Introduction
Globoid cell leukodystrophy (GLD), also known as Krabbe’s disease (KD), is a neurodegenerative lysosomal storage disease affecting both the central nervous system (CNS) and peripheral nervous system (PNS). The disease results from a deficiency in the galactosylcerebrosidase (GALC) enzyme responsible for the degradation of galactosylceramide (GalCer), an important glycolipid component of myelin, and psychosine, a toxic metabolite [1, 2]. The GALC deficiency leads to progressive accumulation of psychosine, which is detrimental to oligodendrocytes and Schwann cells responsible for myelination [3–5]. KD causes severe demyelination and degeneration in the CNS and PNS, leading to an early death in patients born with KD.
Currently, the only clinical treatment for KD is bone marrow or umbilical cord-blood cell transplantation for late-onset and pre-symptomatic patients [6]. The rationale for using bone marrow transplantation (BMT) to treat a neurological disease such as KD is that marrow-derived macrophages from unaffected donors will migrate to the brain and secrete wild-type GALC enzyme, which can then be taken up by neighboring cells to correct the enzymatic deficiency via a cross-corrective mechanism [7]. If patients receive the transplantation prior to the display of symptoms, most will survive the first three years with improved neurological function compared to patients who did not receive a transplant [8]. However, one of the limitations to performing bone marrow transplants is the patient risks developing graft versus host disease (GVHD), in which functional immune cells in the transplanted donor marrow recognize the recipient host as “foreign” and mount an immunologic attack. Also, transplantation of umbilical cord blood in babies with infantile KD was only beneficial if performed before the onset of symptoms [8]. Moreover, recent reports indicate that unfortunately the majority of these patients have developed subsequent motor and language deterioration [9].
The twitcher mouse is an authentic murine model of KD and is a natural mutant of the C57Bl/6J strain [10]. The twitcher presents similar biochemical and histopathological symptoms to the human form of KD such as infiltration of periodic acid-Schiff (PAS) - positive cells in the CNS and PNS, loss of oligodendrocytes, and demyelination [11]. Symptoms become apparent at 3 weeks of age and include “twitching,” weight loss, progressive hind limb paralysis, and loss of coordination [12, 13]. Most twitcher mice die between post-natal days (PND) 30–40.
BMT following high dose radiation can prolong the lifespan of the twitcher mouse to approximately 80–90 days [14–16]. Other current therapies being investigated in the twitcher mouse as treatments for KD include enzyme replacement therapy, stem cell therapy, gene therapy, substrate reduction, or combinations thereof [6, 12, 13, 17–26]. These approaches have been limited in their success in that they only delayed the onset of the symptoms.
This study investigates the transplantation of adult mesenchymal-lineage stem cells as a potential therapy for KD in the twitcher mouse. Theories behind why transplantation of mesenchymal stem cells (MSCs) may have an impact on the pathology of KD include the secretion of functional GALC enzyme in a cross-corrective mechanism, differentiation of these cells to replace damaged oligodendrocytes, or perhaps most importantly, their immunosuppressive and anti-inflammatory effects. MSCs derived from both bone marrow and adipose tissue seem to be not only hypo-immunogenic and thus may be suitable for allogeneic transplantation, but are also able to produce immunosuppression upon transplantation [27–33]. Recently, MSCs have been shown to decrease expression of a wide panel of inflammatory cytokines and chemokines, decrease infiltration of inflammatory cells, and to increase expression of growth factors in models of inflammation [34–39]. The ability of MSCs to suppress inflammatory responses may be an important component to any stem cell therapeutic treatment in the twitcher mouse since an important aspect of this disease is the chronic state of inflammation [40–44].
Adipose- and bone marrow- derived mesenchymal stem cells (ASCs and BMSCs, respectively) used in this study were derived from transgenic C57Bl/6 mice expressing the enhanced green fluorescent protein (eGFP) (eGFPTgASCs and eGFPTgBMSCs, respectively). These cells were isolated, expanded in culture, and characterized prior to intracerebroventricular transplantation into neonatal twitcher mice on PND 3 or 4 [45]. Lifespan, weight, twitching frequency/severity, and motor function were recorded, and tissues were collected from all mice in the study. Twitcher mouse brains were analyzed for markers of inflammation as well as GALC activity. Since the transplanted stem cells were eGFP+, an attempt was also made to monitor the transplanted cells to evaluate the persistence of the cells after administration. The eGFPTgASCs and eGFPTgBMSCs were also compared by examining potential differences and similarities in cellular persistence, differentiation, and therapeutic effects.
Materials and Methods
Animals
Breeder pairs of twitcher mice carrying a naturally occurring mutation of GALC were initially obtained from Jackson Laboratories (Bangor, ME, USA). A colony was established and maintained under standard housing conditions. All procedures conform to the requirements of the Animal Welfare Act and protocols approved by the Institutional Animal Care and Use Committee at Tulane University. The twitcher mutation was confirmed by polymerase chain reaction (PCR) on DNA obtained from anal swabs.
Murine eGFPTgASCs and eGFPTgBMSCs
Murine ASCs from inguinal fat pads and BMSCs were obtained from 2–4 month old mice from the inbred transgenic strain C57Bl/6-Tg(UBC-GFP)30Scha/J that ubiquitously expresses enhanced green fluorescent protein and were a generous gift from Dr. David Welsh of Louisiana State University Health Sciences Center (Jackson Laboratories). All donors were 2–4 months old and were individually euthanized by CO2. Cells were isolated, cultured, and cryopreserved as previously described [45].
Neonatal Stereotaxic Injections
eGFPTgASCs or eGFPTgBMSCs were harvested and prepared at a concentration of 20,000 cells/μl in Hank’s Balanced Salt Solution (HBSS) (Fisher Scientific, Pittsburgh, PA) and kept on ice before injection. On PND3 or 4, the pups (twitchers and unaffected littermate controls) were anesthetized using cryoanesthesia for approximately 10 minutes. Once anesthetized, the pups were placed in a stereotaxic frame and received bilateral intraventricular injections, 1 μl per hemisphere (20,000 cells), at a rate of 0.5 μl/min through a 30G stainless steel needle (Hamilton, Reno, NV) in a steady stream using a syringe pump (KD Scientific, 780100V). Control pups were injected with HBSS only. The stereotaxic coordinates were as follows: anterior/posterior = −0.5 mm from the posterior edge of the eye; medial/lateral = +1.0/−1.0 mm from the midline; dorsal/ventral = −1.7 mm. Coordinates were determined prior to experiments involving cell injections by dye injections into the lateral ventricles of control mice followed by sectioning of their brains to track the location of the dye within the ventricles. After each injection there was a 2 minute dwell time before the needle was slowly removed. The mice were warmed by placing them on a heating pad, stimulated to recover, and returned to the mother.
