Abstract
OBJECTIVE
To determine if there is an association between visceral adiposity measured on CT colonography (CTC) and colorectal polyps.
MATERIALS AND METHODS
The study was HIPAA-compliant and approved by our Institutional Review Board and Office of Human Subjects Research. 1186 patients who underwent CTC and same day optical colonoscopy were analyzed. Visceral adipose tissue volumes (VAV) and volume percents relative to total internal body volume (VAV%) were measured on slices in the L2–L3 regions on supine CTC scan with validated fully-automated software. Student t-test, odds ratio (OR), logistic regression and receiver operating characteristic analyses were performed.
RESULTS
For subjects with and without adenomatous polyps, means and s. d. of VAV% were 31.2 ± 10.8% (n=345) and 28.2% ± 11.3% (n=841) (p<0.0001), respectively. For subjects with and without hyperplastic polyps they were 31.8% ± 10.7% (n=244) and 28.3% ± 11.2% (n=942) (p<0.0001), respectively. Comparing the lowest and highest quintiles of VAV%, the ORs for having at least one adenomatous polyp or hyperplastic polyp versus no polyp were 2.06 (95% CI: 1.36–3.13) and 1.71 [1.08, 2.71] and the prevalence of having adenomatous polyps or hyperplastic polyps increased 14% and 8%, respectively.
CONCLUSION
Subjects with higher visceral adiposity measurements on CTC have a greater risk for the presence of colonic polyps.
Keywords: CT, colon; CT, virtual imaging; visceral fat measurement; colonic polyps
Colorectal cancer is the second leading cause of cancer death in Americans [1]. Identification of risk factors for adenomatous polyps can aid in the prevention of colorectal cancer (CRC). Obesity and adenomatous polyps are known independent risk factors for CRC [2, 3]. However, the relation between visceral adipose tissue and adenomatous polyps is still controversial.
Studies have reported an association between total body measurements of obesity (such as body mass index (BMI)) and adenomatous polyps [3, 4]. Abdominal obesity has been shown to be more strongly related to adenomas than overall obesity [5]. Abdominal adiposity is composed of subcutaneous and visceral compartments. Visceral adiposity is more metabolically active than subcutaneous adiposity and produces several humoral factors that have been implicated in carcinogenesis. CT can distinguish subcutaneous and visceral fat whereas anthropometric measurements like BMI and waist circumference cannot [6].
CT colonography (CTC) has been proven to accurately detect colorectal polyps [7, 8]. The purpose of this study was to determine whether there is an association between visceral adiposity measured on CT colonography (CTC) and colorectal polyps.
MATERIALS AND METHODS
Patient population
The patient population consisted of 1233 consecutive asymptomatic adults between 40 and 79 years of age who underwent same day CTC and optical colonoscopy at one of three medical centers. The patients were predominantly of average risk (97.4%) although 32 had family histories positive for colorectal cancer (7). 47 of the 1233 patients were excluded because of incomplete optical colonoscopy, inadequate preparation or failure of the CT colonographic system. Characteristics of the remaining 1186 patients are given in Table 1.
TABLE 1.
Patient characteristics, visceral adipose volume and visceral adipose volume fraction
| Any polyp*,** | Adenomatous* | Advanced Adenomatous* | Non-advanced Adenomatous | Hyperplastic | |
|---|---|---|---|---|---|
| Number (n = 1186) | 497/689 | 345/841 | 53/1133 | 319/867 | 244/942 |
| Male (n = 700) | 323/377 | 229/471 | 35/665 | 216/484 | 164/536 |
| Age (mean±s.d.) | 58.8±7.0/57.1±7.3 | 59.2±7.0/57.2±7.2 | 60.2±7.2/57.7±7.2 | 59.2±6.9/57.3±7.3 | 58.6±7.1/57.6±7.2 |
| Polyp size (range; median) (cm) | 0.1 – 4.2; 0.4 | 0.1 – 4.2; 0.5 | 0.5 – 4.2; 1.1 | 0.1 – 0.9; 0.4 | 0.1 – 2.0; 0.4 |
| VAV (mean±s.d.) (cm3) | 1173±645/973±620 p<0.0001 |
1193±648/1001±625 p<0.0001 |
1301±609/1045±637 p=0.0007 |
1197±657/1005±623 p=0.0001 |
1216±637/1016±632 p<0.0001 |
| VAV% (mean±s.d.) | 31.0%±10.8%/27.6%±11.3% p<0.0001 |
31.2%±10.8%/28.2% ± 11.3% p<0.0001 |
33.5%±9.2%/28.8%±11.2% p=0.004 |
31.1%±10.9%/28.3%±11.2% p<0.0001 |
31.8%±10.7%/28.3%±11.2% p<0.0001 |
Data are for patients with/without one or more of the indicated type of polyp. Advanced adenomas are adenomas that are villous or tubulovillous, have high-grade dysplasia or are 1 cm or larger at optical colonoscopy. P values are given for VAV and VAV%.
Includes two adenocarcinomas
Adenomatous or hyperplastic
Abbreviations: VAV visceral adipose volume VAV% visceral adipose volume divided by internal body volume
The patient population and CTC and optical colonoscopy results used for this study were from the IRB-approved project published in Ref. [7]. Retrospective use of the data was approved by our institution’s Office of Human Subjects Research.
Bowel Preparation
Patients underwent a 24-hour colonic preparation that consisted of oral administration of 90 ml sodium phosphate (Fleet 1 preparation, Fleet Pharmaceuticals), 10 mg bisacodyl, 500 ml of barium sulphate (2.1% by weight; Scan C, Lafayette Pharmaceuticals) and 120 ml of diatrizoate meglumine and diatrizoate sodium (Gastrografin, Bracco Diagnostics) given in divided doses.
