Abstract
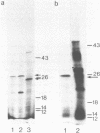
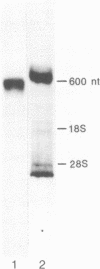
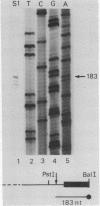
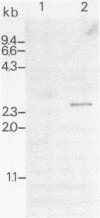
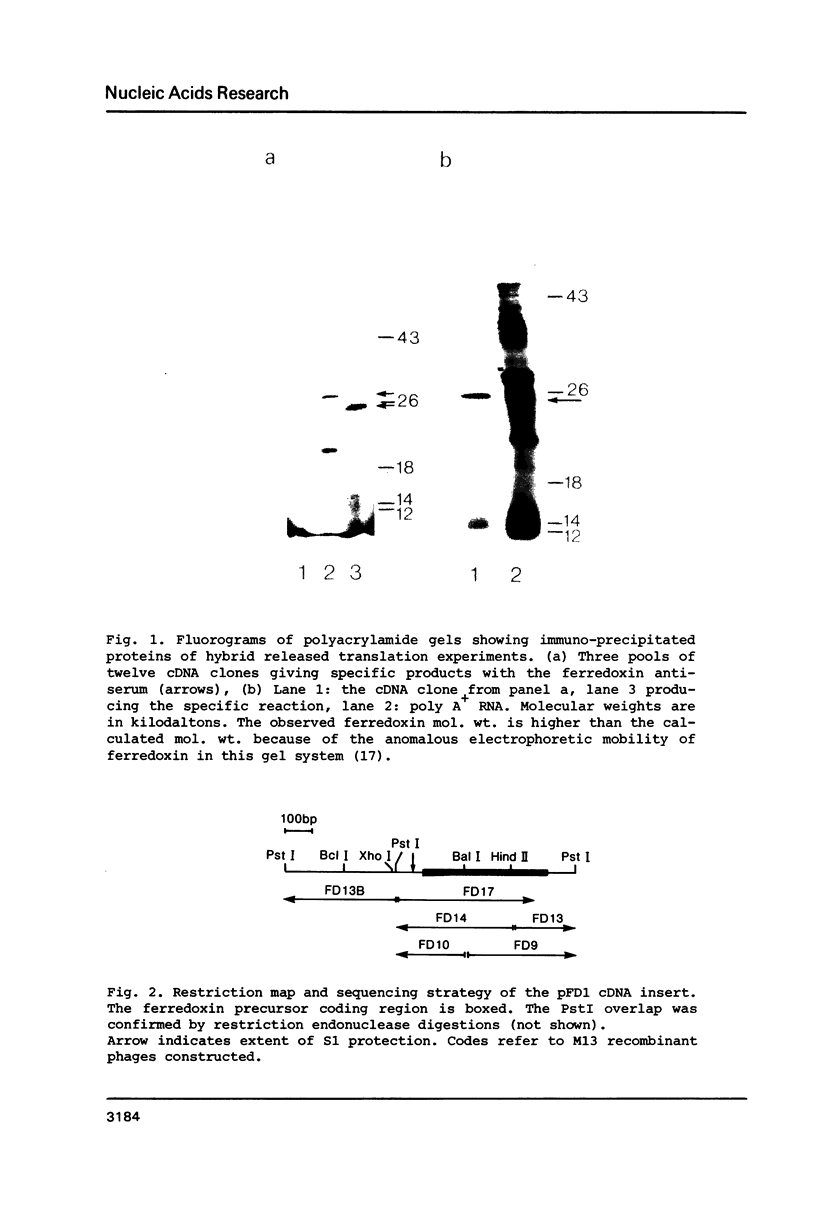
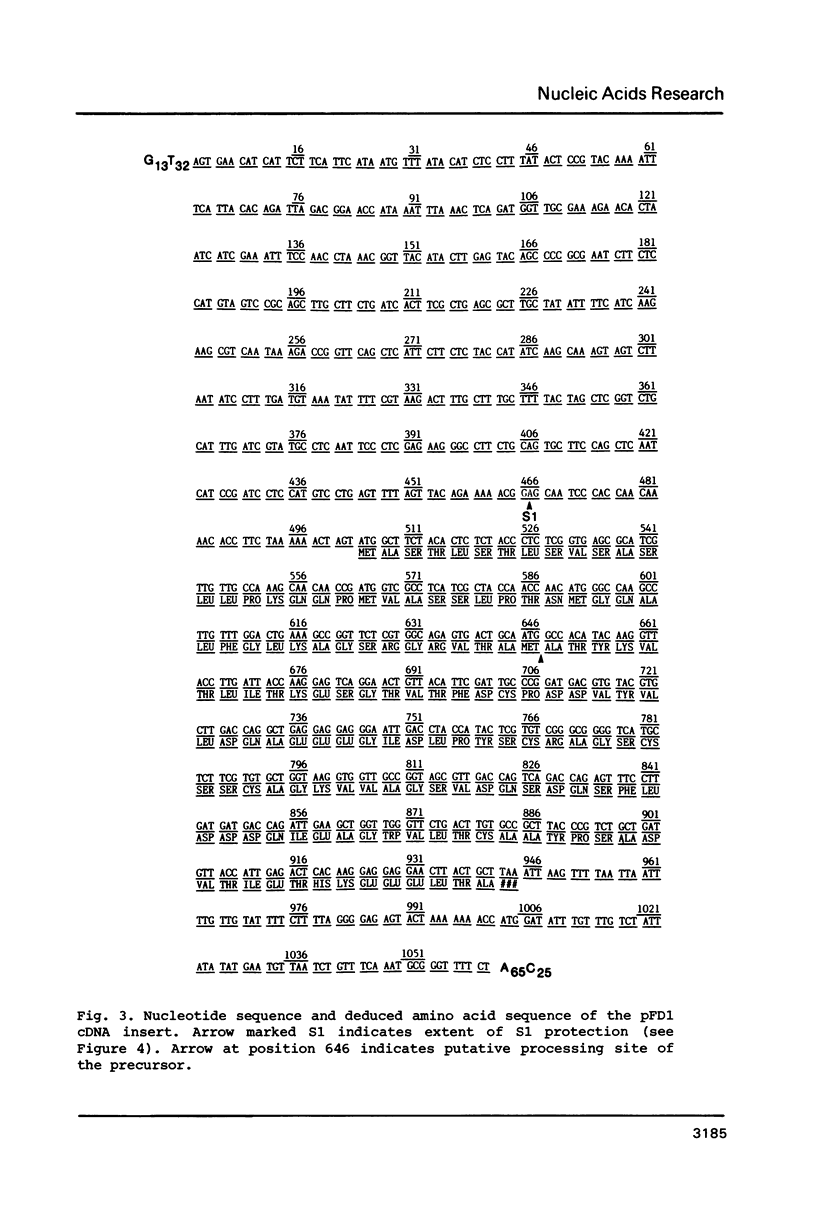
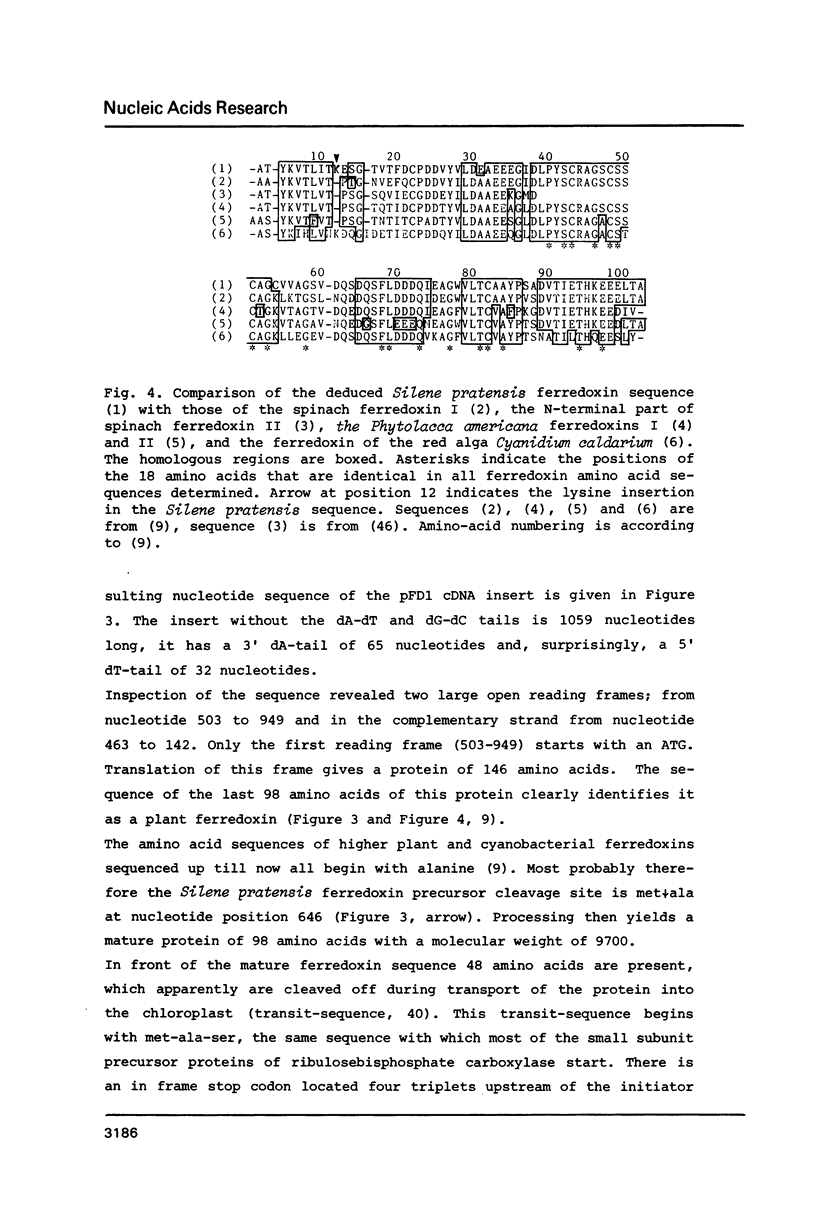
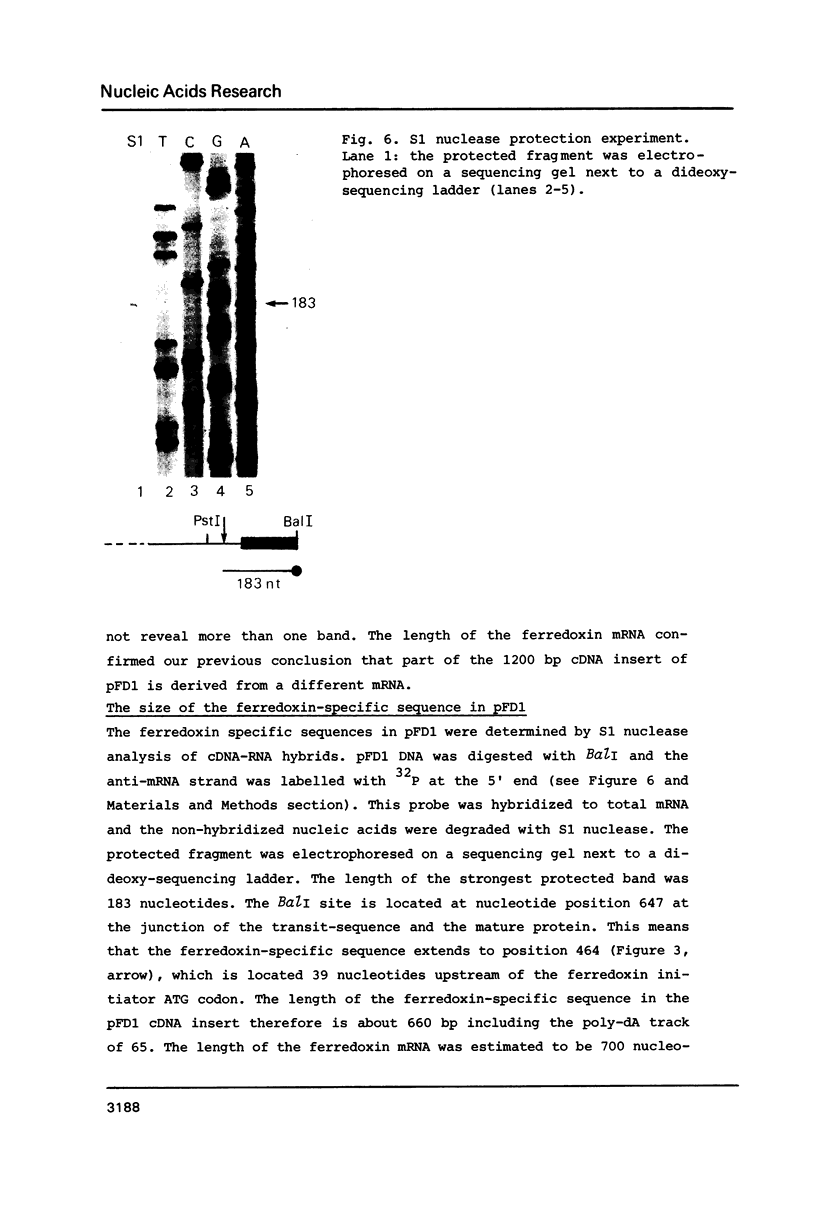
A cDNA clone (pFD1) derived from Silene pratensis ferredoxin mRNA was selected from a cDNA-library using the hybrid released translation technique. Nucleotide sequence analysis showed the cDNA insert to contain the complete coding region of the ferredoxin precursor protein. The ferredoxin precursor has a mol.wt. of 15 300, the transit-peptide has a mol.wt. of 5600. The length of the ferredoxin mRNA was found to be 700 nucleotides whereas the cDNA insert was about 1200 basepairs. S1 nuclease protection experiments showed the ferredoxin-specific DNA to be 660 basepairs in length and to start 39 nucleotides upstream of the ferredoxin coding sequence. Southern blot analysis of genomic DNA revealed the presence of only one fragment with homology to the ferredoxin cDNA probe, so it is probably a single-copy gene. Comparison of the ferredoxin transit-sequence with transit sequences of another stromal protein, the small subunit of ribulosebisphosphate carboxylase showed no apparent homology, except for a stretch of three amino acids near the processing site.