Assessment of Physiological Effects
The lifespan of the twitcher mice was measured by noting the date of euthanization. According to our experimental design, twitcher mice were euthanized once they lost 20% of their maximum body weight or became moribund. Body weight was measured three times per week beginning on PND15 and measurements continued until the date of euthanization. Twitching frequency and severity was scored three times per week beginning on PND15 using the following scoring system: Frequency – rare (1), intermittent (2), constant (3); Severity – fine (1), mild (2), moderate (3), and severe (4) [46].
Beginning on PND15, motor function tests were performed on mice in all groups to examine motor strength and coordination three times per week. Mice were trained to perform the motor function tests prior to the initiation of the assessments. The wire hang maneuver provides a measurement of motor function. Mice were suspended by the tail and lowered onto a horizontal wire and then released. A mouse received a score of 0 when it could swing its hind legs around to grasp the wire, 1 when it grasped the wire with some struggling, 2 when the mouse was unable to grasp the wire with its hind legs, 3 when it fell within 3 seconds, and 4 when it fell immediately onto the soft bedding below [22]. Hind stride length was measured by applying food coloring to the paws of the mice and allowing them to walk through a tube lined with graph paper. The hind stride length of both the left and right back paws was measured and averaged together.
GALC activity
Fresh brain tissue was collected from mice euthanized by CO2. The tissue was homogenized in 4 volumes of 20 mM acetate buffer (pH 4.5). The substrate solution for GALC containing oleic acid, sodium taurocholate, triton-X-100 and [3H]GalCer was prepared as previously published [47]. Radioactive [3H]GalCer, the substrate for GALC, is cleaved into [3H]galactose and ceramide by GALC. Brain homogenate (50 μl) was added to 50 μl of the substrate solution (~10,000 cpm) and the mixture incubated at 37°C for 8 hours. The reaction was stopped by the addition of 5 ml of chloroform: methanol (2:1), 0.1 ml of 1 mg/ml galactose, and 0.8 ml water. After centrifugation of the mixture at 3,000 rpm for 10 minutes, the radioactivity in 0.9 ml of the aqueous phase was measured with a scintillation counter (TRI-CARB 1600CA, PACARD). GALC activity is expressed as nmoles of [3H]GalCer hydrolyzed per hour per mg of protein (nmol/h/mg protein).
Real Time PCR
For eGFP+ cell tracking, DNA was extracted from brain tissue from mice at various time points after cell injection using a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). DNA was examined for the presence of the eGFP gene using real-time PCR by the standard curve method for absolute quantification with Sybr Green (Applied Biosystems, Foster City, CA) using the following primers: Fwd 5′-GGG CAC AAG CTG GAG TAC AAC-3′; Rev 5′-CAC CTT GAT GCC GTT CTT CTG-3′ using an AB 7900HT Real-Time sequence analyzer (Applied Biosystems). A standard curve was generated with Ct values corresponding to the signal derived from DNA extracted from eGFP+ cells loaded in serial dilution. Output was analyzed using the corresponding software, SDS 2.3 (Applied Biosystems).
Real Time RT-PCR
For inflammatory cytokine mRNA expression analysis using real time RT-PCR, RNA was isolated using an RNeasy mini kit (Qiagen) with Trizol for 10 minutes at room temperature instead of lysis buffer. The RNA was first treated with DNase and then converted into cDNA using the ImProm-II Reverse Transcription System (Promega, Madison, WI). The reaction mixture contained 500 ng of RNA with 1x Reaction Buffer, 2.5 mM MgCl2, 0.5 mM dNTPs, 0.25 μg Oligo(dT)15 primer, 20 U Recombinant RNasin Ribonuclease Inhibitor, 1 μl Reverse Transcriptase, and Nuclease Free Water with a total reaction of 20 μl. The samples were subsequently placed in a PTC-200 Peltier Thermal Cycler (MJ Research, Ramsey, MN) at 25°C for 5 minutes, 42°C for 1 hour followed by 70°C for 15 minutes. The real-time PCR reactions were amplified with a 7900HT Sequence Detection System (Applied Biosystems). Each 20 μl amplification reaction contained 1 μl of commercially available TaqMan Gene Expression Assay primer/probe sets (Applied Biosystems), 10 μl TaqMan Gene Expression Master Mix (Applied Biosystems), 4 μl cDNA template (diluted 1:10), and 5 μl RNase-free water. All samples were normalized to β-actin content.
Multiplex Assay
An aliquot of brain homogenate from twitcher mice in each cell injected and control groups as well as from wild-type mice was centrifuged at 10,000 × g for 5 minutes to obtain a cleared lysate. 25 μl of each sample was loaded onto a Milliplex Mouse Cytokine 32 Plex premixed Immunoassay plate (Millipore, MPXMCYTO70KPMX32). The levels of a panel of 32 cytokines/chemokines involved in inflammatory response pathways were measured and analyzed according to the manufacturer’s protocol on a BioRad Bio-plex 200 with Bio-Plex Manager 5.0 software.
Western Blotting
An aliquot of brain homogenate in acetate buffer from twitchers in each cell injected group and control twitchers as well as from wild-type mice was centrifuged at 10,000 × g for 5 minutes to obtain a cleared lysate. For detection of CD163 or iNOS, 20 μg of protein from each sample was loaded onto an 8% SDS-PAGE gel and then transferred to a PVDF membrane. Membranes were incubated with primary antibody overnight at 4°C with anti-iNOS (1:500, Abcam, Cambridge, MA: ab49999) or anti-CD163 (1:500, AbD Serotec, Raleigh, NC: MCA342R) according to manufacturer recommendations. For detection of myelin or Iba-1(ionized calcium-binding adaptor molecule 1), an aliquot of brain homogenate in RIPA buffer (Fisher) from twitchers in both cell injected groups and control twitchers as well as from wild-type mice was centrifuged at 13,200 × g for 15 minutes to obtain a cleared lysate. 20 μg of protein from each sample was loaded onto a 4–20% polyacrylamide gel and then transferred to a PVDF membrane. Membranes were incubated with anti-myelin basic protein (anti-MBP) (1:2500, Millipore: MAB386) overnight at 4°C. 130 μg of brain lysate in RIPA buffer was loaded onto an 8% polyacrylamide gel and incubated overnight at 4°C with anti-Iba-1(1:500, Wako Chemicals, Richmond, VA: 016-20001). Membranes were probed with anti-GAPDH (1:500, Abcam: ab9485) overnight at 4°C for normalization.
Immunofluorescence
Mice were perfused under 5% isoflurane anesthesia at various time points with heparinized PBS followed by 4% para-formaldehyde (PFA). Brains were then post-fixed with 4% PFA overnight at 4°C and then cryo-protected with 30% sucrose. The brains were cut into 2 mm wide blocks and then flash-frozen in OCT using liquid nitrogen and stored at −80°C. Cryosections of 16 μm thickness were cut from each block and used for immunohistochemistry (IHC).