CTC Scanning
The colon was distended with patient-controlled insufflation of room air. CT scanning occurred during one breathhold in each of the prone and supine positions using a four-or eight-channel CT scanner (General Electric LightSpeed or LightSpeed Ultra). CT scanning parameters included 1.25 – 2.5 mm section collimation, 15 mm per second table speed, 1 mm reconstruction interval, 100 mAs and 120 kVp.
Optical Colonoscopy
Patients underwent same-day optical colonoscopy by one of 17 colonoscopists. The colonoscopies were performed using segmental unblinding, wherein CTC results were revealed to the colonoscopists during the examination to create an enhanced reference standard. Polyp sizes were determined at optical colonoscopy using a calibrated guidewire. The polyp findings at optical colonoscopy after segmental unblinding served as the reference standard for polyps in this study.
Visceral fat measurement
In the literature, fat volumes are typically assessed at the L2–L3 spinal level [9]. The fat volumes on all slices in the L2–L3 region on supine CTC scans were analyzed with fully automated software developed at our institution [10]. The CTC images were first processed by fully automated spine segmentation and labeling software that finds the slices which correspond to the top and bottom of each lumbar vertebral body [11]. An adipose tissue (AT) measurement algorithm was then used to measure each slice from the top of L2 to the bottom of L3. Total volumes for this region were computed by summing the volumes computed in each slice.
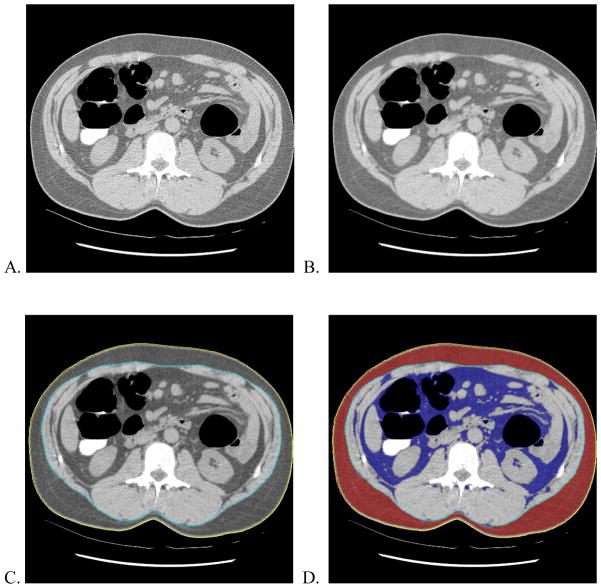
The fully automated AT measurements algorithm consisted of five steps: body masking, noise reduction, AT labeling, visceral and subcutaneous adipose tissue separation, and quantification (Figure 1). The body mask was created by a region growing algorithm on the image background. The region growing algorithm initially segmented the low intensity pixels outside the body and then in a second pass removed the CT table. Once the body mask was created, an anisotropic diffusion filter was used to reduce noise and voxels between −274 HU and −49 HU were labeled AT.
Fig. 1.
Illustration of fully-automated method to measure adipose tissue. A) Two-dimensional transaxial CT colonography image at L2 level of a 52 year-old man. B) Smoothed image after anisotropic filtering. C) External (yellow) and internal (light blue) contours generated by automated software. D) Segmented subcutaneous (red) and visceral (dark blue) adipose tissue. The volume of the visceral adipose tissue normalized to internal body volume (VAV%) measured by the automated software was 25.3%.
A contour around the outside of the body, the “external contour”, was initialized. Active contour models were then used to iteratively modify the external contour to find the inner boundary of the subcutaneous adipose tissue. This results in a contour along the abdominal wall, the “internal contour”.
Quantification was performed by multiplying voxel counts by the voxel volumes (pixel area times slice thickness) to get visceral adipose volumes (VAV) and internal body volumes (IBV). VAV was defined as the volume of all AT voxels inside of the internal contour. IBV was defined as the volume of all voxels inside the internal contour. VAV% was defined as VAV divided by IBV.
Validation Experiment
A validation experiment was performed to assess the accuracy of the automated measurement of adipose tissue. 50 random CTC cases were selected from the dataset described above.
Each case was manually traced by two experienced radiology technologists who routinely perform manual assessment of adipose tissue on CT for clinical purposes. The manual tracings were done along the inner boundary of the subcutaneous adipose tissue. Manual tracings and automated analyses were done on a single section at the L2–L3 level for each case.
Statistical Analysis
For the validation experiments, pairwise comparisons were made of VAV and VAV% measured by the two technologists and by the automated software. Linear regression and Bland-Altman analyses were performed using Excel (Microsoft, Version 2007).
All analyses regarding the presence or absence of polyps were based on detection of polyps of any size as determined at segmentally unblinded optical colonoscopy. Polyp findings at CTC were used only for the segmental unblinding procedure.
Means and standard deviations were reported for VAV and VAV%. The Student t-test (unpaired, unequal variance) was used to compare means of adipose tissue measurements between patients with and without any, adenomatous, advanced adenomatous, non-advanced adenomatous and hyperplastic polyps.
Subjects were divided into quintiles (separated by sex) of VAV and VAV% then the men and women were recombined. The odds ratios (OR) for presence/absence of at least one adenomatous polyp for each quintile was computed by dividing the odds for the quintile by the odds of the lowest quintile. ORs were also computed for prevalence of at least one advanced adenomatous, non-advanced adenomatous, hyperplastic and adenomatous or hyperplastic polyp. An approximate 95% confidence interval for the population log odds ratio was calculated by the formula exp(log odds ratio ± 1.96×SE), where SE is the standard error for the log odds ratio. To test the hypothesis that the population odds ratio equals one, the p-value was computed as where Z = log(OR)/SE denotes a standard normal random variable and erf is the error function. This analysis assumes that log(OR) has a normal distribution. Odds ratios were computed using a program written in Matlab, version R2010a.
