Summary
Despite their efficacy in myeloma, corticosteroids have acute and chronic toxicities. Newer agents with significant anti-myeloma activity permit the development of steroid-free regimens. We designed a Phase II clinical trial to study the toxicity and efficacy of a steroid-free combination of bortezomib and thalidomide as a first-line treatment in patients with symptomatic myeloma. Patients received bortezomib 1·3 mg/m2 on days 1, 4, 8 and 11 every 21 d and thalidomide 150 mg/d for a maximum of eight cycles. Amongst 27 evaluable patients, the overall response was 81·5% with 25·8% near complete response or greater. The response rate was comparable to most other two drug combinations for upfront therapy but lower than that obtained with the use of three drugs. The most common grade 3 toxicities were peripheral neuropathy (22%), pneumonia (15%), fatigue (7%) and anaemia (7%). Peripheral neuropathy completely resolved in 80% of the patients upon completion of therapy, but not in the remaining 20% of patients. No venous thromboembolic events were observed even in the absence of prophylactic anticoagulation. The median progression-free survival was 16·8 months (95% confidence interval 8·7–21·6 months). Median overall survival has not yet been reached at a median follow up of 39 months. The 3-year overall survival was 74%. This study demonstrates: (i) the efficacy of a steroid-free regimen; (ii) mostly reversible treatment-related peripheral neuropathy; and (iii) the absence of venous thrombotic events.
Keywords: bortezomib, thalidomide, multiple myeloma
A major advance in multiple myeloma in the last decade was the increase in overall response rates (ORRs) and improvement in overall survival (OS) largely attributable to proteosome inhibitors and immunomodulatory drugs. Bortezomib is a potent, selective, reversible inhibitor of the 26s proteosome (Adams et al, 1999; Orlowski et al, 2002). In previously untreated patients, bortezomib has been used as a single agent and in combination with dexamethasone with a response rate of 40% and 88% respectively (Jagannath et al, 2005). In three additional trials for newly diagnosed myeloma, bortezomib and dexamethasone as induction therapy yielded post-induction response rates of 67–86% (Harousseau et al, 2006, 2007; Corso et al, 2007a). More recently, combinations of bortezomib with thalidomide and prednisone (VTP) or melphalan and prednisone (VMP) have been explored (Mateos et al, 2009), yielding response rates of 81% and 80% respectively. These induction studies have been expanded to include maintenance regimens with either thalidomide (VT) or prednisone (VP) with an increased complete response (CR) rate from 23% to 42%. With a median duration of maintenance of 13 months, there is a trend in favour of VT compared to VP in terms of 1-year time-to-progression (84% vs. 71%; P = 0·05).
While corticosteroids have historically been the backbone of most myeloma-targeted therapies, their efficacy must be balanced with their considerable side-effect profile. Pre-clinical evidence supports the synergistic anti-myeloma effect of immunomodulatory drugs (IMIDs) with bortezomib (Mitsiades et al, 2002). Taken together, we sought to develop a steroid-free regimen combining an IMID with bortezomib as first-line treatment in patients with symptomatic multiple myeloma to determine its efficacy in a steroid-free regimen.
This design enabled the determination of: (i) the efficacy of this steroid-free combination; (ii) the toxicity profile of a steroid-free regimen; and (iii) the effect of two neuropathic agents on the development and progression of peripheral neuropathy (reported elsewhere (Chaudhry et al, 2008).
Methods
Study design
This was a single institution, single arm, safety and efficacy trial to determine the treatment effect as measured by the anti-tumour response [partial response (PR) + very good partial response (VGPR) + near (n)CR + CR] and assess toxicity associated with the combination of bortezomib + thalidomide. The trial used a Simon two-stage design to test the null hypothesis that the overall objective response rate was 35% versus an alternative response rate of 65% with 90% power at a significant level of 0·05. Twelve or more objective responders among a total of 25 patients would be considered positive results for the trial. The trial was designed to enrol up to 35 patients to ensure 25 evaluable patients who completed four cycles of the treatment. A total of 30 patients actually signed consents for the clinical trial.
Patient eligibility and selection
Newly diagnosed, untreated patients or treated with less than one full cycle of a prior regimen with Durie Salmon Stage II/ III, and Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 were eligible for this study. All patients were required to have measurable disease as defined by a detectable paraprotein in either serum or urine by electropheresis or measurable light chains by the serum free light chain assay. Patients were excluded from the study if they had baseline peripheral neuropathy ≥ grade 2, corticosteroid administration within 14 d of enrollment or were human immunodeficiency virus positive.
