3B) is also consistent with a lactam (4-methyl-2-hydroxy-5-oxoproline) configuration. Ions observed at m/z values of 360 and 258 for M-KGM-3TMS are ascribed to M+-CH3 and M+-CO2Si(CH3)3, respectively. Note that during cyclization of M-KGM, nucleophilic attack of the amide nitrogen at the α carbon can occur from either side, resulting in a D,L configuration at the α carbon. Moreover, the γ carbon in the lactam is asymmetric (L configuration). Therefore, the lactam derived from M-KGM exists in solution as a pair of diastereoisomers (with L,L and L,D configurations).
In earlier work, 2HC11 was used as IS (retention time, 9.45 min) for analysis of several urinary metabolites [24]. When values for urinary KGM were calculated post hoc from preexisting data sets, in which 2HC11 was used as an IS, and compared to new data sets using the same urine samples and M-KGM as IS, the results were comparable (n= 67, two-tailed paired t test, p=0.22; linear regression analysis, R2=0.85). This finding indicates that KGM is stable in the urine samples during storage in solution at −20 °C. Since M-KGM has a structure very similar to KGM but not to 2HC11, M-KGM was deemed to be a more appropriate IS than 2HC11. Note also that one diastereoisomer of M-KGM has the exact retention time as KGM (compare Figs. 2A and 3A). This overlap is not a problem as there is no possible contribution of an ion with m/z value of 258 originating from KGM or of an ion with an m/z value of 244 originating from M-KGM (compare Figs. 2B and 3B). Thus, M-KGM was used as an IS in all the data reported in the present work.
Levels of KGM relative to creatinine in urine from control subjects are shown in Table 1. There is no significant difference (unpaired t test) among KGM values obtained from control age groups 1 (4–28 days), 2 (1–12 months), and 3 (1–3 years, 11 months; Table 1). Despite a few gaps in age in the series of controls, the data in Table 1 clearly show a significant decline in the urinary KGM/creatinine ratio with age after age 3 into early adulthood. The average and median urinary KGM values in group 4 (4 years, 10 months–9 years, 11 months) tended to be lower than that of combined groups 1–3 (p=0.1). However, the mean and median levels of urinary KGM in group 5 (11 years, 1 month–16 years, 10 months), and group 6 (20 years, 3 months–29 years, 3 months) were highly significantly different from the values obtained for the combined group 1–3 (Table 1).
Table 1.
Baseline levels of urinary KGM (millimole per mole creatinine)
| Group | Age range | KGM | Median | Lower quartile | Upper quartile | n |
|---|---|---|---|---|---|---|
| 1 | 4–28 days | 2.26±1.06 (1.12–4.60)a | 2.42 | 2.15 | 3.12 | 10 |
| 2 | 1–12 months | 2.42±1.09 (1.17–4.20) | 2.47 | 1.26 | 3.48 | 12 |
| 3 | 1 year–3 years 11 months | 2.56±1.40 (0.71–5.63) | 2.34 | 1.71 | 3.08 | 13 |
| 1–3 combined | 4 days–3 years 11 months | 2.54±1.18 (0.71–5.63) | 2.36 | 1.59 | 3.20 | 35 |
| 4 | 4 years 10 months–9 year 11 months | 2.09±0.56 (1.22–2.99) | 1.91 | 1.49 | 2.48 | 12 |
| 5 | 11 years 1 month–15 years 10 months | 1.30±0.52* (0.53–2.25) | 1.20 | 0.97 | 1.69 | 9 |
| 6 | 20 years 3 months–29 years 3 months | 0.80±0.18** (0.58–1.25) | 0.78 | 0.69 | 0.79 | 10 |
p=0.0019 versus combined groups 1–3
p=0.00005 versus combined groups 1–3
Values in parenthesis represent the range
Urinary KGM levels in patients with primary hyperammonemia are shown in Table 2. In the OTC patients, the blood ammonia levels on the day of urine sampling ranged from moderately elevated to very high (Table 2). Of note is the highly significant increase in urinary KGM (n=5) relative to controls (i.e., combined groups 1–3; Table 1; p=1.5×10−6). In one patient (OTC3) with very high blood ammonia levels (155 μM), the millimole KGM per mole creatinine and millimole orotate per mole creatinine ratios in urine were very high — 13 and 910, respectively (Table 2). However, it is notable that when prophylactic treatment was initiated in this patient so that blood ammonia levels were much lower (65 μM), the millimole KGM per mole creatinine and millimole orotate per mole creatinine ratios in urine also were much lower — 4.6 and 6.0, respectively (Table 2). In another OTC patient (OTC5), the mole KGM per mole creatinine ratio was very high (35.4) when the blood ammonia concentration was high (100 μM; Table 2). When prophylactic treatment lowered the blood ammonia level to almost normal (41 μM) the concentration of millimole KGM per mole creatinine ratio was also much lower (13.0). Interestingly, however, in patient OTC5, the ratio of millimole orotate per mole creatinine did not improve as the blood ammonia level improved. In a 13-year-old child (OTC6; data not shown) with mild OTC deficiency whose symptoms and hyperammonemia were controlled by a low protein diet, the urinary KGM level (1.91 mmol/mol creatinine) was within normal range for his/her age group (0.53–2.25 mmol KGM/mol; Table 1, group 5).