Five multiple logistic regression analyses were done using an online calculator (http://statpages.org/logistic.html). The dependent variables were the presence of one or more adenomatous, advanced adenomatous, non-advanced adenomatous, hyperplastic or adenomatous or hyperplastic polyps (“any polyps”), respectively. In each analysis, the independent variables were age, gender and VAV%. VAV% was expressed as a number ranging from 0 to 1. Gender was coded as male=1 and female=0. These data indicate the estimated adjusted odds ratio of having polyps associated with each independent variable after adjusting for the other independent variables.
Receiver operating characteristic (ROC) curves were calculated by varying a threshold of VAV% and prediction probability from logistic regression analysis. The true and false positive fractions of patients above the thresholds having any polyp, adenomatous polyps, advanced adenomatous polyps, non-advanced adenomatous polyps or hyperplastic polyps were determined. The areas under the ROC curves were computed using ROCKIT, version 9.1 [12].
P-values less than 0.05 were considered significant.
RESULTS
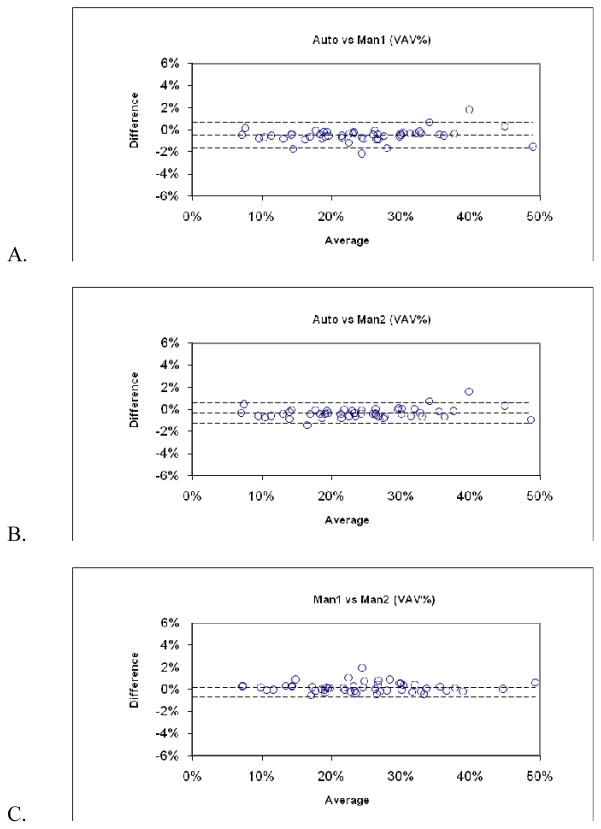
The results for the validation experiments are shown in Table 2. Correlation coefficients for the linear regressions between the manual and automated measurements exceeded 0.99. Bland-Altman 95% limits of agreement between manual and automated assessments were within ±2% for VAV% and were comparable to that between two manual assessments. Bland-Altman plots for VAV% are shown in Figure 2.
TABLE 2.
Results of validation experiments
| Bland-Altman 95% Limits of Agreement | Coefficient of Determination (r2) from Linear Regression | |||
|---|---|---|---|---|
| VAV (cm3) | VAV% (%) | VAV | VAV% | |
| Automated vs. Manual 1 | −1.64, 0.34 | −1.68, 0.67 | 0.997 | 0.996 |
| Automated vs. Manual 2 | −1.21, 0.20 | −1.26, 0.60 | 0.998 | 0.997 |
| Manual 1 vs. Manual 2 | −0.48, 0.77 | −0.71, 1.06 | 0.998 | 0.997 |
Abbreviations:
VAV visceral adipose volume
VAV% visceral adipose volume divided by internal body volume
Measurements were made on a single 1 mm CTC section.
Fig. 2.
Bland-Altman plots of validation experiment (n=50). (A–C) Plots compare pairwise assessments of visceral adipose tissue as a percentage of internal body volume (VAV%) determined by automated software (Auto) and by two observers using manual tracing (Man1 and Man2). The dashed lines indicate the ± two standard deviation limits of agreement. Maximum disagreement was 2% in all three comparisons.
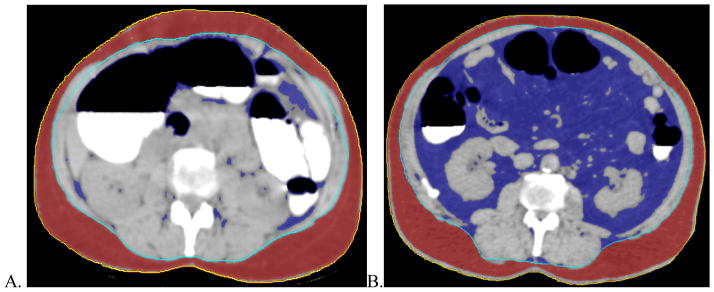
Visceral fat assessments by the automated software were successful for all 1186 patients. The means and standard deviations for VAV and VAV% of the patients with and without polyps are shown in Table 1. Patients with polyps had significantly greater VAV and VAV% for all polyp groupings by histopathology (any, adenomatous, advanced adenomatous, non-advanced adenomatous and hyperplastic polyps). Examples of patients in the lowest and highest quintiles of VAV% are shown in Figure 3.
Fig. 3.
Transaxial CT colonography images at the L2–L3 level of patients in the (A) lowest and (B) highest quintiles of VAV%. External (yellow) and internal (light blue) contours and subcutaneous (red) and visceral (dark blue) adipose tissue generated by automated software are shown. The volumes of the visceral adipose tissues normalized to internal body volumes (VAV%) measured on these images are (A) 5.65% and (B) 46.15%. The 51 year-old woman in (A) did not have any polyps. The 69 year-old man in (B) had one 10 mm adenomatous polyp in the ascending colon.