All patients were enrolled and treated at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins between September 2004 and October 2006. The study was approved by the Johns Hopkins Institutional Review Board and all patients gave written informed consent before entering the study, in accordance to the Declaration of Helsinki.
Treatment schema
Bortezomib was administered intravenously at an initial dose of 1·3 mg/m2 on days 1, 4, 8, 11 every 21 days. A known adverse effect of thalidomide is neurotoxicity. Although 200 mg is often considered as the standard dose of thalidomide, because it was being combined with another agent with known neurotoxicity, a dose of 150 mg was chosen that was still likely have the therapeutic efficacy but possibly less toxicity. Thalidomide was administered orally at bedtime starting with day 1 of the first cycle of bortezomib at an initial dose of 50 mg and escalated by 50 mg each week to 150 mg/d. Patients were treated for a minimum of four and maximum of eight cycles. Study duration was for a maximum of 8 cycles of bortezomib and thalidomide. All the clinical responses [CR, nCR, VGPR, stable disease (SD) and progressive disease (PD)] were measured during the study period. No maintenance regimen was built into the study although patients continued on therapy if they were responding and tolerating the treatment. Twenty-one patients completed eight cycles of bortezomib and thalidomide therapy.
Deep venous thrombosis prophylaxis was not administered considering this was a non-steroid containing regimen. No zoster prophylaxis was required. The maximum total planned doses for bortezomib and thalidomide were 41·6 mg/m2 and 24·1 g respectively. Five patients (18·5%) received a total planned dose of bortezomib and two patients (7·4%) received the total planned dose of thalidomide. The mean total bortezomib dose was 33·3 mg/m2 (11·4–41·6 mg/m2) or 80% of the total planned dose. The mean total thalidomide dose was 16·4 g (3·3–24·1 g) or 68% of the total planned dose. This corresponded to an average bortezomib dose of 1·1 mg/m2 and average daily thalidomide dose of 110 mg.
Cytogenetics
Overnight, unstimulated bone marrow cultures were performed according to standard protocols. Twenty cells were analysed for each case.
Fluorescence in situ hybridization (FISH) was performed with probes for 3cen (D3Z1), 4p16·3 (FGFR3), 7cen (D7Z1), 9cen (D9Z1), 11q13 (CCND1-XT), 13q14 (Rb1), 13q34 (LAMP1), 14q32 (5′IGH, 3′IGH), 14q32 (IGH-XT), 15cen (D15Z4), 16q23 (c-MAF), 17p12 (p53) and 17cen (D17Z1). Two-hundred interphase nuclei were evaluated for each probe and performed at the Mayo Clinic labs (Fonseca et al, 2003).
Toxicity and response evaluation
Dose adjustments of both agents were performed based on neuropathy (Table I). Therapy was discontinued if there was evidence of progressive disease. Adverse events were reported and graded using the National Cancer Institute Common Toxicity Criteria Version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). In addition, neuropathy was monitored using Total Neuropathy Score reduced (TNSr) and has been reported previously (Chaudhry et al, 2008).
Table I.
Bortezomib and thalidomide dose-reduction steps.
| Bortezomib (mg/m2) | Thalidomide | |
|---|---|---|
| Starting dose | 1·3 | 150 mg/d |
| Dose level-1 | 1 | 100 mg/d |
| Dose level-2 | 0·7 | 50 mg/d |
| Dose level-3 | 0·5 | 50 mg day before and day of bortezomib |
Clinical evaluations of disease response were determined with each cycle. Bone marrow biopsies were done at baseline and at study termination. Clinical responses were defined by the International Myeloma Working Group criteria (Durie et al, 2006). Briefly, complete response (CR) was defined as absence of detectable paraprotein by electrophoresis and immunofixation. Stringent CR was defined as CR plus normalization of the free light chain ratio and absence of clonal plasma cells in the bone marrow. nCR was defined as the absence of detectable paraprotein by electrophoresis but detectable by immunofixation. Very good partial response (VGPR) was a 90% reduction in the paraprotein and partial response (PR) was a 50% reduction in paraprotein. Progressive disease (PD) was a 25% increase in paraprotein with an absolute increase ≥5·0 g/l for serum or ≥200 mg/24 h for urine. Stable disease (SD) was defined as not meeting criteria for CR, nCR, VGPR, PR and PD. All results were confirmed with a second test at a subsequent date.