Cryosections from brains collected were washed with PBS containing fish skin gelatin (FSG, Sigma: G-7765) and Triton x-100 (Tx100, Sigma: x-100) for 30 minutes at room temperature (RT). Sections were blocked with 10% normal goat serum (Invitrogen, Carlsbad, CA) in PBS-FSG for 1 hour at RT and then with anti-NeuN (1:50, Millipore, Temecula, CA: MAB377), S100 (1:1000, Sigma: S-2644), anti-MBP (1:50, Millipore: MAB386), anti-Map2 (1:500, Sigma: M4403), anti-GFAP (glial fibrillary acid protein) (1:200, Sigma: C9205), or anti-Iba-1 (1:100, Wako Chemicals: 019-19741) for 1 hour at RT. Sections were then washed twice with PBS-FSG-Tx100 and then once with PBS-FSG for 10 minutes each. Sections were then incubated with secondary antibodies (1:1000, Invitrogen: Goat α Mouse-Alexa568, A-11031 or A-21124, Goat α Rabbit-Alexa633, A-21071, Goat α Rabbit-Alexa488, A11034, or Goat α Rat-Alexa488, A11006) for 1 hour at RT, washed, and mounted with coverslips for confocal microscopy. Sections immunostained for MBP or Iba-1 were always pre-screened for any presence of eGFP before IHC was performed. For visualization of eGFP+ cells, sections did not need any further staining except with To-Pro-3 to image nuclei (1:2000, Invitrogen: T-3605).
Statistical Analysis
Statistical analysis on the Kaplan-Meier survival curve was done using the log-rank test. Comparison of control and experimental body weight and motor function curves was done using a Chi-squared test followed by a Bonferroni correction. Statistical analysis of three or more groups was performed using ANOVA with Dunnett’s post test, and statistical analysis of cell persistence was done with the student’s t-test. Variance is presented as a measure of standard error.
Results
Lifespan and Body Weight
Lifespan was a measure for determining the therapeutic efficacy of injected eGFPTgASCs and eGFPTgBMSCs (Figure 1A). A Kaplan-Meier survival curve followed by statistical analysis using the log-rank test reveals a cumulative survival of 36% for eGFPTgASC injected and 45% for eGFPTgBMSC injected twitchers at PND40, while HBSS injected twitchers had a 0% cumulative survival at PND40 (p<0.05). The average lifespan for HBSS injected twitchers was 33.3 days +/−1.1, while average lifespan for eGFPTgASC and eGFPTgBMSC injected mice was 37.3 days +/−2.1 and 38.5 days +/−2.2, respectively (p<0.05).
Figure 1. Survival and Body Weight.

A. Kaplan-Meier survival curve illustrating the lifespan of twitcher mice. Cumulative survival was 36% for eGFPTgASC injected, 45% for eGFPTgBMSC injected and 0% for HBSS injected twitchers at PND40, respectively (p<0.05). B. Body weight for twitchers was measured beginning on PND15. Body weight for cell injected twitchers was significantly greater than controls (p<0.001).
Body weight was used as an objective measure of disease progression. After PND21, control and treated twitcher mice gained weight at a reduced rate compared to wild-type mice, whose maximum weight reached 19.0g +/−0.3 at PND40 (data not shown). Maximum body weight for eGFPTgASC injected twitchers was 11.2g +/−0.4, 10.6g +/−0.5 for eGFPTgBMSC injected twitchers, and 8.7g +/−0.7 for HBSS injected twitchers. Differences in body weight monitored through PND40 were statistically significant for cell-injected twitchers compared to control twitchers (Figure 1B) (p<0.001).
Stem Cell Injection Improves Motor Function
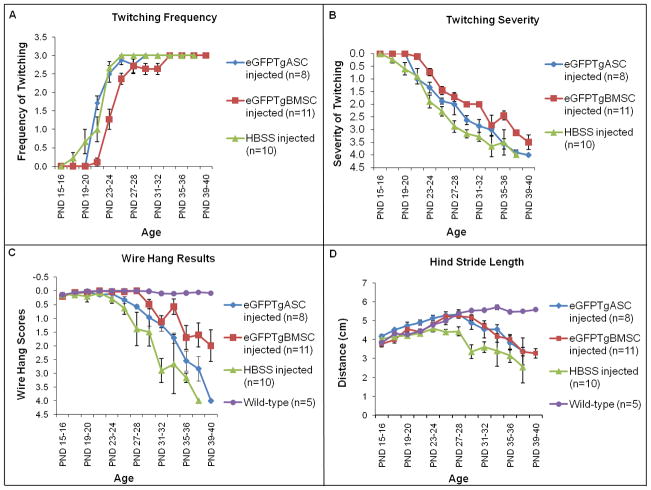
Twitching frequency and severity for twitcher mice was monitored beginning on PND15. Twitching was scored on frequency and severity as previously described [46]. Wild-type mice never displayed any twitching at all. HBSS injected twitchers began displaying symptoms at approximately PND17 and cell injected twitchers at PND21 (Figure 2A). HBSS injected twitchers reached a maximum twitching frequency of 3.0 +/− 0.1 at PND25, while eGFPTgASC and eGFPTgBMSC injected twitchers reached a twitching frequency of 3.0 +/−0.1 at PND29 and PND33, respectively. Only the twitching frequency curve for eGFPTgBMSC injected twitchers is statistically significantly different from controls (p<0.0001). Twitching severity reached a maximum score of 4.0 +/−0.1 for HBSS injected twitchers at PND37, while the maximum twitching severity scores for eGFPTgASC and eGFPTgBMSC injected mice were 3.9 +/−0.1 and 3.1 +/−0.4 respectively at this time point (p<0.01) (Figure 2B).
Figure 2. Motor Function.
A. Comparative analysis of twitching frequency for eGFPTgBMSCs compared to HBSS injected twitchers (p<0.0001). B. Twitching severity curves for both eGFPTgASC and eGFPTgBMSC injected mice are significantly different from HBSS injected controls (p<0.01). C. Mice were suspended by the tail and lowered onto a horizontal wire and then released. The wire hang maneuver curves for cell injected twitchers are significantly different from HBSS injected controls (p<0.01). D. Gait analysis was performed by averaging the hind stride length of both back paws. Cell injected twitchers had a longer hind stride length over time compared to HBSS injected controls (p<0.0001).
The wire hang maneuver measures an animal’s strength and motor function. Each mouse received a score based on its ability to grasp the wire with its hind legs as previously described beginning on PND15 [22]. Wild-type mice had no difficulty grasping the wire with their hind legs. HBSS injected twitchers reached the maximum score of 4.0 +/−0.1 at PND37, while the scores for eGFPTgASC and eGFPTgBMSC injected twitchers were 2.8 +/−0.4 and 1.6 +/−0.4 at this time point, respectively (p<0.01) (Figure 2C).