The ORs for having at least one polyp versus no polyp are given in Table 3 and Table 4 for each quintile of VAV% and VAV, respectively. For VAV% and VAV, the ORs were statistically significantly greater than 1.0 for the fourth and fifth quintiles for all five polyp histologic groupings. The ORs were statistically significantly greater than 1.0 for some of the second and third quintiles as well. In general, ORs were greater for the higher quintiles. The probability or prevalence of having any polyps, adenomatous polyps, advanced adenomatous polyps, non-advanced adenomatous polyps or hyperplastic polyps increased 16%, 14%, 4%, 12% and 8%, respectively, between the lowest and highest quintiles of VAV%. On a proportional basis, the probability or prevalence increased 50% (0.16/0.32), 70% (0.14/0.20), 224% (0.038/0.017), 63% (0.12/0.19) and 50% (0.08/0.16), respectively, comparing the lowest and highest quintiles of VAV%. The odds ratios trended similarly when men and women were assessed separately rather than being recombined, although fewer quintiles reached statistical significance.
TABLE 3.
Odds ratios for having one or more polyps for different groupings of patients by VAV%.
| Any polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.46 | 0.32 | 1.00 | |||
| 2 | 0.67 | 0.40 | 1.45 | 1.91 | 0.03 | [0.99, 2.11] |
| 3 | 0.72 | 0.42 | 1.57 | 2.34 | 0.01 | [1.07, 2.28] |
| 4 | 0.93 | 0.48 | 2.00 | 3.64 | 0.0001 | [1.38, 2.91] |
| 5 | 0.91 | 0.48 | 1.97 | 3.55 | 0.0002 | [1.35, 2.86] |
| Adenomatous polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.25 | 0.20 | 1.00 | |||
| 2 | 0.39 | 0.28 | 1.59 | 2.14 | 0.02 | [1.04, 2.44] |
| 3 | 0.44 | 0.31 | 1.79 | 2.70 | 0.003 | [1.17, 2.73] |
| 4 | 0.49 | 0.33 | 1.98 | 3.20 | 0.0007 | [1.30, 3.01] |
| 5 | 0.51 | 0.34 | 2.06 | 3.39 | 0.0003 | [1.36, 3.13] |
| Advanced adenomatous polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.017 | 0.017 | 1.00 | |||
| 2 | 0.013 | 0.013 | 0.75 | −0.38 | 0.6 | [0.17, 3.37] |
| 3 | 0.063 | 0.059 | 3.64 | 2.25 | 0.01 | [1.18, 11.23] |
| 4 | 0.087 | 0.080 | 5.08 | 2.91 | 0.002 | [1.70, 15.16] |
| 5 | 0.058 | 0.055 | 3.38 | 2.10 | 0.02 | [1.09, 10.52] |
| Non-advanced Adenomatous polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.24 | 0.19 | 1.00 | |||
| 2 | 0.39 | 0.28 | 1.60 | 2.15 | 0.02 | [1.04, 2.46] |
| 3 | 0.37 | 0.27 | 1.53 | 1.93 | 0.03 | [0.99, 2.35] |
| 4 | 0.42 | 0.30 | 1.74 | 2.55 | 0.005 | [1.14, 2.66] |
| 5 | 0.45 | 0.31 | 1.85 | 2.84 | 0.002 | [1.21, 2.82] |
| Hyperplastic polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.19 | 0.16 | 1.00 | |||
| 2 | 0.20 | 0.16 | 1.06 | 0.25 | 0.4 | [0.65, 1.74] |
| 3 | 0.27 | 0.21 | 1.44 | 1.52 | 0.06 | [0.90, 2.30] |
| 4 | 0.35 | 0.26 | 1.87 | 2.70 | 0.004 | [1.19, 2.96] |
| 5 | 0.32 | 0.24 | 1.71 | 2.29 | 0.01 | [1.08, 2.71] |
Subjects were divided into quintiles (separated by gender then recombined) based on the value of VAV%. For each quintile, odds, probability (fraction of patients in the quintile having one or more polyps of a given type), odds ratio (OR), standard normal deviate (Z), P value and 95% confidence interval (CI) of odds ratio are shown for presence of at least one polyp (adenomatous or hyperplastic), adenomatous polyp, advanced adenomatous polyp, non-advanced adenomatous polyp or hyperplastic polyp.
Table 4.
Odds ratios for having one or more polyps for different groupings of patients by VAV.