Data analysis
A total of 27 patients were evaluable for the final analysis. The progression-free survival (PFS) was defined as the time that patients were alive and progression-free from initiation of treatment to the date disease progression was documented. Patients alive and progression-free at the time of this analysis were censored at their last date known to be progression-free. The data analysis cut-off date was May 1, 2009. Overall survival (OS) was defined as the survival time from the date of treatment start to the date of death occurrence. Patients who had not died by the time of this data analysis were censored at their last date known to be alive. The proportions of responses were estimated using binomial distribution (exact method) along with 95% confidence intervals (CIs). The probabilities of PFS and OS were estimated using the Kaplan-Meier method, and 95% CIs were constructed by the formula of Greenwood. Log-rank statistics was used to assess the difference in PFS between the patients with different stages of disease and different clinical responses. All P-values are two-sided with a significant level of 0·05. All analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 30 patients were enrolled between September 8, 2004 and April 11, 2006. Those patients who received at least four cycles of bortezomib and thalidomide were considered evaluable. Three patients were not evaluable for analysis. One patient withdrew consent prior to receiving therapy. A second patient received steroids for haemolytic anaemia after the first two doses of bortezomib. A third patient was unable to obtain thalidomide due to insurance denial and received treatment with dexamethasone. The characteristics of the 27 evaluable patients are shown in Table II. The median age was 58 years (36–83 years). A total of 63% of patients had Durie Salmon Stage III and 33·3% of patients had high-risk disease, as defined by the International Staging System (ISS) (Table II). The average baseline laboratory values including β2 microglobulin and albumin are shown in Table III.
Table II.
Patient and clinical characteristics (N = 27).
| Percentage | Number | |
|---|---|---|
| Male | 33·3 | 9 |
| White | 85·2 | 23 |
| Black | 14·8 | 4 |
| Durie Salmon stage | ||
| Stage II | 37 | 10 |
| Stage III | 63 | 17 |
| International staging system | ||
| Stage I | 37 | 10 |
| Stage II | 29·6 | 8 |
| Stage III | 33·3 | 9 |
| IgA | 22·2 | 6 |
| IgG | 66·6 | 18 |
| Light chain disease | 11·1 | 3 |
| Eastern Cooperative Oncology Group performance score | ||
| 0 | 40·7 | 11 |
| 1 | 51·8 | 14 |
| 2 | 7·4 | 2 |
| Lytic bone lesions | 55·5 | 15 |
Table III.
Baseline laboratory values.
| Variable | Median | Range |
|---|---|---|
| β2 microglobulin (g/l) | 3·4 | 1·8–36·9 |
| Albumin (g/l) | 35 | 23–52 |
| Serum Creatinine (μmol/l) | 79·56 | 53·04–282·88 |
| WBC count (×109/l) | 5·69 | 1·95–11·95 |
| Haemoglobin (g/l) | 109 | 80–140 |
| Platelet count (×109/l) | 230 | 85–707 |
| Serum M spike (g/l) | 41 | 0–95 |
| Urine M spike (mg/24 h) | 95 | 0–9864 |
| Serum free κ chain (mg/l) | 31·8 | 0·1–4190 |
| Serum free λ chain (mg/l) | 11·8 | 0·1–1470 |
Baseline conventional cytogenetic and FISH analyses were performed on 25 of 27 patients. Six patients (24%) had high-risk disease based on presence of either deletion 17p, t(4:14) or t(14:16) by FISH and 13q deletion by conventional cytogenetics. Trisomy 11 was seen in seven patients (28%) and more than one abnormality was found in 23 of patients (92%).