Gait analysis was performed on all groups of mice beginning on PND15 by measuring and averaging hind stride length (Figure 2D). Hind stride length for wild-type mice reached a maximum of 5.5 cm +/−0.19 at PND31. Hind stride length for HBSS injected twitchers reached a maximum of 4.6 cm +/−0.23 at PND23 and then declined with disease progression. Maximum hind stride length for eGFPTgASC and eGFPTgBMSC injected twitchers was 5.3 cm +/−0.18 and 5.3 cm +/−0.13, respectively, at PND27 and then declined with disease progression. Analysis of hind stride length curves reveals that cell injected twitchers had a longer hind stride length over the time period PND15–40 compared to HBSS injected twitchers (p<0.0001).
Analysis of eGFP+ Cell Persistence
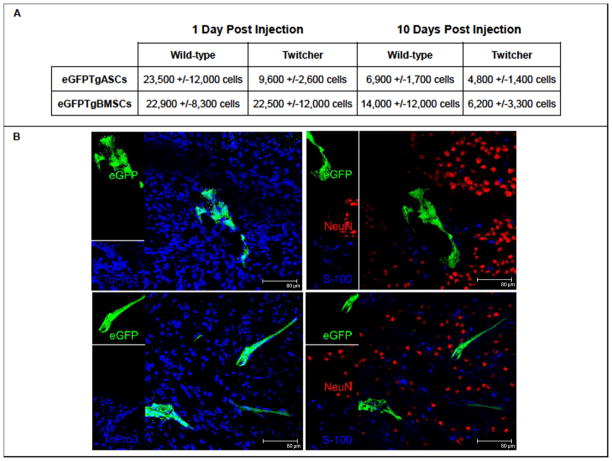
eGFP+ cells were tracked in the injected mouse brains using real-time PCR at various time points. eGFPTgASCs could be found in injected mouse brains up to 16 days post injection, while eGFPTgBMSCs could be found up to 20 days post injection. On day 1 post injection, 23,500 +/−12,000 eGFPTgASCs could be found in wild-type brains (n=3), while only 9,600 +/−2,600 eGFPTgASCs in twitcher brains (n=4). At the same time point, 22,900 +/−8,300 eGFPTgBMSCs could be found in wild-type brains (n=3) and 22,500 +/−12,000 eGFPTgBMSCs in twitcher brains (n=3) (Figure 3A). Thus, one day post injection, 59% of eGFPTgASCs were detected in wild-type mouse brains and 24% in twitcher mouse brains (p>0.05). Also, 57% of injected eGFPTgBMSCs were detected in wild-type mice and 56% in twitcher mice at this time point (p>0.05).
Figure 3.
A. eGFP+ Cell Persistence. eGFP+ cells were tracked in injected mouse brains using real-time PCR at pre-determined time points. 1 day post injection, there was a 59% recovery of eGFPTgASCs in wild-type mouse brains and a 24% recovery in twitcher mice (p>0.05). Also, there was a 57% recovery of eGFPTgBMSCs in wild-type mice and 56% recovery in twitcher mice at this time point (p>0.05). 10 days post injection, there was a 17% recovery of the injected eGFPTgASCs in wild-type brains and a 12% recovery in twitcher mice (p>0.05). Also, there was a 35% recovery of eGFPTgBMSCs in wild-type mice and 16% recovery in twitcher mice at this time point (p>0.05). B. Lack of Transdifferentiation. eGFPTgASCs (top) and eGFPTgBMSCs (bottom) were located in cryosections of injected brains 10 days post injection and immunostained with NeuN (red) and S100 (blue) to investigate cellular differentiation along neural or glial lineages, respectively (right). No co-localization of either antibody was found with the eGFP+. Nuclei were stained with To-Pro-3 (blue) (left).
At 10 days post injection, 6,900 +/−1,700 eGFPTgASCs could be found in wild-type brains (n=3) and 4,800 +/−1,400 eGFPTgASCs in twitcher brains (n=3). 10 days post injection, 14,000 +/−12,000 eGFPTgBMSCs could be found in wild-type brains (n=4) and 6,200 +/−3,300 eGFPTgBMSCs in twitcher brains (n=3). Thus, 10 days post injection, 17% of the injected eGFPTgASCs were detected in wild-type brains and a 12% in twitcher mice (p>0.05). Also, there 35% of injected eGFPTgBMSCs were detected in wild-type mice and 16% in twitcher mice at this time point (p>0.05).
eGFPTgASCs and eGFPTgBMSCs were located in cryosections of injected brains 10 days post injection and immunostained with NeuN (red) and S100 (blue) to investigate cellular differentiation along neural or glial lineages, respectively. No co-localization was found with the eGFP+ cells with either antibody (Figure 3B). In addition, cryosections were also immunostained with the neural marker Map2 and glial markers GFAP and MBP with no co-localization of markers with eGFP+ cells (Supplemental Figure 1).
Presence of Myelin
According to Western blotting results with anti-MBP, the presence of myelin in normal mice was substantially greater than that of twitcher mice. Injection with either eGFPTgASCs or eGFPTgBMSCs did not increase the levels of myelin in the twitcher brain in comparison to HBSS injected twitcher mice as evidenced by both Western blot and IHC (Figure 4).
Figure 4.
A. Western Blotting for Myelin. Protein lysates derived from twitcher brains at euthanization or wild-type mice at PND40 reveal that expression levels of myelin in normal mice is substantially greater than that of twitcher mice. The levels of myelin detected in the brains of either eGFPTgASC or eGFPTgBMSC injected mice were not increased in comparison to HBSS injected twitcher mice. B. Immunohistochemistry. Results from immunohistochemistry (IHC) on cryosections derived from twitcher brains at euthanization or wild-type mice at PND40 stained with anti-MBP (green). IHC results also suggest that the expression of myelin is not increased in stem cell injected twitcher mice compared to HBSS injected twitcher mice.
Stem Cell Injection Decreases Markers of Inflammation
Cytokine Analysis
Real time RT-PCR for several cytokines related to inflammation was performed on mRNA isolated from wild-type and twitcher mouse brains obtained at PND40, or when euthanized, respectively. The mRNA expression of multiple cytokines was down-regulated with stem cell injection. These included interleukin-1α (IL-1α), IL-1β, IL-6, IL-10, tumor necrosis factor-α (TNF-α), granulocyte colony stimulating factor (G-CSF), monocyte chemoattractant protein-1 (MCP-1, also known as CCL2), macrophage inflammatory protein-1α (Mip-1α, also known as CCL3), keratinocyte chemoattractant (KC, also known as CXCL1), and leukocyte inhibitory factor (LIF). Vascular endothelial growth factor (VEGF) was substantially up-regulated by stem cell injection in comparison to wild-type. In addition, IL-10 was significantly down-regulated only by eGFPTgASC injection, while TNFα was significantly down-regulated only by eGFPTgBMSC injection (p<0.05) (Figure 5A). Macrophage colony stimulating factor (M-CSF) was actually up-regulated at the transcriptional level, but this was not reflected at the translational level (p<0.05) (Figure 5A and 5B).