Table 2.
Urinary KGM (millimole per mole creatinine), urinary orotate (millimole per mole creatinine) and blood ammonia levels in patients with primary hyperammonemia
| Disease (Patient ID) | Age | KGM | Orotatea | Blood NH3 (μM)b |
|---|---|---|---|---|
| Ornithine transcarbamylase (OTC) deficiency* | ||||
| OTC1 | 7 days | 47.6 | 1530 | 530 |
| OTC2 | 1 year 2 months | 42.8 | 638 | 302 |
| OTC3 | 1 year 6 months | 13.0 | 910 | 155 |
| OTC3c | 1 year 6 months | 4.6 | 6.0 | 65 |
| OTC4 | 2 years | 11.5 | 358 | 120–180 |
| OTC5 | 3.15 years | 35.4 | 334 | 100 |
| OTC5 | 3.2 years | 13.0 | 428 | 41 |
| Carbamyl phosphate synthetase-I (CPS-I) deficiency** | ||||
| CPS1 | 2 days | 9.9 | 3.9 | 568 |
| CPS2 | 4 days | 11.2 | 3.1 | 588 |
| CPS2 | 5 days | 2.1 | 3.0 | –d |
| CPS3 | 6 days | 3.7 | 0.1 | very high, died day 6 |
| CPS4 | 5 days | 13.0 | 5.0 | 410 (maximum) |
| Argininosuccinate synthase (ASS) deficiency | ||||
| ASS1 | 9 days | 6.96 | 1627 | –d |
| ASS2 | 1 year | 34.3 | 1950 | 304 |
| Argininosuccinate lyase (ASL) deficiency | ||||
| ASL1 | 10 months | 12.2 | 55 | 126 |
| Arginase deficiency | ||||
| ARG1 | 1.5 months | 42.7 | 1370 | 1160 |
| Ornithine transporter I (ORNT-I) deficiency (HHH) | ||||
| ORNT1 | 11 years | 1.81 | 62 | –d |
| ORNT2 | 42 years | 3.88 | 6.2 | –d |
| Lysinuric protein intolerance (LPI) | ||||
| LPI1 | 2 years | 8.12 | 286 | >40 |
| LPI2 | 4.10 years | 22.8 | 6.0 | 89 |
| LPI2 | 4.13 years | 6.52 | 6.7 | –d |
p=1.5×10−6 for KGM values in this group (n=5) versus control values (Table 1; groups 1–3; n=35; for the calculation of the p value the average of the two values for patients OTC3 and OTC5 was used)
p=0.00022 for KGM values in this group (n=4) versus the control values (Table 1; groups 1–3; n=35; for the calculation of the p value the average of the two values for patient CPS2 was used). Acidosis was severe in patients CPS1 and CPS2 on day 4, but was not present in patient CPS4. Patient CPS4 was treated with sodium benzoate and exchange transfusion. Although the exchange transfusion has the possibility of affecting urinary KGM and creatinine levels, the patient CPS4 was included in the patient group because the blood ammonia levels were still very high.