Response to therapy
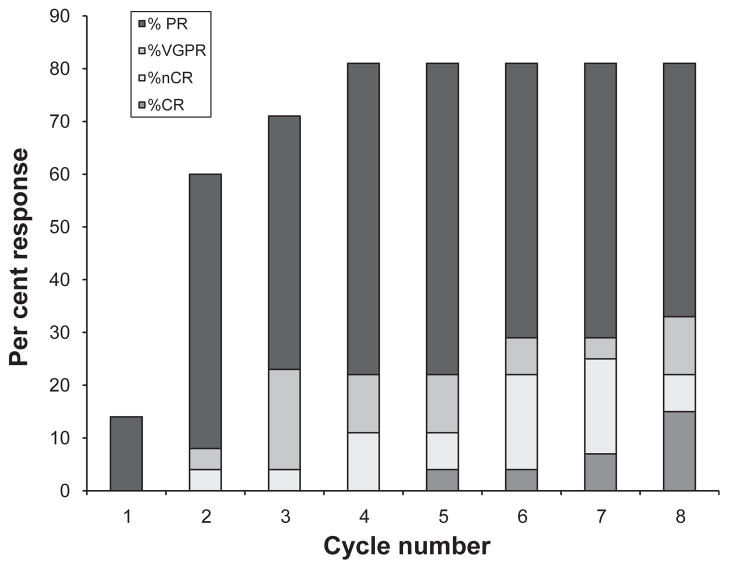
A total of 22 of 27 patients (81·5%, 95% CI: 62–94%) achieved an objective response (at least PR) to therapy (Table IV). On an intent-to-treat analysis 22 of 30 patients (73·3%) achieved an objective response (Table IV). Of 27 patients, 7 (25·9%) achieved nCR or better with 3 (11·1%) obtaining a true CR (absence of M-protein by immunofixation, and a marrow biopsy negative by immunohistochemistry). Amongst the three patients with CR, two achieved a stringent CR. Two patients (7·4%) achieved a 90% reduction in the paraprotein (VGPR) and 13 patients (48·1%) achieved a PR. Median time to first response, as defined by a >50% decrease of M-protein, was 1·2 months (range, 0·4–3·3 months), whereas median time to maximum response was 2 months (range, 0·5–5·8 months). Patterns of response per treatment cycle are depicted in Fig 1. A total of 9 patients (33·3%; 95% CI: 17–54%) achieved a >90% reduction (CR + nCR + VGPR) in M-protein. Four patients (14·81%) had SD and one patient (3·7%) had PD. This patient initially had SD but later developed PD. FISH analysis of bone marrow at diagnosis had shown a t(4;14).
Table IV.
Clinical response to treatment (N = 27 evaluable), (N = 30 Intention to treat (ITT)).
| Response | Number | Percentage (95% CI) Evaluable | Percentage (95% CI) ITT |
|---|---|---|---|
| Complete response | 3 | 11·1 (2–29) | 10 (2–27) |
| Near complete response | 4 | 14·8 (4–34) | 13·3 (4–31) |
| Very good partial response | 2 | 7·4 (1–24) | 6·6 (1–22) |
| Partial response | 13 | 48·1 (29–68) | 43·3 (26–63) |
| Overall response | 22 | 81·5 (62–94) | 73·3 (54–88) |
| Progressive disease | 1 | 3·7 (0–19) | 3·3 (0–17) |
Fig 1.
Patterns of response in 27 patients on bortezomib and thalidomide per treatment cycle. Median time to first response, 1·2 months (range, 0·4–3·3 months); median time to maximal response, 2 months (range, 0·5–5·8 months).
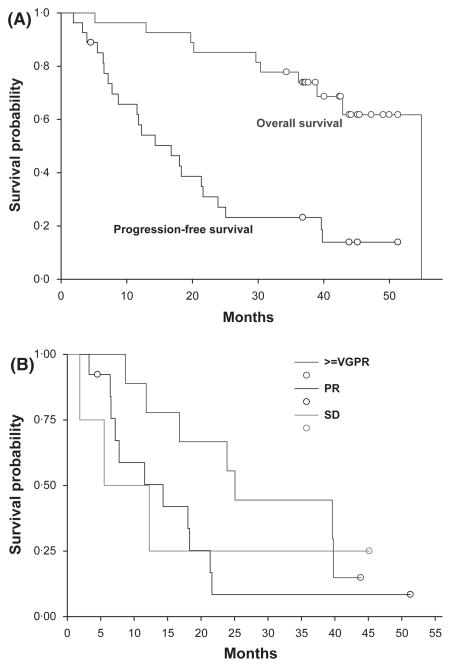
The median PFS was 16·8 months (95% CI: 8·7, 21·6 months). Twenty patients lived more than 3 years from initiation of treatment. Median OS has not been reached at the time of data analysis. The actuarial 3-year OS was 74% (95% CI: 54–89%) (Fig 2A). Four patients (15%) eventually went on to receive an autologous transplant as subsequent therapy. The average time to transplant from diagnosis was 28 months (range, 21–33 months). As such, no patient enrolled in this study underwent transplantation immediately upon completion of the 4–8 cycles of induction therapy.
Fig 2.
Kaplan–Meier Curves for the Time to Disease Progression and Overall Survival. (A) The median progression-free survival was 16·8 months (95% CI 8·7–21·6 months). Median overall survival has not yet been reached at a median follow up of 39 months. The 3-year overall survival was 74% (95%CI: 54–89%). (B) There was a trend to improved progression-free survival for patients achieving a better than very good partial response (VGPR; blue), when compared to those who had a partial response (PR; red) or stable disease (SD; black).