Figure 5.
A. Analysis of Inflammatory Cytokines/Chemokines by Real Time RT-PCR. Total cellular RNA samples derived from twitcher brains at euthanization or wild-type mice at PND40 reveal a marked elevation in expression of inflammatory markers is present in the twitcher mouse brain at this time point. The injection with either eGFPTgASCs or eGFPTgBMSCs markedly down-regulates the expression of several inflammatory mediators. Administration of eGFPTgASCs particularly reduced expression of IL-10 and the eGFPTgBMSCs significantly reduced expression of TNFα (p<0.05). All samples were normalized to β-actin content. B. Analysis of Inflammatory Cytokines/Chemokines by Multiplex Analysis. Protein lysates derived from twitcher brains at euthanization or wild-type mice at PND40 were analyzed on a 32-plex panel multiplex plate, which confirmed that inflammatory mediators are up-regulated in the twitcher brain at the protein level. The administration of both eGFPTgASCs and eGFPTgBMSCs reduced expression of G-CSF, IL-1α, MCP-1, and LIF in a statistically significant manner (p<0.01).
A 32-cytokine multiplex assay was performed using protein lysate from wild-type and twitcher mouse brains obtained at PND40 or when euthanized, respectively. Of the 32 cytokines analyzed by this assay, only 11 cytokines had signals within detection limits (Figure 5B). Both eGFPTgASCs and eGFPTgBMSCs reduced expression of G-CSF, IL-1α, MCP-1, and LIF in a statistically significant manner (p<0.01). However, many additional inflammatory cytokines appeared to be influenced by stem cell injection, but not in a statistically significant manner at the time points analyzed. These down-regulated pro-inflammatory cytokines normally function as potent attractants for monocytes/macrophages or activate other pro-inflammatory cells, and down-regulation may decrease the levels of inflammation in the twitcher mouse brain.
Stem Cell Injection Decreases Expression of Inducible Nitric Oxide Synthase
Inducible nitric oxide synthase (iNOS) is often up-regulated when chronic inflammation is present and is known to be markedly up-regulated as KD worsens [44]. Western blot analysis on protein lysates obtained from the brains of wild-type mice at PND40 and twitcher mice at the time of euthanization revealed that iNOS was substantially up-regulated in the twitcher mouse at the terminal stage compared to wild-type mice. Injection of eGFPTgASCs decreased expression of iNOS by a factor of 0.68 and injection of eGFPTgBMSCs decreased expression of iNOS by a factor of 0.53. Therefore it appears that injection of these stem cells down-regulates the expression of iNOS in the twitcher brain, even 4–5 weeks after administration (Figure 6).
Figure 6. iNOS Expression.
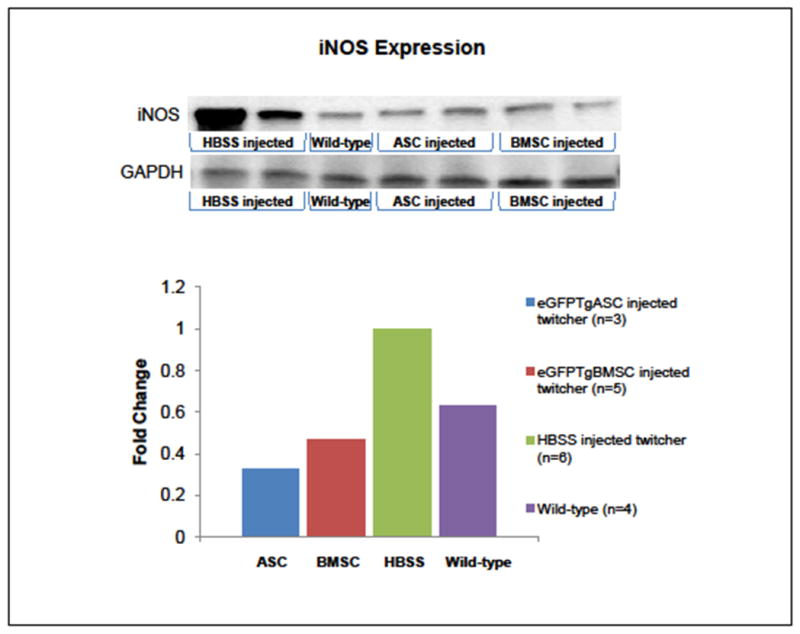
Protein lysates derived from twitcher brains at euthanization or wild-type mice at PND40 were analyzed by Western blot and illustrate inducible nitric oxide synthase (iNOS) is substantially up-regulated in the twitcher mouse compared to wild-type mice. Injection of eGFPTgASCs and eGFPTgBMSCs substantially decreased expression of iNOS, even 4–5 weeks after administration.
Macrophage Infiltration and Microglial Activation
CD163 is a marker for perivascular macrophages [44, 48]. Western blot analysis on protein lysates obtained from the brains of wild-type mice at PND40 and twitcher mice at the time of euthanization revealed that CD163 was substantially up-regulated in the twitcher mouse at the terminal stage compared to wild-type mice. Injection of eGFPTgASCs decreased expression of CD163 by a factor of 0.35 and injection of eGFPTgBMSCs decreased expression of CD163 by a factor of 0.37 compared to HBSS injected twitchers. Therefore it appears that injection of these stem cells decreased the infiltration and/or proliferation of macrophages in the twitcher brain (Figure 7A).
Figure 7.
A. Macrophage Infiltration. Protein lysates derived from twitcher brains at euthanization or wild-type mice at PND40 were analyzed by Western blot using anti-CD163 and revealed increased macrophage infiltration in the twitcher brain. Injection of eGFPTgASCs and eGFPTgBMSCs substantially decreased expression of CD163, even 4–5 weeks after administration. B. Microglial Activation. Protein lysates derived as previously mentioned were analyzed by Western blot using anti-Iba-1 for activated microglia and illustrate the increased presence of activated microglia in the twitcher brain compared to wild-type brains. These results suggest a small decrease in the numbers of activated microglia with eGFPTgASC injection and a much larger decrease with injection of eGFPTgBMSCs in the twitcher brain in comparison to HBSS injected twitchers. C. Immunohistochemistry for Iba-1. Results from IHC on cryosections derived from twitcher brains at euthanization or wild-type mice at PND40 stained with anti-Iba-1(green) support Western blotting results.