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Straus J. W., Dudock B. S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- Armstrong J. J., Moll B., Surzycki S. J., Levine R. P. Genetic transcription and translation specifying chloroplast components in Chlamydomonas reinhardi. Biochemistry. 1971 Feb 16;10(4):692–701. doi: 10.1021/bi00780a022. [DOI] [PubMed] [Google Scholar]
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Buchanan B. B. The ferredoxin/thioredoxin system: a key element in the regulatory function of light in photosynthesis. Bioscience. 1984 Jun;34(6):378–383. [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Hall D. O. Metalloproteins in the evolution of photosynthesis. Biosystems. 1981;14(1):57–80. doi: 10.1016/0303-2647(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G., Wu R. An improved procedure for utilizing terminal transferase to add homopolymers to the 3' termini of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4173–4188. doi: 10.1093/nar/9.16.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvitz S. C., Bacchetti S., Rainbow A. J., Graham F. L. A rapid and efficient procedure for the purification of DNA from agarose gels. Anal Biochem. 1980 Aug;106(2):492–496. doi: 10.1016/0003-2697(80)90553-9. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Hase T., Wada K., Matsubara H. Horsetail (Equisetum arvense) ferredoxins I and II Amino acid sequences and gene duplication. J Biochem. 1977 Jul;82(1):277–286. doi: 10.1093/oxfordjournals.jbchem.a131680. [DOI] [PubMed] [Google Scholar]