Normal reference values for orotate are: upper limit (average+2SD) for ages <1 month, 1–12 months, 1–4 years, and >4 years are 4.8, 5.2, 6.98, and 4.37 mmol/mol creatinine, respectively
Blood ammonia levels were determined at the clinic or hospital where the patient was being treated. Normal blood ammonia concentrations from the Clinical Laboratory of Kanazawa Medical University Hospital are<40 μM
The blood and urine chemistries were obtained after IV treatment with argimate administration and rectal infusion of lactulose had begun (argimate is a mixture of arginine and glutamate used to treat hyperammonemic patients in Japan)
Not measured
All four patients with CPS-I deficiency had very high blood ammonia levels and patients CPS1, CPS4, and (initially) CPS2 had correspondingly high levels of urinary KGM (Table 2). In patient CPS2, treatment was initiated on day 4 and by day 5 urinary KGM was normal. Patient CPS3 died shortly after a urine specimen was obtained. The level of urinary KGM in this patient was high normal, but may have been affected by the agonal state. Overall, however, there is a highly significant increase in urinary KGM in children with CPS-I deficiency compared to age-matched controls (p=0.00022). Of interest is the finding that in patients CPS1 and CPS2, the Z score for urinary glutamine was +5, whereas that for citrate was −2.
A defect in ASS results in classical hypercitrullinemia (i.e., type 1). High concentrations of urinary KGM in two children with this deficiency were noted (Table 2). High levels of urinary KGM were also found in a patient with ASL deficiency and in a patient with arginase deficiency (Table 2).
When data in Table 2 for urea cycle disorders are combined (n=12) and compared to age-matched controls (n=35; groups 1–3 in Table 1), the probability that elevated urinary KGM is a marker for primary hyperammonemia associated with defects of enzymes of the urea cycle is highly significant (p<10−6).
A defect in the ornithine transporter I (ORNT-I) gives rise to the HHH phenotype. The urinary KGM level was high normal in patient ORNT1 (1.81 mmol KGM/mol creatinine) relative to the control group (0.53–2.25 mmol/mol creatinine; Table 1, group 5), whereas the level of urinary KGM (3.88 mmol KGM/mol creatinine) in patient ORNT2 was considerably outside the range of the control group (0.58–1.25 mmol KGM/mol creatinine; Table 1, group 6), but no blood ammonia levels were available for the two patients (Table 2). It is interesting, however, that the Z score for urinary glutamine in patient ORNT1 was −2, whereas Z scores for urinary citrate, glutamate and 5-oxoproline were 0.5, 3.8, and 2.7, respectively. Three patients with LPI syndrome had high urinary KGM levels (Table 2). In two of these patients, the blood ammonia level was high, but blood ammonia was not measured in a third patient.
Urinary KGM levels in several patients with inborn errors that can result in secondary hyperammonemia are shown in Table 3. A patient with a mild form of propionic acidemia (PCCD2) had normal urinary KGM and normal blood ammonia levels. However, a patient with severe propionic acidemia (PCCD1) had greatly elevated blood ammonia, but normal to high normal KGM. Two patients with methylmalonic acidemia appear to have normal or high normal urinary KGM despite the moderately elevated blood ammonia concentrations (Table 3). One possible explanation for the relatively low urinary KGM levels is that excess propionyl CoA interferes with acetyl CoA metabolism and hence decreases the availability of 5-carbon units for glutamine/KGM synthesis. In this regard, Filipowicz et al. [25] noted that plasma glutamine levels are decreased in patients with propionic acidemia, and that there is an inverse relationship between plasma ammonia levels and plasma glutamine levels. These authors also noted an increased excretion of methylcitrate and decreased excretion of citrate. We found that the Z score of urinary glutamine in patients PCCD1 and PCCD2 was −2. In patient PCCD1, the Z scores for citrate and α-ketoglutarate were −3 and −2, respectively, consistent with a major block in the tricarboxylic acid (TCA) cycle and therefore of diminished 5-carbon units for glutamine and KGM formation.
Table 3.