| Any polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.47 | 0.32 | 1.00 | |||
| 2 | 0.65 | 0.39 | 1.37 | 1.63 | 0.05 | [0.94, 2.00] |
| 3 | 0.76 | 0.43 | 1.62 | 2.51 | 0.006 | [1.11, 2.35] |
| 4 | 0.80 | 0.44 | 1.69 | 2.73 | 0.003 | [1.16, 2.45] |
| 5 | 1.03 | 0.51 | 2.17 | 4.08 | <0.0001 | [1.50, 3.16] |
| Adenomatous polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.27 | 0.22 | 1.00 | |||
| 2 | 0.37 | 0.27 | 1.35 | 1.39 | 0.08 | [0.88, 2.06] |
| 3 | 0.38 | 0.28 | 1.40 | 1.57 | 0.06 | [0.92, 2.13] |
| 4 | 0.48 | 0.32 | 1.76 | 2.68 | 0.004 | [1.16, 2.65] |
| 5 | 0.58 | 0.37 | 2.12 | 3.61 | 0.0002 | [1.41, 3.18] |
| Advanced adenomatous polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.022 | 0.021 | 1.00 | |||
| 2 | 0.009 | 0.008 | 0.39 | −1.10 | 0.9 | [0.08, 2.06] |
| 3 | 0.072 | 0.067 | 3.34 | 2.32 | 0.01 | [1.20, 9.28] |
| 4 | 0.072 | 0.068 | 3.36 | 2.33 | 0.01 | [1.21, 9.32] |
| 5 | 0.063 | 0.059 | 2.91 | 2.02 | 0.02 | [1.03, 8.22] |
| Non-advanced adenomatous polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.27 | 0.21 | 1.00 | |||
| 2 | 0.35 | 0.26 | 1.33 | 1.30 | 0.1 | [0.87, 2.03] |
| 3 | 0.30 | 0.23 | 1.12 | 0.53 | 0.3 | [0.73, 1.73] |
| 4 | 0.42 | 0.30 | 1.57 | 2.10 | 0.02 | [1.03, 2.38] |
| 5 | 0.53 | 0.35 | 1.98 | 3.25 | 0.0006 | [1.31, 2.98] |
| Hyperplastic polyps | ||||||
|---|---|---|---|---|---|---|
| Quintiles | Odds | Probability | OR | Z | P | 95% CI of OR |
| 1 | 0.17 | 0.14 | 1.00 | |||
| 2 | 0.22 | 0.18 | 1.29 | 1.0 | 0.2 | [0.79, 2.11] |
| 3 | 0.29 | 0.22 | 1.71 | 2.22 | 0.01 | [1.06, 2.75] |
| 4 | 0.31 | 0.24 | 1.85 | 2.55 | 0.005 | [1.15, 2.96] |
| 5 | 0.33 | 0.25 | 1.98 | 2.86 | 0.002 | [1.24, 3.16] |
Subjects were divided into quintiles (separated by gender then recombined) based on the value of VAV. For each quintile, odds, probability (fraction of patients in the quintile having one or more polyps of a given type), odds ratio (OR), standard normal deviate (Z), P value and 95% confidence interval (CI) of odds ratio are shown for presence of at least one polyp (adenomatous or hyperplastic), adenomatous polyp, advanced adenomatous polyp, non-advanced adenomatous polyp or hyperplastic polyp.
The odds ratios for the various coefficients in the logistic regression are given in Table 5. VAV% was a significant factor for predicting all five histologic groups of polyps with ORs ranging from 3.63 to 28.01. Age (OR 1.03) and gender (ORs 1.33 – 1.52) were significant factors for predicting any, adenomatous and non-advanced adenomatous polyps.
Table 5.
Odds ratios for coefficients of multiple logistic regression
| Any Polyps | Adenomatous Polyps | Advanced Adenomatous | Non-advanced Adenomatous | Hyperplastic Polyps | |
|---|---|---|---|---|---|
| Age | 1.03 [1.01, 1.05]* | 1.03 [1.02, 1.05]* | 1.04 [1.00, 1.08] | 1.03 [1.01, 1.05]* | 1.01 [0.99, 1.03] |
| Gender | 1.33 [1.03, 1.73]* | 1.39 [1.05, 1.84]* | 1.08 [0.58, 2.00] | 1.52 [1.14, 2.04]* | 1.31 [0.95, 1.79] |
| VAV% | 7.23 [2.28, 22.92]* | 4.93 [1.41, 17.25]* | 28.01 [1.67, 470.69]* | 3.63 [1.01, 13.02]* | 9.86 [2.40, 40.56]* |
The odds ratio and 95% confidence intervals are given for each coefficient in the multiple logistic regression where the response variable was the presence of one or more of the given polyp type. These data indicate the estimated adjusted odds ratio of having polyps associated with each independent variable after adjusting for the other independent variables.
p < .05
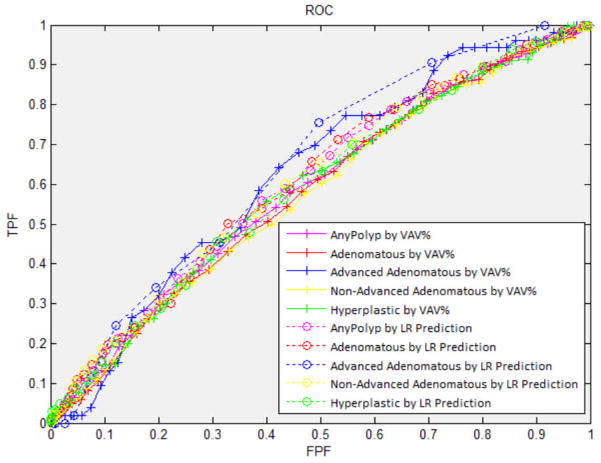
The receiver operating characteristics curves are shown in Figure 4. The areas under the ROC curves are similar for the different polyp types and using either the multiple regression prediction variables or VAV% (0.57 – 0.66). The higher odds ratios for the fifth quintiles (as shown in Table 3) are reflected in the steeper slopes of the curves at the lower left of Figure 4 compared to the shallower slopes at the upper right.
Fig. 4.
ROC curves for the presence in a patient of at least one polyp. The AUC and standard deviation of AUC for any, adenomatous, advanced adenomatous, non-advanced adenomatous, and hyperplastic polyps are 0.583±0.017, 0.579±0.018, 0.624±0.034, 0.574±0.018 and 0.584±0.020, respectively, at different thresholds of VAV% (solid lines). The AUC and standard deviation of AUC for any, adenomatous, advanced adenomatous, non-advanced adenomatous and hyperplastic polyps are 0.606±0.016, 0.611±0.018, 0.658±0.033, 0.612±0.018 and 0.596±0.020, respectively, at different thresholds of prediction probability using logistic regression (LR) analysis (dotted lines).
DISCUSSION
Visceral fat has been implicated in a variety of disorders, including cardiovascular diseases, diabetes and carcinoma [13–15]. However, its role as a risk factor in colorectal adenoma, a precursor of cancer is still under investigation. We found that patients with high visceral adipose volume had a greater risk of having adenomatous and hyperplastic polyps. In the multiple regression analyses, when VAV% was adjusted for, older patients and men had a greater risk of having adenomatous polyps, but neither age nor gender were significantly associated with having hyperplastic polyps. Patients in the highest quintile of VAV% were 50%, 70% and 50% more likely to have any polyp, adenomatous polyps or hyperplastic polyps than those in the lowest quintile. Although advanced adenomatous polyps are uncommon, patients in the highest quintile of VAV% were twice as likely to have adenomatous polyps compared to those in the lowest quintile. The predictive values of VAV and VAV% were similar, on the basis of similar odds ratios and confidence intervals. As shown by the ROC analyses and as one would predict based on prior research, visceral adipose volume alone cannot be used to predict with certainty which patients have polyps.