Iba-1 is a marker for activated microglia [48]. Western blot analysis on protein lysates obtained from the brains of wild-type mice at PND40 and twitcher mice at the time of euthanization reveals that Iba-1 is substantially up-regulated in the twitcher mouse at the terminal stage compared to wild-type mice. Injection of eGFPTgASCs decreased expression of Iba-1 by a factor of 0.19, and injection of eGFPTgBMSCs decreased expression of Iba-1 by a factor of 0.69 compared with HBSS injected twitchers. The Western blotting results suggest a small decrease in the numbers of activated microglia with eGFPTgASC injection and a much larger decrease with injection of eGFPTgBMSCs in the twitcher brain in comparison to HBSS injected twitchers (Figure 7B). IHC results for Iba-1 support the Western blotting results (Figure 7C).
GALC Activity
GALC activity was measured in twitcher mouse brains collected at the time of euthanization from all groups as well as wild-type mice euthanized at PND40. All twitcher mice, regardless of stem cell injection, had a GALC activity that was approximately 10% of wild-type. Therefore, neither eGFPTgASCs nor eGFPTgBMSCs managed to increase GALC activity in the brains of twitcher mice deficient in this enzyme at the time of euthanasia (Supplemental Figure 2) even though these cells exhibited a 5-fold increase in GALC activity compared to twitcher MSCs in vitro (data not shown).
Discussion
In this study, a total of 40,000 eGFPTgASCs or eGFPTgBMSCs were injected into the intracerebroventricular space of neonatal mouse pups in order to assess their therapeutic value in reducing the pathology in the twitcher mouse model of KD. Treated twitcher mice did exhibit significant improvements in lifespan, body weight, and motor function, though none of these mice approached the body weight or functional capabilities of wild-type mice. However, any improvements in aspects of this disease that could be extrapolated to increased “quality of life” in a clinical setting are noteworthy improvements and indicate a positive therapeutic impact.
The functional improvements observed in twitcher mice treated with these MSCs raises questions about mechanism of action. In this study, “cell replacement,” enzyme cross-correction, and anti-inflammatory effects were examined as possible mechanisms for the improvement seen in the treated twitcher mice. MSCs were investigated as a therapeutic option in these mice due to the possibility of multiple mechanisms of repair versus a classic anti-inflammatory pharmacological treatment which only offers a single benefit of suppressing inflammation, though these anti-inflammatory agents have offered some benefit in prolonging twitcher lifespan [49].
These cells did not appear to confer any endogenous GALC enzyme to surrounding cells as there was no increase in GALC activity in the brains of the treated twitcher mice at the terminal time point. Also, eGFPTgASCs and eGFPTgBMSCs were not found in injected mouse brains longer than 16 days or 20 days post injection, respectively. In addition, neither cell type appeared to transdifferentiate along neural or glial lineages. This lack of long-term engraftment and differentiation suggests that “cell replacement” is also not a mechanism which improved the function of the twitcher mice since the stem cells used in this study did not differentiate to replace cells damaged or destroyed by KD.
Recently scientists have been investigating the anti-inflammatory properties associated with mesenchymal-lineage stem cells and the subsequent improvements in disease pathology associated with this activity. Inflammation is clearly present in the twitcher mouse brain when markers of inflammation are evaluated and compared with those of wild-type mice. Even though only a relatively small number of eGFPTgASCs or eGFPTgBMSCs were injected compared with the total number of cells in the brain, these stem cells appear to exert powerful anti-inflammatory effects in the twitcher mouse brain. These anti-inflammatory effects even seem to persist for at least 1–2 weeks after the disappearance of the cells since the twitcher brain samples evaluated were taken when the mice were at the terminal stage.
Information gathered from real-time RT-PCR results suggest the stem cells down-regulated several markers of inflammation at the mRNA level such as IL-1α, IL-1β, IL-6, IL-10 (p<0.05), TNF-α (p<0.05), G-CSF, MCP-1, Mip-1α, KC, and LIF, and up-regulate the pro-angiogenic growth factor VEGF. However, only G-CSF, IL-1α, MCP-1, and LIF were affected by eGFPTgASCs and eGFPTgBMSCs at the protein level in a statistically significant manner even though an apparent trend of down-regulation seemed present with additional cytokines. Many of the cytokines/chemokines down-regulated with stem cell treatment are involved in activation, differentiation, or recruitment of immuno-regulatory cells such as neutrophils, basophils, B cells, T cells, granulocytes, or cells noted for exacerbating inflammation such as monocytes/macrophages. iNOS, another marker for chronic inflammation, was also down-regulated in treated twitcher brains. This enzyme responsible for synthesizing nitric oxide is often up-regulated in microglia or macrophages after the cells are stimulated with IL-1 or TNFα and is a major instigator in disease progression [44, 50–52].
Since certain cytokines/chemokines which recruit macrophages (MCP-1) or can be secreted by macrophages or microglia (G-CSF, IL-1α, IL-6, LIF) were down-regulated with stem cell treatment, the next step was to examine the infiltration of macrophages and activation of microglia in the twitcher brain. Expression of CD163, a marker for macrophages, was up-regulated in the twitcher brain compared to the wild-type brain. However, injection of eGFPTgASCs or eGFPTgBMSCs appeared to decrease expression of CD163 in the twitcher brain indicating decreased recruitment and/or infiltration of macrophages into the brain, which corresponds with the cytokine analysis data. Expression of Iba-1, a marker for activated microglia, was also up-regulated in the twitcher brain compared to the wild-type brain. Injection with eGFPTgASCs appeared to slightly decrease expression of Iba-1 while the eGFPTgBMSCs had a much more substantial impact on the presence of activated microglia in the twitcher brain. Perhaps the eGFPTgBMSCs had a greater therapeutic impact in the twitcher mice due to increased distribution of cells throughout the brain (data not shown) and increased cellular persistence in the brain compared to the eGFPTgASCs.
Mechanisms by which mesenchymal lineage stem cells mediate immunosuppression are still under investigation. However, evidence points to activation of this immunosuppressive response by MSCs by combinations of certain cytokines such as IFN-γ, TNFα, IL-1α, or IL-1β that could be present in an inflammatory niche of injured tissue [53]. Once activated, the MSCs may decrease proliferation of target cells or release of pro-inflammatory cytokines by cell-to-cell contact, secretion of soluble factors such as HLA-G5, IL-6, IL-10, TGFβ, HGF, nitric oxide, indoleamine 2,3-dioxygenase, or prostaglandins, or some combination thereof [54–60]. Chemokine dependent upregulation of iNOS by MSCs has been particularly implicated as a possible mechanism for immunosuppression [60]. Pro-inflammatory cytokines such as TNFα, IL-1α, and IL-1β were up-regulated in the twitcher brain and may have activated the immunosuppressive capabilities of the injected MSCs which in turn led to the down-regulation of many of the inflammatory mediators examined in this study.