- Haslett B. G., Cammack R., Whatley F. R. Quantitative studies on ferredoxin in greening bean leaves. Biochem J. 1973 Nov;136(3):697–703. doi: 10.1042/bj1360697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman J. G., Moorman A. F., Verkley F. N. In vitro synthesis of chloroplast ferredoxin as a high molecular weight precursor in a cell-free protein synthesizing system from wheat germs. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1121–1131. doi: 10.1016/0006-291x(78)90303-0. [DOI] [PubMed] [Google Scholar]
- Huisman J. G., Stapel S., Muijsers A. O. Two different plant-type ferredoxins in each of two petunia species. FEBS Lett. 1978 Jan 15;85(2):198–202. doi: 10.1016/0014-5793(78)80454-2. [DOI] [PubMed] [Google Scholar]
- Huisman J. G., Stapel S., Muijsers A. O. Two different plant-type ferredoxins in each of two petunia species. FEBS Lett. 1978 Jan 15;85(2):198–202. doi: 10.1016/0014-5793(78)80454-2. [DOI] [PubMed] [Google Scholar]
- Hutson K. G., Rogers L. J., Haslett B. G., Boulter D., Cammack R. Comparative studies on two ferredoxins from the cyanobacterium Nostoc strain MAC. Biochem J. 1978 Jun 15;172(3):465–477. doi: 10.1042/bj1720465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov B. N., Red'ko T. P., Shmeleva V. L., Mukhin E. N. Uchastie ferredoksina v psevdotsiklicheskom elektronnom transporte v izolirovannykh khloroplastakh gorokha. Biokhimiia. 