Several articles have been published on the association of visceral fat with colorectal polyps and cancer (Table 6). Only the studies by Kang et al., in a population of Koreans, and Yamaji et al., in a population of Japanese, had numbers of patients comparable to that of the current research [16, 17]. One study utilized CTC, the others routine CT. Johnson et al. found using multiple logistic regression an odds ratio of about 2 for the presence of large colorectal adenomas on CTC in patients with higher visceral fat; this is within the error bounds of our results for advanced adenomatous polyps [18]. However, their result was not statistically significant perhaps because of the small sample size of 25 patients and 25 controls. They also found slightly higher areas under the ROC curves, ranging from 0.74±0.05 to 0.77±0.05, depending upon the anatomic level at which the visceral fat was assessed. Their anatomic levels were the kidneys, the iliac crests or the acetabuli rather than at the L2–L3 vertebral level and so their results are not directly comparable to ours. Studies that utilized routine CT have produced conflicting results. While some studies have found a strong association between visceral adipose area and adenomas [16, 17, 19, 20], others have not [21–24] (Table 6). Our study found that the odds of having at least one adenomatous polyp is increased 2.06 times in the highest VAV% quintile as compared to the lowest quintile. This value is comparable to those found in other studies in the literature which showed a positive association between visceral adipose area and adenomatous polyps [16, 17, 19, 20]. In the study by Schoen et al. [22], patients with nonadvanced adenomas had greater visceral fat compared to controls while those with advanced adenomas did not. We found that patients with increased visceral fat were at greater risk of having both advanced and nonadvanced adenomas. In Sass et al. [21], the authors focused on recurrent rather than primary adenomas. Yamamoto et al. [24] found no association between visceral adiposity and colorectal adenoma, but found a strong association between colorectal cancer and visceral adiposity. Our study focused on screening patients more likely to have primary adenomas rather than recurrent adenomas or carcinomas as patients with a prior history of optical colonoscopy within the previous 10 years or a history of adenomatous polyps were excluded (7). Finally, our study measured visceral fat volume; the other studies assessed a single CT slice, with the exception of Yamaji et al. who measured visceral fat volume in the entire abdominopelvic region [17].
Table 6.
Odds ratios for the association of visceral adipose area and adenomatous polyps reported in the literature
| Reference | Number of Subjects with and without Adenomas | Odds Ratio |
|---|---|---|
| Johnson et al. [18] | 25, 25 | 1.9 [0.5, 7.3] |
| Oh et al. [19] | 53, 147 | 4.07 [1.01, 16.43] |
| Otake et al. [20] | 51, 52 | 2.19 [1.47, 3.28] |
| Kang et al. [16] | 1122, 1122 | 3.09 [2.19, 4.36] |
| Yamaji et al. [17] | 637, 568 | 1.58 [1.14, 2.21] |
| Sass et al. [21] | 60, 59 | 1.0 [0.3, 3.3] |
| Schoen et al. [22] | 202, 256 | 0.77 [0.44, 1.33] |
| Erarslan et al. [23] | 31, 50 | * |
| Yamamoto et al. [24] | 86, 258 | 1.02 [0.46, 2.24] |
Note -- Visceral adipose tissue is sometimes reported as a volume even when measured on only a single image. Odds ratios are for patients in the highest group of visceral fat relative to those patients in the lowest group.
Odds ratios not reported; visceral fat area not significantly different between patients with adenomas and controls.
We found that the prevalence of colorectal adenomas increased from 20% for the lowest quintile to 34% for the highest quintile. These prevalences are greater than those found in average risk Japanese in Japan using quartiles of BMI, 15.4% for the lowest quartile and 24.2% for the highest quartile; however, the percentage increase in prevalence from the lowest to the highest categories are comparable (70% [0.14/0.20] versus 57% [.088/.154]) [25].
Anthropometric markers such as BMI, waist circumference and waist-to-hip ratio have known positive associations with risk of CRC [26–30][14, 31–33]. However, there are important metabolic and biochemical differences between subcutaneous fat and visceral fat [34]. Unlike CT, anthropometric markers such as BMI, waist circumference and waist-to-hip ratio cannot distinguish excesses in total body fat from increases in lean body mass or differences in fat distribution [3].
BMI was also assessed on the cohort reported in our study [4]. In a multivariate analysis that included age and gender, the odds ratio for adenomas or cancers was 1.34 [1.02, 1.77], p = .03, when patients were dichotomized into two groups at a BMI cutoff of 25. The odds ratio for hyperplastic polyps was not statistically significant. In contrast, Martinez et al. found an elevated odds ratio for hyperplastic polyps when patients in the highest BMI quartile were compared to those in the lowest [35]. We found that elevated visceral adipose volume was associated with an increased probability of hyperplastic polyps on the basis of the statistically significant odds ratios and logistic regression coefficients. In other studies, correlations of visceral fat and hyperplastic polyps were not assessed. An increased risk of hyperplastic polyps may have relevance for the alternate serrated adenoma pathway to colorectal carcinoma [36].
In the logistic regression analysis, we found that VAV%, gender and age were independent significant factors for predicting adenomatous polyps. Greater age is well known to be associated with an increased risk of polyps and cancers. Our finding that gender was associated with adenomatous polyps agrees with that of the National Polyp Study in which men were found more likely to have adenomatous polyps [37]. The National Polyp Study did not consider the effect of visceral fat on adenoma risk.