Conclusion
The eGFPTgASCs and eGFPTgBMSCs utilized in this study for evaluation of mesenchymal stem cells as a therapeutic option for the treatment of KD provided some modest improvements in lifespan and motor function. They did not afford this improvement through enzyme replacement or cell replacement mechanisms, but by reducing markers of inflammation, macrophage infiltration, and microglial activation. The reduction of inflammation due to anti-inflammatory properties of the injected stem cells was apparently substantial enough to slow deterioration in the twitcher brain. The anti-inflammatory effects demonstrated by these mesenchymal-lineage stem cells in the twitcher mouse may be translatable to other models of neurodegeneration when chronic inflammation is a critical component of the pathology.
It is desirable and necessary to achieve greater improvements in the pathology of this disease in order to prevent or retard onset of symptoms in a clinical setting. The approach used in this study evidently only targets the inflammation in the CNS. Therefore, combining the use of these stem cells with other targeted approaches such as enzyme replacement therapy, BMT, substrate reduction therapy, or gene therapy may prove advantageous and merits further investigation.
Supplementary Material
Injected cells (green), eGFPTgASCs (top) and eGFPTgBMSCs (bottom), were located in cryosections of injected brains 10 days post injection and immunostained with the following antibodies (red): GFAP (left), MBP (middle), and Map2 (right) to investigate cellular differentiation along glial or neural lineages. None of the markers exhibited co-localization with the eGFP+ cells. Nuclei were stained with To-Pro-3 (blue).
GALC activity (nmol/h/mg protein) was measured in twitcher mouse brains when euthanized as well as wild-type mice at PND40. All twitcher mice, regardless of stem cell injection, had a GALC activity that was approximately 10% of wild-type. Neither eGFPTgASCs nor eGFPTgBMSCs managed to increase GALC activity in the brains of twitcher mice.
Acknowledgments
The authors would like to acknowledge the expertise or resources rendered by Roxanne Reger (stereotaxic injections), Dina Gaupp and Claire Llamas (histology), Dr. William Wimley (statistics), Dr. David Welsh (eGFP+ mice), and Dr. Lisa Morici (multiplex assay). The project described was supported by Award Number F31NS062588 from National Institutes of Neurological Disorders and Stroke (NINDS), NIH/NINDS R21-NS059665, Louisiana Gene Therapy Research Consortium, Pennington Biomedical Research Center, and Tulane University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or National Institutes of Health.
Grants and Funding:
The project described was supported by Award Number F31NS062588 from the National Institutes of Neurological Disorders and Stroke (NINDS), NIH/NINDS R21-NS059665, Louisiana Gene Therapy Research Consortium, Pennington Biomedical Research Center, and Tulane University.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
Author Contribution Summary
Cynthia B. Ripoll: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing
Mette Flaat: Conception and design, collection and/or assembly of data, data analysis and interpretation
Jessica Klopf-Eiermann: Collection and/or assembly of data
Jeanne M. Fisher-Perkins: Collection and/or assembly of data
Cynthia B. Trygg: Collection and/or assembly of data
Brittni A. Scruggs: Collection and/or assembly of data
Marjorie L. McCants: Collection and/or assembly of data
Helen Paige Leonard: Collection and/or assembly of data
Amy F. Lin: Collection and/or assembly of data
Shijia Zhang: Collection and/or assembly of data
Michelle E. Eagle: Collection and/or assembly of data
Xavier Alvarez: Collection and/or assembly of data
Yu Teh Li: Collection and/or assembly of data
Su Chen Li: Collection and/or assembly of data
Jeffrey M. Gimble: Conception and design
Bruce A. Bunnell: Conception and design, financial support, administrative support, final approval of manuscript
References
- 1.Suzuki K, Suzuki Y. Globoid cell leucodystrophy (Krabbe’s disease): deficiency of galactocerebroside beta-galactosidase. Proc Natl Acad Sci U S A. 1970;66:302–309. doi: 10.1073/pnas.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum Mutat. 1997;10:268–279. doi: 10.1002/(SICI)1098-1004(1997)10:4<268::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 1980;21:53–64. [PubMed] [Google Scholar]
- 4.Igisu H, Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984;224:753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- 5.Taniike M, Mohri I, Eguchi N, et al. An apoptotic depletion of oligodendrocytes in the twitcher, a murine model of globoid cell leukodystrophy. J Neuropathol Exp Neurol. 1999;58:644–653. doi: 10.1097/00005072-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Dolcetta D, Perani L, Givogri MI, et al. Design and optimization of lentiviral vectors for transfer of GALC expression in Twitcher brain. J Gene Med. 2006;8:962–971. doi: 10.1002/jgm.924. [DOI] [PubMed] [Google Scholar]
- 7.Wenger DA, Rafi MA, Luzi P, et al. Krabbe disease: genetic aspects and progress toward therapy. Mol Genet Metab. 2000;70:1–9. doi: 10.1006/mgme.2000.2990. [DOI] [PubMed] [Google Scholar]
- 8.Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 9.Duffner PK, Caviness VS, Jr, Erbe RW, et al. The long-term outcomes of presymptomatic infants transplanted for Krabbe disease: report of the workshop held on July 11 and 12, 2008, Holiday Valley, New York. Genet Med. 2009;11:450–454. doi: 10.1097/GIM.0b013e3181a16e04. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, Yamanaka T, Jacobs JM, et al. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease) Brain Res. 1980;202:479–483. doi: 10.1016/0006-8993(80)90159-6. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K. The twitcher mouse: a model for Krabbe disease and for experimental therapies. Brain Pathol. 1995;5:249–258. doi: 10.1111/j.1750-3639.1995.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin D, Donsante A, Macauley S, et al. Central nervous system-directed AAV2/5-mediated gene therapy synergizes with bone marrow transplantation in the murine model of globoid-cell leukodystrophy. Mol Ther. 2007;15:44–52. doi: 10.1038/sj.mt.6300026. [DOI] [PubMed] [Google Scholar]
- 13.Pellegatta S, Tunici P, Poliani PL, et al. The therapeutic potential of neural stem/progenitor cells in murine globoid cell leukodystrophy is conditioned by macrophage/microglia activation. Neurobiol Dis. 2006;21:314–323. doi: 10.1016/j.nbd.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Yeager AM, Brennan S, Tiffany C, et al. Prolonged survival and remyelination after hematopoietic cell transplantation in the twitcher mouse. Science. 1984;225:1052–1054. doi: 10.1126/science.6382609. [DOI] [PubMed] [Google Scholar]