There was a trend towards improved PFS for patients with nCR or better (Fig 2B). The median PFS for patients who achieved a VGPR or better was 25·1 months (95% CI: 16·8–39·8 months) as compared to 14·3 months (95% CI: 7·2–18·3 months) for patients who achieved a PR or 8·9 months for patients with SD. The differences in PFS between these three groups were not statistically significant, probably due to the small sample size although there was a trend towards improved PFS in patients with at least a VGPR. The PFS was not affected by the Durie Salmon stage (P = 0·15) or ISS stage (P = 0·49).
Adverse events
Grade 2 and higher toxicities are summarized in Table V. There were no grade 4 toxicities. The most common grade 3 toxicity was peripheral neuropathy. The results and characteristics of bortezomib- and thalidomide-induced peripheral neuropathy have been previously reported (Chaudhry et al, 2008). Six (22%) patients developed grade 3 neuropathy and 13 (48%) patients had grade 2 neuropathy. The mean time for development of > grade 2 neuropathy was 2·1 months. The neuropathy was reversible with complete resolution of the symptoms in 80% of patients upon discontinuation of treatment.
Table V.
Grade 2 and higher adverse events.
| Events | Grade 2 (%) | Grade 3 (%) |
|---|---|---|
| Haematological disorders | ||
| Anaemia | 3 (11) | 2 (7) |
| Neutropenia | 3 (11) | 0 |
| Gastrointestinal disorders | ||
| Anorexia | 4 (15) | 2 (7) |
| Diarrhoea | 9 (33) | 1 (4) |
| Constipation | 14 (52) | 0 |
| Nervous system disorders | ||
| Peripheral neuropathy | 13 (48) | 6 (22) |
| Musculoskeletal | ||
| Leg cramps | 5 (19) | 1 (4) |
| Infections | ||
| Herpes zoster | 3 (11) | 1 (4) |
| Pneumonia | 2 (7) | 4 (15) |
| Cardiac | ||
| Hypotension | 6 (22) | 0 |
| Other | ||
| Fatigue | 13 (48) | 2 (7) |
| Rash | 5 (19) | 0 |
The most common grade 2 gastrointestinal toxicity was constipation seen in 14 (52%) patients. Constipation is a known adverse effect of thalidomide. One patient had grade 3 diarrhoea, probably from autonomic dysfunction, which resolved upon discontinuation of treatment.
Herpes zoster reactivation developed in 4 (15%) patients. Zoster prophylaxis was not mandated by the study. Six patients developed pneumonia, of which two required hospitalization. Other notable grade 2 adverse effects were fatigue (48%), hypotension (22%) and leg cramps (19%). With the exception of peripheral neuropathy, the incidence of grade 3 adverse events was low. Importantly, no deep venous thrombosis or pulmonary embolism occurred in the absence of thromboembolic prophylaxis. Interestingly, other induction regimens with thalidomide and dexamethasone (TD) have reported incidences of thromboembolic events ranging from 14% to 26% (Rajkumar et al, 2002, 2006; Cavo et al, 2004).
Discussion
This phase 2 trial utilized bortezomib and thalidomide for treatment-naive, symptomatic myeloma and showed 81·5% ORR with a combined CR/nCR rate of 25·9%. The combination therapy was well tolerated. None of the patients in this study went on to autologous stem cell transplantation immediately upon completion of 4–8 cycles. The disease-free survival (DFS) thus reflects the durable efficacy of this regimen. The absence of hyperglycaemic or thromboembolic events is attributable to the absence of corticosteroids in this regimen. Despite the absence of steroids, the time to response did not appear significantly delayed. The median time to a first response was 1·2 months and median time for best response was 2 months. This compares favourably with steroid containing regimens, such as VMP and VD, with a median time to response of 1·4 months and 2 months respectively (Jagannath et al, 2005; San Miguel et al, 2008), and TD of 1·1–1·9 months (Rajkumar et al, 2006, 2008; Ludwig et al, 2009). This steroid-free combination can achieve rapid responses similar to those observed with steroid-containing regimens yet eliminate the major steroid-related toxicities – specifically, thromboembolic events and hyperglycaemia.