The visceral fat measurements were made using fully-automated software. Validation experiments showed that the automated measurements were comparable to manual measurements. For example, the automated and manual measurements were highly correlated (r2 > 0.99) and the Bland-Altman 95% limits of agreement were small and similar to the inter-observer limits of agreement of two manual measurements. Fully automated visceral fat assessment has also been reported by Zhao et al. on a small dataset of 9 CT scans [38].
The pathogenesis of visceral adipose tissue causing increasing risk of polyps is unclear. Possible mechanisms include the association of visceral adipose tissue with insulin resistance, hyperinsulinemia, insulin-like growth factors (IGF-1), leptin and adiponectin [39][40][20, 41][42][20, 41, 43].
There are several potential clinical implications of the association between VAV and colonic polyps. Patients who are more predisposed to having colonic adenomas on the basis of visceral adiposity could be identified and scrutinized more closely for adenomas and undergo more frequent surveillance. Identification of such patients could be accomplished by automated assessment of visceral adiposity by the CT colonography interpretation software. The automated assessment could be incorporated into computer-aided polyp detection (CAD) software. CAD software could be set to identify more polyp candidates on CTC examinations of patients with greater amounts of visceral fat. Colorectal cancer screening guidelines could be modified to consider the greater risk associated with visceral fat. Currently, joint guidelines from the American Cancer Society, the United States Multi-Society Task Force on Colorectal Cancer and the American College of Radiology do not mention that obese patients should be included in the category of patients at higher than average risk [44]. The American College of Gastroenterology recognized that obesity is a risk factor and that obese patients may require earlier screening, but that modification of the screening guidelines should await results of more studies [45]. Aside from its implications for assessing polyp risk, measurement of visceral adiposity on CTC also provides potentially clinically useful information about risks for other cancers, for cardiovascular disease, the metabolic syndrome, diabetes and vitamin D deficiency [46, 47]. Because of its ease of implementation as a fully automated measurement, assessment of visceral adiposity could be added to routine non-colonographic abdominopelvic CT interpretation, particularly in individuals over age 50. Visceral fat measurement may also be useful in the assessment of patients diagnosed with colorectal cancer [48–50].
In this study, we measured visceral fat using validated automated software. We used automated software because of the large number of cases to be analyzed and to show that such automation was feasible and accurate. However, for routine clinical implementation, manual tracing to identify the visceral compartment and measure visceral fat is simple, fast and accurate and is currently widely practiced.
A potential limitation of this study is that subcutaneous fat was not also assessed because often it was partially outside the display field-of-view of the images, leading to inaccuracies in the measurements. This is because for CT colonography, the colon and not the subcutaneous fat is the subject of interest. Therefore, we could not assess the contribution of subcutaneous fat to colorectal polyp risk. However, we could find no published research implicating the importance of subcutaneous fat for predicting colorectal polyps, in contrast to the substantial body of research regarding visceral fat and polyps. Another limitation is that we did not assess the contributions of other risk factors for polyps such as smoking, alcohol or diet.
In summary, visceral adiposity can be determined simultaneously with colorectal cancer screening at CTC. Accurate adiposity measurements can be calculated in a three-dimensional volume on abdominal CT with fully-automated software. Obese patients (those in the highest quintile of VAV%) are about 2.1 times as likely to have at least one adenomatous polyp and about 1.6 times more likely to have at least one hyperplastic polyp compared to thin patients (those in the lowest quintile of VAV%). Our methods may be clinically useful for screening for visceral adiposity and assessing colorectal polyp risk.
Acknowledgments
We thank William R. Schindler, DO, for providing CT colonography and supporting data and Francine Thomas and David Williams for manual measurements on CT images. This research was supported in part by the National Institutes of Health, Clinical Center.
Footnotes
Presented at the RSNA 2010 Annual Meeting
Potential financial interest:
Author Summers and Yao have pending and/or awarded patents for the subject matter described in the manuscript and receive royalty income for a patent license from iCAD. Dr. Summers’ lab is supported in part by a Cooperative Research and Development Agreement with iCAD. Author Pickhardt is on the medical advisory boards of Viatronix, Inc. and Medicsight, Inc., a consultant to Check-Cap and Bracco and co-founder of VirtuoCTC. Author Choi is on the medical advisory boards of Viatronix, Inc and QI and has received research support from E-Z-EM.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–2547. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 3.Burke CA. Colonic complications of obesity. Gastroenterol Clin North Am. 2010;39:47–55. doi: 10.1016/j.gtc.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Hassan C, Pickhardt PJ, Marmo R, Choi JR. Impact of lifestyle factors on colorectal polyp detection in the screening setting. Diseases of the colon and rectum. 2010;53:1328–1333. doi: 10.1007/DCR.0b013e3181e10daa. [DOI] [PubMed] [Google Scholar]
- 5.Shinchi K, Kono S, Honjo S, et al. Obesity and adenomatous polyps of the sigmoid colon. Jpn J Cancer Res. 1994;85:479–484. doi: 10.1111/j.1349-7006.1994.tb02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. The American journal of clinical nutrition. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 7.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 8.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balentine CJ, Marshall C, Robinson C, et al. Validating quantitative obesity measurements in colorectal cancer patients. J Surg Res. 2010;164:18–22. doi: 10.1016/j.jss.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 10.Yao J, Sussman DL, Summers RM. Fully automated adipose tissue measurement on abdominal CT. In: Weaver JB, Molthen RC, editors. SPIE Medical Imaging. Orlando, Florida: SPIE; 2011. p. 79651Z. [Google Scholar]