Urinary KGM (mmol/mol creatinine) and blood ammonia levels in patients with inborn errors of metabolism resulting in secondary hyperammonemia
| Patient ID | Defect | Age | KGM | Blood NH3 (μM) |
|---|---|---|---|---|
| PCCD1 | Propionic acidemia (severe) | 3 days | 2.99 | 206 |
| PCCD1 | Propionic acidemia | 42 days | 4.00 | >235 |
| PCCD2 | Propionic acidemia(mild) | 6 days | 1.48 | <40 |
| MMA1 | Methylmalonic acidemia | 1 year 4 months | 4.19 | 63 |
| MMA1 | Methylmalonic acidemia | 1 year 5 months | 2.75 | 53 |
| MMA2 | Methylmalonic acidemia | 1 year 1 month | 2.59 | <40 |
Urinary orotate is a sensitive biomarker for defects of carbamoylphosphate utilization [26, 27], but not for defects of carbamoylphosphate synthesis [24]. The data shown in Table 2 are consistent with this conclusion. This table shows orotate values in the same urine specimens in which urinary KGM was measured. In all five untreated patients with OTC deficiency reported in Table 2, as expected, there was a very high level of urinary orotate relative to age-matched control values. However, the orotate level in patient OTC3 was normal after treatment was begun, and the levels of urinary KGM and blood ammonia were markedly lower (Table 2). Urinary orotate was not increased in any of the four patients with CPS-I deficiency, whereas urinary KGM increased significantly in three out of the four cases (Table 2). The one exception, as noted above, is to a patient (CPS3) who died shortly after a urine sample was collected. The Z score of urinary glutamine in the same urine specimen from CPS3 was 0 suggesting that glutamine was not increased in this patient. In patient ORNT1, the concentration of orotate was high (62 mmol/mol creatinine) even when that of KGM was within the normal range, suggesting that orotate is a more sensitive indicator of a defect in carbamoylphosphate utilization in this patient. On balance, however, increased urinary KGM is an excellent indicator for primary hyperammonemic diseases and in at least one disorder (i.e., CPS-I deficiency) studied here is a more sensitive indicator of hyperammonemia than is orotate.
In summary, the data in Table 2 show that increased urinary KGM is an excellent indicator of primary hyperammonemia resulting from defects of the urea cycle. Increased urinary KGM also appears to be a good indicator for other diseases that give rise to primary hyperammonemia, such as HHH and LPI. However, because the number of cases analyzed was low (2 and 3, respectively) and blood ammonia levels were not always available more work is needed to verify this conclusion. For propionic acidemia and methylmalonic acidemia, the relationship between urinary KGM levels and hyperammonemia is weak.
The National Institutes of Health (NIH) definition of a biomarker is “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”. We show here that elevated urinary KGM is an indicator of a pathogenic process (i.e., hyperammonemia). Thus, according to the NIH definition, KGM is a biomarker for hyperammonemia associated with urea cycle disorders and probably for most other diseases resulting in hyperammonemia, provided that the TCA cycle is not severely compromised.
The mechanisms by which increased ammonia leads to increased urinary excretion of KGM are unknown. However, one key factor is likely to be increased glutamine production in at least some of the hyperammonemic diseases including inborn errors of the urea cycle. Glutamine synthetase activity is widespread in mammalian tissues [28]. As a consequence, in patients with inborn errors of the urea cycle, the associated hyperammonemia results in increased systemic glutamine synthesis and hyperglutaminemia (e.g., [29]). Therefore, the increased urinary KGM in the patients with inborn errors of the urea cycle may, in part, be a reflection of a general increase in whole body (including presumably the kidney) glutamine synthesis. In this regard, we have noted that urinary glutamine is also generally increased in patients with inborn errors of the urea cycle (data not shown).
Conclusion
Despite the fact that increased KGM is a biomarker for urea cycle disorders and probably for most other hyperammonemic diseases its clinical usefulness at the present time is somewhat limited. A major problem is that KGM is not available commercially. Moreover, since no urine samples were analyzed from patients with illnesses other than those with hyperammonemic diseases, it is not possible to judge the specificity and discriminatory power of increased urinary KGM at the present time as a biomarker solely of hyperammonemic syndromes. Nevertheless, the present article is aimed in part to alert the clinical community to the new and interesting finding that urinary KGM is a good biomarker for most hyperammonemic diseases provided that the TCA cycle is not severely compromised. An understanding of mechanisms contributing to increased urinary KGM production in hyperammonemic diseases may lead to an increased understanding of deranged nitrogen metabolism in these diseases and possibly suggest new effective therapies.
Acknowledgments
This work was supported in part by Kanazawa Medical University (TK) and by NIH grant RO1ES008421 (AJLC). We thank doctors and volunteers throughout Japan who provided urine specimens. We gratefully acknowledge Drs Fuziko Tetsuo and Masahito Tetsuo (Hakuaikai Tetsuo Hospital, Kuchinotsu, Nagasaki, Japan) and the late Dr Toshio Fujita and the late Mrs. Hiroko Fujita (Fujita Obst-Gynecology Clinic, Kamiichi, Toyama Japan) for their support of research for personalized medicine.