- 15.Hoogerbrugge PM, Poorthuis BJ, Romme AE, et al. Effect of bone marrow transplantation on enzyme levels and clinical course in the neurologically affected twitcher mouse. J Clin Invest. 1988;81:1790–1794. doi: 10.1172/JCI113521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K, Hoogerbrugge PM, Poorthuis BJ, et al. The twitcher mouse. Central nervous system pathology after bone marrow transplantation. Lab Invest. 1988;58:302–309. [PubMed] [Google Scholar]
- 17.Lee WC, Courtenay A, Troendle FJ, et al. Enzyme replacement therapy results in substantial improvements in early clinical phenotype in a mouse model of globoid cell leukodystrophy. FASEB J. 2005;19:1549–1551. doi: 10.1096/fj.05-3826fje. [DOI] [PubMed] [Google Scholar]
- 18.Shen JS, Watabe K, Ohashi T, et al. Intraventricular administration of recombinant adenovirus to neonatal twitcher mouse leads to clinicopathological improvements. Gene Ther. 2001;8:1081–1087. doi: 10.1038/sj.gt.3301495. [DOI] [PubMed] [Google Scholar]
- 19.Torchiana E, Lulli L, Cattaneo E, et al. Retroviral-mediated transfer of the galactocerebrosidase gene in neural progenitor cells. Neuroreport. 1998;9:3823–3827. doi: 10.1097/00001756-199812010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Taylor RM, Lee JP, Palacino JJ, et al. Intrinsic resistance of neural stem cells to toxic metabolites may make them well suited for cell non-autonomous disorders: evidence from a mouse model of Krabbe leukodystrophy. J Neurochem. 2006;97:1585–1599. doi: 10.1111/j.1471-4159.2006.03986.x. [DOI] [PubMed] [Google Scholar]
- 21.Meng XL, Shen JS, Watabe K, et al. GALC transduction leads to morphological improvement of the twitcher oligodendrocytes in vivo. Mol Genet Metab. 2005;84:332–343. doi: 10.1016/j.ymgme.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, Fantz CR, Levy B, et al. AAV2/5 vector expressing galactocerebrosidase ameliorates CNS disease in the murine model of globoid-cell leukodystrophy more efficiently than AAV2. Mol Ther. 2005;12:422–430. doi: 10.1016/j.ymthe.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Croitoru-Lamoury J, Williams KR, Lamoury FM, et al. Neural transplantation of human MSC and NT2 cells in the twitcher mouse model. Cytotherapy. 2006;8:445–458. doi: 10.1080/14653240600879152. [DOI] [PubMed] [Google Scholar]
- 24.Biswas S, Biesiada H, Williams TD, et al. Substrate reduction intervention by L-cycloserine in twitcher mice (globoid cell leukodystrophy) on a B6;CAST/Ei background. Neurosci Lett. 2003;347:33–36. doi: 10.1016/s0304-3940(03)00633-5. [DOI] [PubMed] [Google Scholar]
- 25.Biswas S, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res. 2002;51:40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Galbiati F, Givogri MI, Cantuti L, et al. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. J Neurosci Res. 2009;87:1748–1759. doi: 10.1002/jnr.22006. [DOI] [PubMed] [Google Scholar]
- 27.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 28.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Le Blanc K, Ringden O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18:586–591. doi: 10.1016/j.coi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 31.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 33.Yanez R, Lamana ML, Garcia-Castro J, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 34.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 39.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu YP, McMahon EJ, Matsuda J, et al. Expression of immune-related molecules is downregulated in twitcher mice following bone marrow transplantation. J Neuropathol Exp Neurol. 2001;60:1062–1074. doi: 10.1093/jnen/60.11.1062. [DOI] [PubMed] [Google Scholar]
- 41.Giri S, Jatana M, Rattan R, et al. Galactosylsphingosine (psychosine)-induced expression of cytokine-mediated inducible nitric oxide synthases via AP-1 and C/EBP: implications for Krabbe disease. FASEB J. 2002;16:661–672. doi: 10.1096/fj.01-0798com. [DOI] [PubMed] [Google Scholar]
- 42.Kagitani-Shimono K, Mohri I, Fujitani Y, et al. Anti-inflammatory therapy by ibudilast, a phosphodiesterase inhibitor, in demyelination of twitcher, a genetic demyelination model. J Neuroinflammation. 2005;2:10. doi: 10.1186/1742-2094-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Formichi P, Radi E, Battisti C, et al. Psychosine-induced apoptosis and cytokine activation in immune peripheral cells of Krabbe patients. J Cell Physiol. 2007;212:737–743. doi: 10.1002/jcp.21070. [DOI] [PubMed] [Google Scholar]
- 44.Borda JT, Alvarez X, Mohan M, et al. Clinical and immunopathologic alterations in rhesus macaques affected with globoid cell leukodystrophy. Am J Pathol. 2008;172:98–111. doi: 10.2353/ajpath.2008.070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ripoll CB, Bunnell BA. Comparative characterization of mesenchymal stem cells from eGFP transgenic and non-transgenic mice. BMC Cell Biol. 2009;10:3. doi: 10.1186/1471-2121-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsushima GK, Taniike M, Glimcher LH, et al. Absence of MHC class II molecules reduces CNS demyelination, microglial/macrophage infiltration, and twitching in murine globoid cell leukodystrophy. Cell. 1994;78:645–656. doi: 10.1016/0092-8674(94)90529-0. [DOI] [PubMed] [Google Scholar]
- 47.Raghavan S, Krusell A. Optimal assay conditions for enzymatic characterization of homozygous and heterozygous twitcher mouse. Biochim Biophys Acta. 1986;877:1–8. doi: 10.1016/0005-2760(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 48.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luzi P, Abraham RM, Rafi MA, et al. Effects of treatments on inflammatory and apoptotic markers in the CNS of mice with globoid cell leukodystrophy. Brain Res. 2009;1300:146–158. doi: 10.1016/j.brainres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laroux FS, Pavlick KP, Hines IN, et al. Role of nitric oxide in inflammation. Acta Physiol Scand. 2001;173:113–118. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 52.Nathan C, Calingasan N, Nezezon J, et al. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 55.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 56.Groh ME, Maitra B, Szekely E, et al. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 58.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 59.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 60.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Injected cells (green), eGFPTgASCs (top) and eGFPTgBMSCs (bottom), were located in cryosections of injected brains 10 days post injection and immunostained with the following antibodies (red): GFAP (left), MBP (middle), and Map2 (right) to investigate cellular differentiation along glial or neural lineages. None of the markers exhibited co-localization with the eGFP+ cells. Nuclei were stained with To-Pro-3 (blue).
GALC activity (nmol/h/mg protein) was measured in twitcher mouse brains when euthanized as well as wild-type mice at PND40. All twitcher mice, regardless of stem cell injection, had a GALC activity that was approximately 10% of wild-type. Neither eGFPTgASCs nor eGFPTgBMSCs managed to increase GALC activity in the brains of twitcher mice.