- 11.Yao J, O’Connor SD, Summers RM. Automated spinal column extraction and partitioning. 2006 3rd IEEE International Symposium on Biomedical Imaging: From Nano to Macro - Proceedings; 2006. pp. 390–393. [Google Scholar]
- 12.Metz CE. ROCKIT. http://metz-roc.uchicago.edu/
- 13.Kissebah AH, Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 14.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 15.Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288:1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HW, Kim D, Kim HJ, et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am J Gastroenterol. 2010;105:178–187. doi: 10.1038/ajg.2009.541. [DOI] [PubMed] [Google Scholar]
- 17.Yamaji T, Iwasaki M, Sasazuki S, et al. Visceral fat volume and the prevalence of colorectal adenoma. Am J Epidemiol. 2009;170:1502–1511. doi: 10.1093/aje/kwp311. [DOI] [PubMed] [Google Scholar]
- 18.Johnson KT, Harmsen WS, Limburg PJ, Carston MJ, Johnson CD. Visceral fat analysis at CT colonography. Acad Radiol. 2006;13:963–968. doi: 10.1016/j.acra.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Oh TH, Byeon JS, Myung SJ, et al. Visceral obesity as a risk factor for colorectal neoplasm. Journal of gastroenterology and hepatology. 2008;23:411–417. doi: 10.1111/j.1440-1746.2007.05125.x. [DOI] [PubMed] [Google Scholar]
- 20.Otake S, Takeda H, Suzuki Y, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res. 2005;11:3642–3646. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 21.Sass DA, Schoen RE, Weissfeld JL, et al. Relationship of visceral adipose tissue to recurrence of adenomatous polyps. Am J Gastroenterol. 2004;99:687–693. doi: 10.1111/j.1572-0241.2004.04136.x. [DOI] [PubMed] [Google Scholar]
- 22.Schoen RE, Weissfeld JL, Kuller LH, et al. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology. 2005;129:464–475. doi: 10.1016/j.gastro.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 23.Erarslan E, Turkay C, Koktener A, Koca C, Uz B, Bavbek N. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci. 2009;54:862–868. doi: 10.1007/s10620-008-0440-6. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S, Nakagawa T, Matsushita Y, et al. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care. 2010;33:184–189. doi: 10.2337/dc09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaji Y, Okamoto M, Yoshida H, et al. The effect of body weight reduction on the incidence of colorectal adenoma. The American journal of gastroenterology. 2008;103:2061–2067. doi: 10.1111/j.1572-0241.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 26.Rapp K, Schroeder J, Klenk J, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062–1067. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engeland A, Tretli S, Austad G, Bjorge T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control. 2005;16:987–996. doi: 10.1007/s10552-005-3638-3. [DOI] [PubMed] [Google Scholar]
- 28.Lukanova A, Bjor O, Kaaks R, et al. Body mass index and cancer: results from the Northern Sweden Health and Disease Cohort. Int J Cancer. 2006;118:458–466. doi: 10.1002/ijc.21354. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu N, Nagata C, Shimizu H, et al. Height, weight, and alcohol consumption in relation to the risk of colorectal cancer in Japan: a prospective study. Br J Cancer. 2003;88:1038–1043. doi: 10.1038/sj.bjc.6600845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 32.MacInnis RJ, English DR, Hopper JL, Gertig DM, Haydon AM, Giles GG. Body size and composition and colon cancer risk in women. Int J Cancer. 2006;118:1496–1500. doi: 10.1002/ijc.21508. [DOI] [PubMed] [Google Scholar]
- 33.MacInnis RJ, English DR, Hopper JL, Haydon AM, Gertig DM, Giles GG. Body size and composition and colon cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2004;13:553–559. [PubMed] [Google Scholar]
- 34.Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 35.Martinez ME, McPherson RS, Levin B, Glober GA. A case-control study of dietary intake and other lifestyle risk factors for hyperplastic polyps. Gastroenterology. 1997;113:423–429. doi: 10.1053/gast.1997.v113.pm9247459. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien MJ. Hyperplastic and serrated polyps of the colorectum. Gastroenterology Clinics of North America. 2007;36:947–968. doi: 10.1016/j.gtc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Winawer SJ, Zauber AG, O’Brien MJ, et al. The National Polyp Study. Design, methods, and characteristics of patients with newly diagnosed polyps. The National Polyp Study Workgroup. Cancer. 1992;70:1236–1245. doi: 10.1002/1097-0142(19920901)70:3+<1236::aid-cncr2820701508>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Zhao B, Colville J, Kalaigian J, et al. Automated quantification of body fat distribution on volumetric computed tomography. J Comput Assist Tomogr. 2006;30:777–783. doi: 10.1097/01.rct.0000228164.08968.e8. [DOI] [PubMed] [Google Scholar]
- 39.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 40.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 41.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 42.Paz-Filho G, Lim EL, Wong ML, Licinio J. Associations between adipokines and obesity-related cancer. Frontiers in bioscience: a journal and virtual library. 2011;16:1634–1650. doi: 10.2741/3810. [DOI] [PubMed] [Google Scholar]
- 43.Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proceedings of the Japan Academy Series B, Physical and biological sciences. 2010;86:131–141. doi: 10.2183/pjab.86.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 46.Meng K, Lee CH, Saremi F. Metabolic syndrome and ectopic fat deposition: what can CT and MR provide? Acad Radiol. 2010;17:1302–1312. doi: 10.1016/j.acra.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guiu B, Petit JM, Bonnetain F, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59:341–347. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 49.Moon HG, Ju YT, Jeong CY, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Annals of surgical oncology. 2008;15:1918–1922. doi: 10.1245/s10434-008-9891-4. [DOI] [PubMed] [Google Scholar]
- 50.Nitori N, Hasegawa H, Ishii Y, Endo T, Kitagawa Y. Impact of visceral obesity on short-term outcome after laparoscopic surgery for colorectal cancer: a single Japanese center study. Surg Laparosc Endosc Percutan Tech. 2009;19:324–327. doi: 10.1097/SLE.0b013e3181ae5442. [DOI] [PubMed] [Google Scholar]