Contributor Information
Tomiko Kuhara, Department of Biochemistry, Division of Human Genetics, Medical Research Institute, Kanazawa Medical University, Uchinada, Kahoku-gun, Ishikawa 920 0293, Japan.
Yoshito Inoue, Department of Biochemistry, Division of Human Genetics, Medical Research Institute, Kanazawa Medical University, Uchinada, Kahoku-gun, Ishikawa 920 0293, Japan.
Morimasa Ohse, Department of Biochemistry, Division of Human Genetics, Medical Research Institute, Kanazawa Medical University, Uchinada, Kahoku-gun, Ishikawa 920 0293, Japan.
Boris F. Krasnikov, Department of Biochemistry and Molecular Biology, New York Medical College, Valhalla, NY 10595, USA
Arthur J. L. Cooper, Email: Arthur_cooper@nymc.edu, Department of Biochemistry and Molecular Biology, New York Medical College, Valhalla, NY 10595, USA
References
- 1.Meister A, Sober HA, Tice SV, Fraser PE. Transamination and associated deamidation of asparagine and glutamine. J Biol Chem. 1952;197:319–330. [PubMed] [Google Scholar]
- 2.Meister A. Preparation and enzymatic reactions of the keto acid analogues of asparagine and glutamine. J Biol Chem. 1953;200:571–581. [PubMed] [Google Scholar]
- 3.Meister A, Levintow L, Greenfield RE, Abendschein PA. Hydrolysis and transfer reactions catalyzed by ω-amidase preparations. J Biol Chem. 1955;215:441–460. [PubMed] [Google Scholar]
- 4.Cooper AJL, Meister A. Isolation and properties of highly purified glutamine transaminase. Biochemistry. 1972;11:661–671. doi: 10.1021/bi00755a001. [DOI] [PubMed] [Google Scholar]
- 5.Cooper AJL, Meister A. Isolation and properties of a new glutamine transaminase from rat kidney. J Biol Chem. 1974;249:2554–2561. [PubMed] [Google Scholar]
- 6.Cooper AJL, Meister A. Comparative studies of glutamine transaminases from rat tissues. Comp Biochem Physiol. 1981;69B:137–145. [Google Scholar]
- 7.Cooper AJL, Duffy TE, Meister A. α-Keto acid ω-amidase from rat liver. Methods Enzymol. 1985;113:350–358. doi: 10.1016/s0076-6879(85)13048-x. [DOI] [PubMed] [Google Scholar]
- 8.Cooper AJL. The role of glutamine transaminase K (GTK) in sulfur and α-keto acid metabolism in the brain, and in the possible bioactivation of neurotoxicants. Neurochem Int. 2004;44:557–577. doi: 10.1016/j.neuint.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Krasnikov BF, Chien C-H, Nostramo R, Pinto JT, Nieves E, Callaway M, Sun J, Huebner K, Cooper AJL. Identification of the putative tumor suppressor Nit2 as ω-amidase, an enzyme linked to glutamine and asparagine transamination. Biochimie. 2009;91:1072–1080. doi: 10.1016/j.biochi.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hersh LB. Rat liver ω-amidase. Purification and properties. Biochemistry. 1971;10:2884–28891. doi: 10.1021/bi00791a014. [DOI] [PubMed] [Google Scholar]
- 11.Nissim I, Wehrli S, States B, Nissim I, Yudkoff M. Analysis and physiological implications of renal 2-oxoglutaramate metabolism. Biochem J. 1991;277:33–38. doi: 10.1042/bj2770033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy TE, Cooper AJL, Meister A. Identification of α-ketoglutaramate in rat liver, kidney, and brain. Relationship to glutamine transaminase and ω-amidase activities. J Biol Chem. 1974;249:7603–7606. [PubMed] [Google Scholar]
- 13.Vergara E, Plum F, Duffy TE. α-Ketoglutaramate: increased concentrations in the cerebrospinal fluid of patients in hepatic coma. Science. 1974;183:81–83. doi: 10.1126/science.183.4120.81. [DOI] [PubMed] [Google Scholar]
- 14.Duffy TE, Vergara F, Plum F. α-Ketoglutaramate in hepatic encephalopathy. Res Publ Assoc Res Nerv Ment Dis. 1974;53:39–52. [PubMed] [Google Scholar]