These highly active, non cross-reactive drugs have demonstrated significant synergy translating into increased CR rates, improvement in DFS and OS as compared to conventional cytotoxic chemotherapy or their activity as single agents. Bortezomib and dexamethasone has shown a post-induction ORR ranging from 65% to 88%, with an incidence of grade 3 or 4 peripheral neuropathy of 6–12% (Harousseau et al, 2006, 2007, Jagannath et al, 2006; Corso, et al 2007b; Rosinol et al, 2007). The randomized trial of TD compared with dexamethasone showed a response rate of 63% vs. 41% respectively (Rajkumar et al, 2006). Our study using bortezomib and thalidomide produced a high ORR, similar to other two-drug combinations with no venous thromboembolic events even in the absence of prophylactic anticoagulation. The higher response rate observed with thalidomide and bortezomib as compared to thalidomide and dexamethasone may be due to better synergy between bortezomib and thalidomide than between thalidomide and dexamethasone or bortezomib and dexamethasone. The randomized phase III trial comparing TD with VTD (bortezomib, thalidomide and dexamethasone) followed by autologous stem cell transplant in previously untreated myeloma from the Italian Myeloma Group showed a CR rate of 19% and >VGPR rate of 62% (Cavo et al, 2007, 2009). Two other groups have used VTD induction therapy and reported ORR (87–92%), CR (19–21%) and neuropathy (37–39%) (Kaufman et al, 2007; Wang et al, 2007). Despite the use of prophylactic anticoagulation, patients developed venous thromboembolic events. The slightly higher response rate observed with VTD as compared to VT alone is probably due to the additional anti-myeloma activity of dexamethasone. However, it is unclear whether this ultimately translates into improved DFS or OS. Data from the trial comparing high versus low dose dexamethasone in combination with lenalidomide demonstrated superior 2-year OS with low dose dexamethasone despite a higher response rate with high dose steroids. This difference in outcomes was related to steroid toxicities, especially in elderly patients (Rajkumar et al, 2010). Subpopulations of patients clearly exist for whom even low dose steroids are associated with unacceptable toxicities. As such, a steroid-free regimen with significant clinical activity offers significant appeal.
The high incidence of peripheral neuropathy poses a significant barrier to the use of this combination. The mean doses in this study were bortezomib 1·1 mg/m2 and thalidomide 110 mg. Two different approaches are currently employed aimed at reducing the neuropathy. The French are comparing VD-standard dose bortezomib biweekly and dexamethasone (VD) with vTD – low dose bortezomib (1·0 mg/m2 biweekly), thalidomide 100 mg/d and dexamethasone (Harousseau et al, 2009). Alternatively, weekly bortezomib at full dose in combination with other agents is also being utilized and showing a better toxicity profile. Weekly versus twice weekly bortezomib when used in combination with melphalan, prednisone and thalidomide reduced the peripheral neuropathy from 45% to 27% (Gay et al, 2009). Although the CR rate was lower in the once a week bortezomib group (25% vs. 33%), the 2-year PFS and OS were not statistically different in the two groups. Recently, a combination of bortezomib, thalidomide and liposomal doxorubicin resulted in a greater than grade 2 peripheral neuropathy rate of 20% (Sher et al, 2009), which was significantly lower than what we report in our study. Sher et al (2009) administered bortezomib on days 1, 4, 15, and 18 every 28 d as compared to our study, where bortezomib was administered on days 1, 4, 8, 11 of a 21-d cycle. The increased interval between bortezomib doses might account for less neurotoxicity by allowing neurons to recover before subsequent dosing. A Phase III trial was conducted for newly diagnosed myeloma patients, not eligible for transplant (Mateos et al, 2009). Following the initial randomization of VMP or VTP, the maintenance therapy was further randomized to bortezomib and thalidomide (VT) or bortezomib and prednisone (VP) (Mateos et al, 2009). Median PFS was not reached for the VT maintenance arm and was superior to the other arms. This suggests the superiority of bortezomib and thalidomide at least in maintenance therapy.
This report demonstrated the clinical efficacy and durability of the steroid-free combination of thalidomide and bortezomib that represents a viable treatment regimen for patients at high risk of thromboembolic events or with steroid intolerance. Neurotoxicity represents the major adverse event associated with this combination. However, this was reversible in the majority of patients with careful monitoring and aggressive dose adjustments. Neurotoxicity could conceivably be reduced with lower doses of both agents, weekly schedule of bortezomib or, possibly, combinations of bortezomib with newer immunomodulatory drugs, such as lenalidomide.
Recently, a randomized Phase III trial was conducted to compare the efficacy of the steroid-free combination of rituximab and bendamustine to that of rituximab, cyclophosphamide, vincristine, adriamycin and prednisone (R-CHOP) (Rummel et al, 2009). The steroid-free combination of rituximab and bendamustine was superior to R-CHOP with respect to PFS and CR rate. In the current report we provide provocative phase II data about the possibility of treating myeloma without steroids. We suggest that the efficacy and toxicity of a steroid-free regimen should be confirmed in a randomized phase III trial comparing it to a widely used and efficacious steroid-inclusive regimen.