1980 Aug;45(8):1425–1432. [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson R., Messing J. Apple II software for M13 shotgun DNA sequencing. Nucleic Acids Res. 1982 Jan 11;10(1):39–49. doi: 10.1093/nar/10.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Manzano C., Candau P., Gomez-Moreno C., Relimpio A. M., Losada M. Ferredoxin-dependent photosynthetic reduction of nitrate and nitrite by particles of Anacystis nidulans. Mol Cell Biochem. 1976 Feb 25;10(3):161–169. doi: 10.1007/BF01731687. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock S. L., McIver C. M., Monahan J. J. Transformation of E. coli using homopolymer-linked plasmid chimeras. Biochim Biophys Acta. 1981 Sep 28;655(2):243–250. doi: 10.1016/0005-2787(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. The end of the message. Nature. 1982 Aug 5;298(5874):516–517. doi: 10.1038/298516a0. [DOI] [PubMed] [Google Scholar]
- Rawson J. R., Thomas K., Clegg M. T. Purification of total cellular DNA from a single plant. Biochem Genet. 1982 Apr;20(3-4):209–219. doi: 10.1007/BF00484419. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. The precursor of small subunit of ribulose bisphosphate carboxylase is processed to the mature size in two steps. Eur J Biochem. 1984 Jul 16;142(2):343–346. doi: 10.1111/j.1432-1033.1984.tb08292.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Trebst A. The mechanism of photosynthetic sulfate reduction by isolated chloroplasts. Biochim Biophys Acta. 1969 Aug 5;180(3):529–535. doi: 10.1016/0005-2728(69)90031-0. [DOI] [PubMed] [Google Scholar]
- Smith R. V., Noy R. J., Evans M. C. Physiological electron donor systems to the nitrogenase of the blue-green alga Anabaena cylindrica. Biochim Biophys Acta. 1971 Nov 2;253(1):104–109. doi: 10.1016/0005-2728(71)90238-6. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Bedbrook J., Speirs J. Characterisation of three cDNA clones encoding different mRNAs for the precursor to the small subunit of wheat ribulosebisphosphate carboxylase. Nucleic Acids Res. 1983 Dec 20;11(24):8719–8734. doi: 10.1093/nar/11.24.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stiekema W. J., Wimpee C. F., Tobin E. M. Nucleotide sequence encoding the precursor of the small subunit of ribulose 1,5-bisphosphate carboxylase from Lemna gibba L.G-3. Nucleic Acids Res. 1983 Nov 25;11(22):8051–8061. doi: 10.1093/nar/11.22.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Hase T., Wada K., Matsubara H. The second ferredoxin from spinach leaves. J Biochem. 1981 Dec;90(6):1825–1828. doi: 10.1093/oxfordjournals.jbchem.a133662. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bartlett A., Bidwell D. E. Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol. 1978 Jun;31(6):507–520. doi: 10.1136/jcp.31.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]