- 15.Cooper AJL, Gross M. The glutamine transaminase–ω-amidase system in rat and human brain. J Neurochem. 1977;28:771–778. doi: 10.1111/j.1471-4159.1977.tb10626.x. [DOI] [PubMed] [Google Scholar]
- 16.Lash LH, Nelson RM, Van Dyke RA, Anders MW. Purification and characterization of human kidney cytosolic cysteine conjugate β-lyase activity. Drug Metab Dispos. 1990;18:50–54. [PubMed] [Google Scholar]
- 17.Darmaun D, Matthews DE, Bier DM. Glutamine and glutamate kinetics in humans. Am J Physiol. 1986;251:E117–E126. doi: 10.1152/ajpendo.1986.251.1.E117. [DOI] [PubMed] [Google Scholar]
- 18.Krasnikov BF, Nostramo R, Pinto JT, Cooper AJL. Assay and purification of ω-amidase/Nit2, a ubiquitously expressed putative tumor suppressor, that catalyzes the deamidation of the α-keto acid analogues of glutamine and asparagine. Anal Biochem. 2009;391:144–150. doi: 10.1016/j.ab.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto I, Kuhara T. A new chemical diagnostic method for inborn errors of metabolism by mass spectrometry—rapid, practical, and simultaneous urinary metabolites analysis. Mass Spectrom Rev. 1996;15:43–57. doi: 10.1002/(SICI)1098-2787(1996)15:1<43::AID-MAS3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Shoemaker JD, Elliott WH. Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991;562:125–138. doi: 10.1016/0378-4347(91)80571-s. [DOI] [PubMed] [Google Scholar]
- 21.Kuhara T. Diagnosis of inborn errors of metabolism using filter paper urine, urease treatment, isotope dilution and gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;758:3–25. doi: 10.1016/s0378-4347(01)00138-4. [DOI] [PubMed] [Google Scholar]
- 22.Kuhara T, Ohdoi C, Ohse M. Simple gas chromatographic-mass spectrometric procedure for diagnosing pyrimidine degradation defects for prevention of severe anticancer side effects. J Chromatogr B Biomed Sci Appl. 2001;758:61–74. doi: 10.1016/s0378-4347(01)00143-8. [DOI] [PubMed] [Google Scholar]
- 23.Cooper AJL, Dhar AK, Kutt H, Duffy TE. Determination of 2-pyrrolidone-5-carboxylic and α-ketoglutaramic acids in human cerebrospinal fluid by gas chromatography. Anal Biochem. 1980;103:118–126. doi: 10.1016/0003-2697(80)90245-6. [DOI] [PubMed] [Google Scholar]
- 24.Kuhara T. Diagnosis and monitoring of inborn errors of metabolism using urease-pretreatment of urine, isotope dilution, and gas chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2002;781:497–517. doi: 10.1016/s1570-0232(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 25.Filipowicz HR, Ernst SL, Ashurst CL, Pasquali M, Longo N. Metabolic changes associated with hyperammonemia in patients with propionic acidemia. Mol Genet Metab. 2006;88:123–130. doi: 10.1016/j.ymgme.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 26.McCann MT, Thompson MM, Gueron IC, Tuchman M. Quantification of orotic acid in dried filter-paper urine samples by stable isotope dilution. Clin Chem. 1995;41:739–743. [PubMed] [Google Scholar]
- 27.Salerno C, Crifò C. Diagnostic value of urinary orotic acid levels: applicable separation methods. J Chromatogr B Anal Technol Biomed Life Sci. 2002;781:57–71. doi: 10.1016/s1570-0232(02)00533-0. [DOI] [PubMed] [Google Scholar]
- 28.Wu C. Glutamine synthetase. I a comparative study of its distribution in animals and its inhibition by dl-allo-δ-hydroxylysine. Comp Biochem Physiol. 1963;34:335–351. doi: 10.1016/0010-406x(63)90169-5. [DOI] [PubMed] [Google Scholar]
- 29.Scaglia F, Lee B. Clinical, biochemical, and molecular spectrum of hyperargininemia due to arginase I deficiency. Am J Med Genet C Semin Med Genet. 2006;142C:113–120. doi: 10.1002/ajmg.c.30091. [DOI] [PMC free article] [PubMed] [Google Scholar]