References
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Research. 1999;59:2615–2622. [PubMed] [Google Scholar]
- Cavo M, Zamagni E, Tosi P, Cellini C, Cangini D, Tacchetti P, Testoni N, Tonelli M, de Vivo A, Palareti G, Tura S, Baccarani M. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologica. 2004;89:826–831. [PubMed] [Google Scholar]
- Cavo M, Patriarca F, Tacchetti P, Galli M, Perrone G, Petrucci MT, Brioli A, Bringhen S, Pantani L, Tosi P, Crippa C, Zamagni E, Di Raimondo F, Narni F, Cellini C, Ceccolini M, Pescosta N, Goldaniga MC, Montefusco V, Callea V, De Stefano V, Caravita T, Boccadoro M, Baccarani M. Bortezomib (Velcade(R))-thalidomide-dexamethasone (VTD) vs thalidomide-dexamethasone (TD) in preparation for autologous stem-cell (SC) transplantation (ASCT) in newly diagnosed multiple myeloma (MM) Blood (ASH Annual Meeting Abstracts) 2007;110:73. [Google Scholar]
- Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, Raimondo FD, Crippa C, Bringhen S, Offidani M, Narni F, Montefusco V, Zamagni E, Spadano T, Pescosta N, Baldini L, Cellini C, Caravita T, Ledda A, Falcone A, Tosi P, Nozzoli C, Zambello R, Masini L, Agostini P, Fiacchini M, Baccarani M. A phase III study of double auto-transplantation incorporating bortezomib-thalidomide- dexamethasone (VTD) or thalidomide-dexamethasone (TD) for multiple myeloma: superior clinical outcomes with VTD compared to TD. Blood (ASH Annual Meeting Abstracts) 2009;114:351. [Google Scholar]
- Chaudhry V, Cornblath DR, Polydefkis M, Ferguson A, Borrello I. Characteristics of bortezomib- and thalidomide-induced peripheral neuropathy. Journal of the Peripheral Nervous System. 2008;13:275–282. doi: 10.1111/j.1529-8027.2008.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso A, Barbarano L, Mangiacavalli S, Montalbetti L, Brasca P, Zappasodi P, Carella A, Spriano M, Alessandrino E, Cairoli R, Petrò D, Varettoni M, Bernasconi P, Lazzarino M, Morra E. Bortezomib with high-dose dexamethasone as first line therapy in patients with multiple myeloma candidates to high-dose therapy. Blood. 2007a;110:3595. [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA, Dewald GW, Van Ness B, Van Wier SA, Henderson KJ, Bailey RJ, Greipp PR. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101:4569–4575. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- Gay F, Brirnghen S, Genuardi M, Rossi D, Ria R, Romano A, Ferrara F, Di Renzo N, Dominietto A, Andriani A, Rizzi R, Vallone R, Mele G, Storti S, Podda L, Aitoro G, Mettivier V, Annibali O, Rossini F, Gentilini P, Pavone V, Giuliani N, Rauco A, Baraldi A, Capaldi A, Gherlinzoni F, Gaidano G, Boccadoro M, Palumbo A. The weekly infusion of bortezomib reduces peripheral neuropathy. Blood (ASH Annual Meeting Abstracts) 2009;114:3887. [Google Scholar]
- Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, Hulin C, Benboubker L, Fuzibet JG, Renaud M, Moreau P, Avet-Loiseau H. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91:1498–1505. [PubMed] [Google Scholar]
- Harousseau JL, Mathiot C, Attal M, Marait G, Caillot D, Mohty M, Hullin C, Facon T, Webb I, Moreau P. VELCADE/Dexamethasone (Vel/D) versus VAD as induction treatment prior to autologous stem cell transplantion (ASCT) in newly diagnosed multiple myeloma (MM): updated results of the IFM 2005/01 trial. Blood. 2007;110:139a. [Google Scholar]
- Harousseau JL, Avet-Loiseau H, Facon T, Attal M, Doyen C, Hulin C, Garderet L, Tiab M, Lepeu G, Aranjo C, Cailliot D, Petillon m-O, Mathiot C, Mary JY, Moreau P. Bortezomib plus dexamethasone (VD) versus reduced-dose bortezomib plus thalidomide plus dexametasone (vTD) as induction treatment prior to autologous stem-cell transplantation (ASCT) in newly diagnosed multiple myeloma (MM) Blood (ASH Annual Meeting Abstracts) 2009;114:354. [Google Scholar]
- Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. British Journal of Haematology. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J. Longterm follow up of patients treated with bortezomib alone or in combination with dexamethasone as frontline therapy for multiple myeloma. Blood. 2006;108:238a–239a. [Google Scholar]
- Kaufman JL, Gleason C, Heffner L, Lonial S. Bortezomib, thalidomide, and dexamethasone as induction therapy for patients with symptomatic multiple myeloma. Blood (ASH Annual Meeting Abstracts) 2007;110:3605. doi: 10.1002/cncr.25143. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Hajek R, Tothova E, Drach J, Adam Z, Labar B, Egyed M, Spicka I, Gisslinger H, Greil R, Kuhn I, Zojer N, Hinke A. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113:3435–3442. doi: 10.1182/blood-2008-07-169565. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Oriol A, Martinez J, Cibeira MT, Gutierrez NC, Terol MJ, de Paz R, Garcia-Larana J, Bengoechea E, Garcia-Sancho AM, Martinez R, Palomera L, de Arriba F, Gonzalez Y, Hernandez J, Sureda A, Bello JL, Lahuerta JJ, Blade J, San-Miguel JF. A prospective, multicenter, randomized, trial of bortezomib/melphalan/prednisone (VMP) versus bortezomib/thalidomide/prednisone (VTP) as induction therapy followed by maintenance treatment with bortezomib/thalidomide (VT) versus bortezomib/prednisone (VP) in elderly untreated patients with multiple myeloma older than 65 years. Blood (ASH Annual Meeting Abstracts) 2009;114:3. [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP, Anderson KC. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ, Pien CS, Guerciolini R, Anderson JK, Depcik-Smith ND, Bhagat R, Lehman MJ, Novick SC, O’Connor OA, Soignet SL. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. Journal of Clinical Oncology. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Hayman S, Gertz MA, Dispenzieri A, Lacy MQ, Greipp PR, Geyer S, Iturria N, Fonseca R, Lust JA, Kyle RA, Witzig TE. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. Journal of Clinical Oncology. 2002;20:4319–4323. doi: 10.1200/JCO.2002.02.116. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Rosinol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, Olesnyckyj M, Yu Z, Knight R, Zeldis JB, Blade J. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. Journal of Clinical Oncology. 2008;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncology. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinol L, Oriol A, Mateos MV, Sureda A, Garcia-Sanchez P, Gutierrez N, Alegre A, Lahuerta JJ, de la Rubia J, Herrero C, Liu X, Van de Velde H, San Miguel J, Blade J. Phase II PETHEMA trial of alternating bortezomib and dexamethasone as induction regimen before autologous stem-cell transplantation in younger patients with multiple myeloma: efficacy and clinical implications of tumor response kinetics. Journal of Clinical Oncology. 2007;25:4452–4458. doi: 10.1200/JCO.2007.12.3323. [DOI] [PubMed] [Google Scholar]
- Rummel MJ, Niederle N, Maschmeyer G, Banat A, Gruenhagen Uv, Losem C, Heil G, Welslau M, Balser C, Kaiser U, Ballo H, Weidmann E, Duerk HA, Kofahl-Krause D, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W. Bendamustine plus rituximab is superior in respect of progression free survival and CR rate when compared to CHOP plus rituximab as first-line treatment of patients with advanced follicular, indolent, and mantle cell lymphomas: final results of a randomized phase III study of the StiL (Study Group Indolent Lymphomas, Germany) Blood (ASH Annual Meeting Abstracts) 2009;114:405. [Google Scholar]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. New England Journal of Medicine. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- Sher T, Miller KC, Ailawadhi S, Manfredi D, Wood M, Tan W, Wilding G, Liu H, Czuczman MS, Hernandez F, Hong F, Sood R, Soniwalla S, Lawrence W, Kouides PA, Lee KP, Chanan-Khan A. Bortezomib in combination with pegylated liposomal doxorubicin and thalidomide (VDT), an effective steroid independent regimen for previously untreated multiple myeloma patients: final result of a phase II study. Blood (ASH Annual Meeting Abstracts) 2009;114:618. [Google Scholar]
- Wang M, Giralt S, Delasalle K, Handy B, Alexanian R. Bortezomib in combination with thalidomide-dexamethasone for previously untreated multiple myeloma. Hematology. 2007;12:235–239. doi: 10.1080/10245330701214236. [DOI] [PubMed] [Google Scholar]